Comparative growth, dry matter accumulation and photosynthetic rate of seven species of Eucalypt in response to phosphorus supply
2014-04-20PengfeiWuXiangqingMaMulualemTigabuYongHuangLiliZhouLipingCaiXiaolongHouPerChristerOden
Peng-fei Wu • Xiang-qing Ma • Mulualem Tigabu • Yong Huang Li-li Zhou • Liping Cai • Xiao-long Hou • Per Christer Oden
Introduction
The ever-increasing demand for wood, fibre and pulp coupled with efforts to mitigate greenhouse gas