Effect of livestock grazing and human uses on herbaceous species diversity in oriental beech (Fagus orientalis Lipsky) forests, Guilan, Masal, northern Iran
2014-04-20SepideSadatEbrahimiHassanPourbabaeiDavidPotheirAliOmidiJavadTorkaman
Sepide Sadat Ebrahimi • Hassan Pourbabaei • David Potheir Ali Omidi • Javad Torkaman
Introduction
Plant diversity plays key ecological roles in forest ecosystems, influencing succession, resilience and nutrient cycling (Nilsson and Wardle 2005; Gilliam 2007; Hart and Chen 2008). Therefore, vegetation studies are important to assess the current state of forest ecosystems and to make projections for the near future. In the last few decades, especially after the Rio de Janeiro conference on global biodiversity, loss of biodiversity caused by human activities became a major source of concern for forest ecologists (UNCED 1992). Such disturbances can profoundly alter plant communities in terms of density and composition. Pressures on ecosystems, including grazing and human utilization, cause degradation and reduce genetic diversity, especially in forests, which are considered one of the most sensitive ecosystems. Direct effects of grazing and trampling on species diversity depend on abiotic factors such as weather conditions and soil characteristics that can significantly affect the structure, canopy and soil properties of forests along with air temperature and humidity. Ultimately, depending on biotic and abiotic factors associated with environmental conditions and grazing, the functions of entire ecosystems can change (Sætersdal et al. 2004; Cingolani 2005).
Many studies of vegetation response concluded that high grazing intensity reduced plant diversity by decreasing evenness in plant communities (Tillman and Downing 1994). Conversely, other studies indicated that low grazing intensity positively affected ecosystem functions and vegetation diversity depending on the type and abundance of livestock as well as grazing period and vegetation type (Bouahim et al. 2011; Frank and McNaughton 1993; Augustine and Frank 2001; Casasus et al. 2007; Loucougaray et al. 2004; Van Uytvanck and Hoffmann 2009).
Forests cover 12 million hectares of the land surface of Iran (8% of the total land area), of which about 1.8 million hectares are located in the northern part of the country, i.e. the Hyrcanian or Caspian Forest ecoregion. This forest type is situated on the northern slopes of the Alborz Mountains along the Caspian Sea (Sagheb-Talebi et al. 2004). Some parts of this forest are under pressure from grazing and human utilization. Among the forest communities in northern Iran, plant communities dominated by oriental beech (Fagus orientalis Lipsky) are more valuable for society because they constitute a major sink of carbon (Hall et al. 2001) and are important for socio-economic activities, soil protection and recreation resources (Wardle 2005).
Forests dominated by oriental beech cover about 565,000 ha and represent the total area of indigenous forests in Guilan Province. Considering the socio-economic importance of these ecosystems and their conservation, the general objective of this study was to investigate the effects of grazing and human activities on herbaceous species diversity of these forests. The results can contribute to development and application of sustainable management in the forests of this region within which plant diversity has never been studied.
Material and methods
Study areas
This study was carried out in a 100-ha forest area of the Masal of Guilan province in northern Iran at latitude 37°14'00" to 37°19'20" N and longitude 48°55̕'19" to 49°02̕'00" E. Elevation ranges from 300 to 2000 m, a.s.l. and the area is mainly characterized by east-facing slopes. Mean annual precipitations and temperature are 990 mm and 16°C, respectively (information from station of Hydrology and Meteorology at Shanderman). Common forest soils are acidic with pH of 5.5 to 6.5. Parent materials include shill, sandstone and limestone.
There was no permanent residential land in the study area, but dairy farmers and locals generally used the area for animal husbandry for about 2-4 months during spring and summer each year. The forests were under heavy pressure of livestock grazing, girdling, and excessive cutting of trees and shrubs to supply fuel wood. All these activities were thought to change the primary structure of these forests that are uneven-aged and composed of mixed deciduous broadleaved trees or, more rarely, of oriental beech only. The protected area was dominated by oriental beech which was less abundant in the unprotected area due to destructive activities such that a significant proportion of the area completely lacked trees. To restore forests and reduce grazing pressure, 50 ha of these forests were fenced in 2005 to prevent the entry of livestock and humans.
Data collection
We surveyed 50 ha of protected area and 50 ha of unprotected area. Survey sites were selected along the sides of a road near each other. The protected and unprotected areas were similar in terms of elevation, slope and aspect. At each survey area, 25 circular plots of 1000-m2area were established following a random-systematic network using a 100 m × 200 m grid (Zobeiry 2002). Elevation, aspect and slope gradient were recorded at each sampling point. In addition, we measured litter depth at five locations within each plot (Adel et al. 2013) and we estimated the number of trees per hectare and the percent canopy cover of trees. Because the 1000 m2plots were too large for detailed measurements of herbaceous species, we used the Whittaker's nested plot sampling and minimal area method to determine an optimal subplot size. This resulted in subplots of 64 m2being sampled for herbaceous species measurements and in each of these subplots, percent cover of each species was estimated according to the Domin criterion (Mueller and Ellenberg 1989).
Data analysis
To evaluate herbaceous diversity, we computed different components of diversity, evenness and richness. First, species diversity was assessed with the Shannon-Wiener (H΄) and McArthur and Hill΄s indices (Ludwig et al. 1988; Krebs 1999).
Shannon-Wiener index:

where, Piis the relative frequency of the ithspecies
McArthur index:

where, N1is an equal number of common species that create diversity similar to the H'. The e is logarithm, H'is Shanon-Wiener.
Hill΄s index:

where, λis the dominance index of Simpson and N2is the inverse of Simpson’s dominance index.
Second species richness was estimated (Humphries et al. 1996) according to the R=S index in which S is the number of species. Finally evenness was assessed using the Smith-Wilson index, the modified index of Nee (EQ) and the modified index of Hill (E5) (Krebs 1999):
Smith-Wilson index:

where, niis the number of individuals of the ithspecies in a plot, njis number of individuals of the jthspecies, and S is the total number of species;
Modified index of Hill:

where, N1is an equal number of common species that yield diversity similar to that expressed by Hˊ, N2is the inverse of Simpson’s dominance;
Modified Nee index:

where, b is the gradient of Dominance - Diversity curves. All diversity indices were computed with Ecological Methodology software for Windows, version 6.0 (Krebs 1989). Kolmogorov–Smirnov tests were used to study the normality of data distribution. To detect differences in diversity indices between protected and unprotected areas, Student t-tests were used in the case of normally distributed data while the non-parametric equivalent (Mann-Whitney U-test) was applied to other cases. These analyses were conducted with SPSS 16.0 software.
Results
Mean leaf litter depth and mean percent canopy cover of trees in the protected area were both significantly higher than in the unprotected area. Mean percent herbaceous cover was higher in the unprotected area than in the protected area (Table 1). In the combined areas, we recorded 64 species of 35 plant families, with 51 species of 33 families in the protected area and 49 species of 31 families in the unprotected area (Appendix 1).

Table 1: Characteristics of the protected and unprotected study area.
Although many species were common to both areas, some were only present in the protected area (Hordeum spp., Hordeum spontaneum, Sanicula europaeal, Alium spp., Malva spp., Hypericum perforatum, Lathyrus spp., Orobus spp., Solanum nigrum, Convulva L, Nasturtium officinal) while others were only present in the unprotected area (Crocus sativus, Conyza canadensis, Tanacetum spp., Crisium avvense, Cricium congestum, Taraxacum sp, Gundeliatour enfortti, Acropetilon repens, Asperula stylosa, Bromus L, Teucrium hyrcanicum, Cephalanthera sp).
In the protected area, the Poaceae family was represented by the highest number of species (5) while Asplenaceae and Lamiaceae were represented by four species each. Hypericaceae, Polygonaceae, Asteraceae, Solanaceae, Rosaceae, Brassicaceae, Apiaceae each had two species and other families’ one species. In the unprotected area, the family with most species was Asteracea at seven species while Poaceae was represented by five species, Aspleniaceae by four species, Rosaceae by three species, and Lamiaceae, Solanaceae, Polygonacea, and Apiaceae by two species each. In the protected area, the highest species frequencies were associated with Euphorbia amygdaloides (100), Pteridium aquilinum (80), Ceterach officinarum (80), Primula hetrochroma (72), Fragaria vesca L. (72), and Rumex sp (60). In the unprotected area, highest frequencies were Viola sylvestris (92), Prunella vulgaris (84), Petasites hybrids (76), Rubus fruticosus (76), Potentilla recta (72), Sambacus ebulus (64), Sedum stoloiferum (60), and Rumex spp.. Six species in the protected area and one species in the unprotected area occurred at frequencies less than five.
In the protected area, highest percent cover was recorded for Euphorbia amygdalodies (17.7%), Lathyrus spp. (12%), Lamium alba (11%), Circaea lutetiana L (10%), Primula hetrochrom (9.2%), and Phytolacca American (7.2%). In the unprotected area, highest percent cover was recorded for (Table 2): Sambacus ebulus (39.1%), Pteridium aquilinum (33.2%), Chenopodium album (13.7%), Prunella vulgaris (12.3%), Asplenium trichomanes (9.2%), Potentilla recta (8.3%) and Teucrium hyrcanicum (8.3%).
One species in the protected area and 15 species in the unprotected area accounted for less than 1% of herbaceous cover. Percent cover of 15 species differed significantly between the areas. With the exception of Pteridium aquilinum, Asplenium trichomanes, Sedum stoloniferum and Rumex spp., percent cover of the above-listed species was higher in the protected than in the un-protected area. Values of diversity indices H′, N1, N2aVarand E5were greater in the protected area than in the unprotected area, and all differences except for the EVarindex were significant at the 5% level. Richness was higher in the protected area than in the unprotected area, but the difference was not significant (Table 2).

Table 2: Means, Standard deviation (SD) and Standard Error (SE) of biodiversity indices in protected and unprotected areas.
Pearson correlations between diversity indices and both evenness and richness indices are presented in Table 3. In the protected area, E5index was positively and significantly (0.01%) correlated with H′, N1and N2indices whereas EVarand Nee indices had significant correlations with N2index (at 5% level). Also, EVarindex was significantly (5%) correlated with N1index while R=S index was significantly related to H′ and N1indices at the 5% level (Table 3). In the unprotected area, EVarwas positively correlated with diversity indices whereas R=S index was correlated with H′ and N1indices, but not with N2index. E5index was positively and significantly correlated with N2index at the 1% (Table 3).
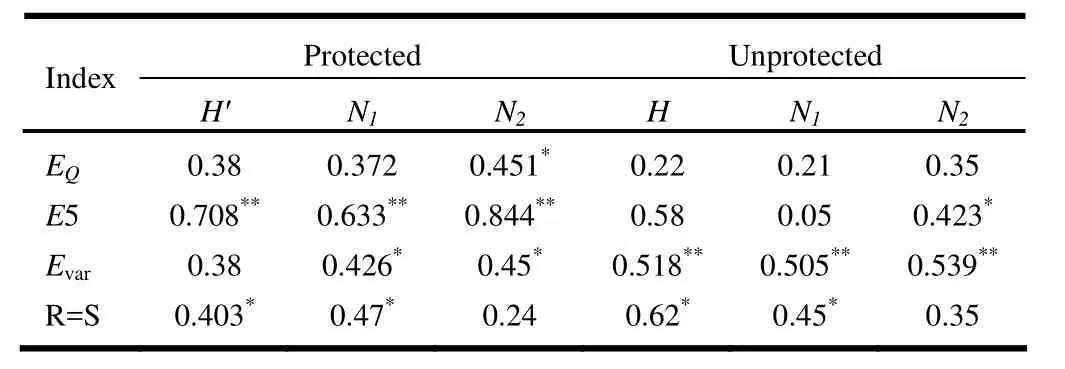
Table 3: Pearson correlations between diversity indices with richness and evenness.
Discussion
Diversity, richness and evenness were lower in areas that were subject to intensive human exploitation and livestock grazing. Many other studies have shown that the number and diversity of forest herbaceous species are sharply reduced by similar destructive factors such that many sensitive species have been eradicated (Keeley et al. 2003; Bouahim et al. 2011; Krzic et al. 2003; Hendricks et al. 2005; Milgo 2006; Cesa et al. 2011). Conversely, when forests receive protection from grazing, plant and species numbers increase markedly (Bertoncini and Rodrigues 2008; Bengtsson et al. 2002; Schmidt 2005; Caspersen and Pacala 2001).
Species richness was slightly higher in the protected than in the unprotected areas and this could be predicted because grazing is known to be a cause of reduced species richness (Roberts and Zhu 2002; Schumann et al. 2003; Pykälä 2005; Shackleton et al. 2000; Altesor et al. 2006; Loydi and Distel 2010; Schultz et al. 2011). In both areas, diversity was more strongly correlated with evenness than richness. This result is supported by observations of the important role of evenness in increasing diversity as compared to species richness (Casado et al. 2004; Anderson et al. 2007; Arévalo et al. 2007; Fernandez-Lugo et al. 2009). In the unprotected area, grazing reduced both evenness and species diversity by removing and reducing the coverage of some sensitive species (Webster et al. 2005; Milgo 2006). Therefore, species diversity was maximum when grazing pressure was minimum, as reported for other ecosystems (Jouri 2009; Nikbole and Ojima 2004; Yingzhang et al. 2004; Ta´rrega et al. 2006).
Average litter depths were 9.6 and 1.4 cm in the protected and unprotected areas, respectively. Accordingly, higher values of litter depth and percent cover of herbaceous species were reported for protected areas in other studies (Potvin and Harrison 1994; Berg et al. 1997). In our protected area, the high density of herbaceous species was an important factor explaining greater litter depth and the high density of deciduous trees. The absence of grazing accounted for the greater litter depth in the protected area. In contrast, in the unprotected area, grazing was an important factor reducing litter depth and vegetation cover (Xie et al. 2007; McEvoy et al. 2006). The destruction and the excessive use of herbaceous cover probably reduced the growth and the reproduction capacity of many species while some sensitive species probably disappeared over time and were replaced by invader species as reported for other sites by Onainadia et al. (2004) and Webster et al. (2005). Reduced litter depth can also be attributed to the mechanical effects of trampling and grazing that stimulate litter decomposition by crushing litter into small pieces. Together with changes in forest density and composition, reduction in litter depth caused by grazing negatively impacted forest ecosystems through changes in organic matter that promote soil erosion (Belsky and Blumenthal 1997).
Grazing has a negative effect on threatened species (Campbell and Donlan 2005; Carrete et al. 2009). Lathyrus sylvatica was a common and dominant species in the protected area, but its abundance was sharply reduced when subject to grazing (Mitchell and Kirby 1990). Also, Hedra helix and Primula hetrochroma are palatable species for livestock, but are resistant to grazing and are indicator species for protected areas (Kuiters and Slim 2003; Kirby 2001).
Some species were only recorded in the unprotected area (Table 2), suggesting that grazing could have promoted colonization by a number of rare species or unpalatable species for livestock. Actually, these species successively competed with other species and their growth was completed before livestock entered the area. Grazing can cause changes in the pattern of species dominance (Muthuramkumar et al. 2006; Aikens et al. 2007; Rasingam et al. 2009). For example, the dominant Asteraceae family in the unprotected area was replaced in the unprotected area by Cricium congestum, Crisium avvense, Uritica dioica, Prunella vulgaris or Rumex sp, which can be considered as indicator species of the unprotected area. On the other hand, Euphorbia amygdaloides can be considered as an indicator species of the protected area (McEvay et al. 2006).
Percent cover of herbaceous species reached 91.6 and 99.0% in the protected and the unprotected areas, respectively, while tree canopy cover was lower in the unprotected than the protected area. On forest sites, herbaceous species richness is mostly influenced by canopy openness which increases with site degradation. The reduction of canopy cover increases solar irradiation at the forest floor and, by providing the energy-rich material for photosynthesis, increases plant production (Ebrahimzade 1990; Boer, 1998). Decomposition, on the other, increases temperature and moisture, both key factors for controlling litter decomposition (Lousier and Parkinson 1975; Moore 1986), and thereby stimulates (Riterr 2005; Hopmans 2006) and ultimately leads to increased availability of nutrients. This could explain the higher herbaceous cover in the unprotected area. However, disturbances such as grazing and human pressure can lead to changes in ecological conditions, especially competition among plant species and can alter vegetation composition and promote the emergence of invasive species (Keeley et al. 2003; Anderson 2007; Vera et al. 2000).
Invasive species, characterized by rapid seed dispersal and germination, high competitive capacity, short life span and fast growth, strongly compete with native species and have caused changes in the structure and processes of many ecosystems (Godefroid et al. 2005; Martin et al. 2009). Establishment of invasive species in natural ecosystems is usually slow, but this process is accelerated by human intervention and site degradation (Martin et al. 2009).
In the unprotected area, the dominance of Pteridium aquilinum and Sambucus ebulus increased gradually such that in many locations, their coverage was 100% to form a closed cover above the ground. These species were mainly present in gaps resulting from human activities. On the other hand, due to the tall height of invasive species, competition for light increases and thus richness and percent cover decline for many forest floor species (Howard and Lee 2003). In addition, Sambucus ebulus can reduce growth of other species by allelopathy and chemical release (Pourbabaei et al. 2004). Of species in the unprotected area, none exceeded 5% in cover except Potentila recta, Viola sylvestris, Asplenium trichomanes, Polygonatum orientale, Prunella vulgaris, Acropetilon repens, Teucrium hyrcanicum.
The percent cover of 15 plant species differed between the protected and the unprotected areas. For example, the percent cover of typical species of beech communities in northern Iran such as Euphorbia amygdaloides, Lamium album L, Viola sylvestris, Carex sp, Sanicula europaeal, Chenopodium album L. and Galium sp (Taheri and Pilevar 2008) was lower in the unprotected than in the protected areas, suggesting that they were negatively affected by grazing and human activities. Since species diversity and richness are closely associated with grazing and traditional human activities, conservation programs should be developed in collaboration with local people in order to promote education and correct land utilization to help conserve natural resources and biodiversity. In addition, we recommend controlling livestock access and exploitation by local people in sensitive forest areas. Finally, studies should be undertaken to determine the parameters of conservation and restoration of these ecosystems.
Adel MN, Pourbabaei H, Omidi A, C Dey Daniel. 2013. Forest structure and woody plant species composition after a wildfire in beech forests in the north of Iran. Journal of Forestry Research, 24(2): 255-262.
Aikens Melissa L, Ellum D, McKenna John J. 2007. The effects of disturbance intensity on temporal and spatial patterns of herb colonization in a southern New England mixed-oak forest. Forest Ecology and Management, 252: 144–158.
Altesor A, Piñeiro G, Lezama F, Jackson RB, Sarasola M, Paruelo JM. 2006. Ecosystem changes associated with grazing in subhumid South American grasslands. Journal of Vegetation Science, 17: 323–332.
Anderson PML, Hoffman MT. 2007. The impacts of sustained heavy grazing on plant diversity and composition in lowland and upland habitats across the Kamiesberg mountain range in the Succulent Karoo South Africa. Journal of Arid Environment, 70: 686–700.
Arévalo JR, Chinea E, Barquín E. 2007. Pasture management under goat grazing on Canary Islands. Agriculture Ecosystem and Environment, 128: 291–296.
Augustine DJ, Frank DA. 2001. Effects of migratory grazers on spatial heterogeneity of soil nitrogen properties in a grassland ecosystem. Ecology, 82: 49–62.
Belsky JA, Blumenthal D. 1997. Effect of Livestock Grazing on Stand Dynamics and Soils in Upland Forests of the Interior West. Conservation Biology, 11(2): 315–327.
Bengtsson J, Engelhart K, Giller P. 2002. The scaling components of biodiversity ecosystem functioning relations, Oxford: Oxford University Press, p. 220.
Berg WA, Bradford JA, Sims PL. 1997. Long-term soil nitrogen and vegetation change on sand hill rangeland. Journal of Range Management, 50(5): 462–466.
Bertoncini AP, Rodrigues RR. 2008. Forest restoration in an indigenous land considering a forest remnant influence (Avaí, São Paulo State, Brazil). Forest Ecology and Management, 255: 513–521.
Boer BE, Sargent DO. 1998. Desert perennial as plant and soil indicator in Eastern Arabia. Journal of Plant and Soil. 199: 261–266.
Bouahim S, Rhazi L, Mathevet R, Ernoual L, Amami B, Saber E, Muller SD, Grillas P. 2011. Analysis of perception of temporal pools in western of Morocco by the local stakeholders and the interest of sustainable development. Journal of Materials and Environment Science, 2 (S1): 451–454.
Campbell KJ, Donlan CJ. 2005. A review of feral goat eradication on islands. Conservation Biology, 19: 62–74.
Carrete M, Serrano D, Illera JC, López G, Vögeli M, Delgado A, Tella J. 2009. Goats, birds, and emergent diseases: apparent and hidden effects of exotic species in an island environment, Ecological Applications, 19: 840–853.
Casado MA, Castro I, Ramírez-Sanz L, Costa-Tenorio M, de Miguel JM, Pineda FD. 2004. Herbaceous plant richness and vegetation cover in Mediterranean grasslands and shrub lands. Plant Ecology, 170: 83–91.
Casasus I, Bernues A, Sanz A, Villalba D, Riedel JL, Revilla R. 2007. Vegetation dynamics in Mediterranean forest pastures as affected by beef cattle grazing. Agriculture Ecosystem and Environment, 121: 365–370.
Caspersen J, Pacala S. 2001. Successional diversity and forest ecosystem function. Ecological Research, 16: 895–903.
Cesa A, Paruelo JM. 2011. Changes in vegetation structure induced by domestic grazing in Patagonia (Southern Argentina), Journal of Arid Environment, 75: 1129–1135.
Cingolani AM, Noy-Meir I, Díaz S. 2005. Grazing effects on rangeland diversity: a synthesis of contemporary models. Ecological Application, 15 (2): 757–775.
Ebrahimzade H. 1990. Plant Physiology (Nutrition and assimilation). Iran (Tehran): Tehran University Press, p. 492.
Fernández-Lugo S, de Nascimento L, Mellado M, Bermejo L.A, Arévalo J.R. 2009. Vegetation change and chemical soil composition after four years of goat grazing exclusion in a Canary Islands pasture. Agriculture Ecosystem and Environment, 132: 276–282.
Frank DA, McNaughton SJ. 1993. Evidence for the promotion of above ground grassland production by native large herbivoresin Yellowstone National Park. Oecology, 96: 157–61.
Gilliam FS. 2007. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience, 57: 845–858.
Godefroid S, Massant W, Weyembergh G, Koedam N. 2005. Impact of fencing on the recovery of th ground flora on heavily eroded slopes of a deciduous forest. Environment Management, 32: 62–76.
Hall GMJ, Wiser SK, Allen RB, Beets PN, Goulding CJ. 2001. Strategies to estimate national carbon stocks from inventory data: the 1990 New Zealand baseline. Global Change Biology, 7: 389–403.
Hart SA, Chen HYH. 2008. Fire, logging, and overstory affect understory abundance, diversity and composition in boreal forest. Ecological Monograph, 78: 123–140.
Hendricks HH, Bond WJ, Midgley JJ, Novellie PA. 2005. Plant species richness and composition a long livestock grazing intensity gradients in a Namaqualand (South Africa) protected area. Plant ecology, 176: 19–33.
Hopmans JW, 2006. Soil physical properties, processes and associated root–soil interactions. In: D´Odorico P, Porporato, A. (Eds.), Dryland Ecohydrology. Netherlands: Springer Netherlands, p. 13–29.
Howard LF, Lee TD. 2003. Temporal patterns of vascular plant diversity in southeastern New Hampshire forests. Forest Ecology and Management, 185: 5–20.
Humphries CJ, Williams PH, Vane-Wright RI. 1996. Measuring biodiversity value for conservation. Annual Review of Ecology and Systematic, 26: 93–111.
Jouri MH, Temzad B, Shokri M, Banihashemi B. 2009. Comparison of Diversity and Richness Indices for Evaluation of Mountain Rangeland Health (Case study: Rangelands of Javaherdeh of Ramsar). Journal of rangeland, 2(4): 344–356 (In Persian).
Keeley JE, Lubin D, Fotheringham CJ. 2003. Fire and grazing impacts on plantdiversity and alien plant invasions in the southern Sierra Nevada. Ecological Applications, 13: 1355–1374.
Kirby KJ. 2001. The impact of deer on the ground flora of British broadleaved woodland. Forestry, 74(3): 219–229.
Krebs CJ. 1989. Ecological Methodology 1stedition: New York: Harper and Row Publishers, p. 654.
Krebs CJ. 1999. Ecological methodology (2 nd edition): Addison Wesley Longman Menlo Park. California. British: Columbia Technische University Press, p. 607.
Krzic M, Newman RF, Broersma K. 2003. Plant species diversity and soil quality in harvested and grazed boreal aspen stands of northeastern British Columbia. Forest Ecology and Management, 182: 315–325.
Kuiters AT, Slim PA. 2003. Tree colonisation of abandoned arable land after 27 years of horse-grazing: the role of bramble as a facilitator of oak wood regeneration. Forest Ecology and Management, 181: 239–251.
Loucougaray G, Bonis A, Bouzillé JB. 2004. Effects of grazing by horses and or cattle on the diversity of coastal grasslands in western France. Biological Conservation, 116 (1): 59–71.
Lousier JD, Parkinson D, 1975. Litter decomposition in a cool temperate deciduous forest. Canadian Journal of Botany, 54: 419–436.
Loydi A, Distel RA. 2010. Floristic diversity under different intensities of large herbivore grazing in mountain grasslands of the Ventania System, Buenos Aires. Ecología Austral, 20: 281–291.
Martins da Silva P, Aguiar CAS, Niemela J, Sousa JP, Serrano ARM. 2009. Cork-oak woodlands as key-habitats for biodiversity conservation in Mediterranean landscapes: a case study using rove and ground beetles (Coleoptera: Staphylinidae, Carabidae). Biodiversity Conservation, 18: 605–619.
McEvoy PM, Flexan M, McAdam JH. 2006. The effects of livestock grazing on ground flora in broadleaf woodlands in Northern Ireland. Forest Ecology and Management, 225: 39–50.
Mitchell FJG, Kirby KJ. 1990. The impact of large herbivores on the conservation of semi-natural woods in the British uplands. Forestry, 63: 333–353.
Mligo C. 2006. Effect of grazing pressure on plant species Composition and diversity in the semi-arid rangelands of Mbulu district, Tanzania. Agricultural Journal, 1 (4): 277–283.
Moore AM. 1986. Temperature and moisture dependence of decomposition rates of hardwood and coniferous leaf litter. Soil Biology and Biochemistry, 18: 427–435
Mueller DD, Ellenberg H. 1989. Aims and methods of vegetation ecology. New York: NY: Wiley, p. 547.
Muthuramkumar S, Ayyappan N, Parthasarathy N, Mudappa D, Shankar Raman TR, ArthurSelwyn M. 2006. Plant community structure in tropical rainforest fragments of the Western Ghats, India. Biotropica, 38: 143–160.
Nikbole NB, Ojima DS. 2004. Changes in plant functional groups, litter quality, and soil carbon and nitrogen mineralization with sheep grazing in anInner Mongolian grassland. Journal of Range Management, 57: 613–619.
Nilsson MC, Wardle DA. 2005. Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forests. Frontiers in Ecology and the Environment, 3: 421–428.
Potvin MA, Harrison AT. 1994. Vegetation and litter changes of a Nebraska sand hills prairie protected from Grazing. Journal of. Range Management, 37 (1): 55–58.
Pourbabaei H, Ahani H. 2004. Biodiversity of woody species in Acer platanoides sites in the Shafaroud forests, Guilan. Rostaniha, 5(2): 147–158.
Pykälä J. 2005. Plant species responses to cattle grazing in mesic semi-natural grassland. Agriculture ecosystem and environment, 108: 109–117.
Rasingam L, Parthasarathy N. 2009. Diversity of understory plants in undisturbed and disturbed tropical lowland forests of Little Andaman Island, India. Biodiversity Conservation, 18: 1045–1065.
Riterr E. 2005. Litter decomposition and nitrogen mineralization in newly formed gaps in a Danish beech (Fagus sylvatica) forest. Soil Biology and Biochemistry. 37: 1237–1247
Roberts MR, Zhu L. 2002. Early response of the herbaceous layer to harvesting in a mixed coniferous-deciduous forest in New Brunswick, Canada. Forest Ecology and Management, 155: 17–31.
Sætersdal M, Gjerde I, Blom HH, Eide E, Ihlen PG, Pommeresche R, Skartveit J, Solhøy T, Aas O. 2004. Vascular plants as a surrogate species group in complementary site selection for bryophytes, macrolichens, spiders, carabids, staphylinids, snails, and wood living polypores in a northern forest. Biological Conservation, 115: 21–31.
Sagheb-Talebi K, Sajedi T, Yazdian F. 2004. Forests of Iran. Tehran: Research Institute of forests and Rangelands, p.28.
Schmidt W. 2005. Herb layer species as indicators of biodiversity of managed and unmanaged beech forests. Forest Snow Landscape Research, 79: 111–125.
Schultz NL, Morgan JW, Lunt ID. 2011. Effects of grazing exclusion on plant species richness and phytomass accumulation vary across a regional productivity gradient. Journal of Vegetation Science, 22: 130–142.
Schumann ME, White AS, Witham JW. 2003. The effects of harvest created gaps on plant species diversity, composition savanna South Africa. Biological Conservation, 94: 273–285.
Ta´rrega R, Calvo L, Marcos E, Taboada A. 2006. Forest structure and understory diversity in Quercus pyrenaica communities with different human uses and disturbances. Forest Ecology and Management, 227: 50–58.
Taheri abkenar K, Pilevar B. 2008. Silviculture. Iran (Rasht): Haghshenas publishing, p.300.
Tilman D, Downing JA. 1994. Biodiversity and stability in grassland. Nature, 367: 363–365.
Van Uytvanck J, Hoffmann M. 2009. The role of large herbivores in woodland regeneration patterns, mechanisms and processes. Belgium (Brussels): Research Institute for Nature and Forest (INBO), p. 241.
Vera FWM. 2000. Grazing ecology and forest history. Oxon: CABI Publishing, p. 528.
Wardle DA. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs, 75: 3–35.
Webster CR, Jenkins MA, Rock JH. 2005. Long-term response of spring flora to chronic herbivory and deer exclusion in Great Smoky Mountains National Park, USA. Biological Conservation, 125: 297–307.
Xie Y, Becker U, Witting R. 2007. Vegetation of the Stipa loess steppe un Ningxia (northern China) in relation to grazing intensity. Grassland Science, 53: 143–-154.
Xie Y, Wittig R. 2004. The impact of grazing intensity on soil characteristics of Stipagrandis and Stipa bungeana stepps in northern China (autonomous region of Ningxia). Acta Oecologia, 25(3): 197–204.
Zobeiry M. 2002. Forest inventory (Measurement of tree and forest). Iran (Tehran): Tehran university publishing, p. 401.

Appendix 1: Frequency and mean percent cover of herbaceous species in protected and unprotected areas.
杂志排行
Journal of Forestry Research的其它文章
- Carbon sequestration in Chir-Pine (Pinus roxburghii Sarg.) forests under various disturbance levels in Kumaun Central Himalaya
- Regional differences of water conservation in Beijing’s forest ecosystem
- Spatial modeling of the carbon stock of forest trees in Heilongjiang Province, China
- Spatial heterogeneity of factors influencing forest fires size in northern Mexico
- Community ecology and spatial distribution of trees in a tropical wet evergreen forest in Kaptai national park in Chittagong Hill Tracts, Bangladesh
- Diversity, regeneration status and population structure of gum- and resin-bearing woody species in south Omo zone, southern Ethiopia
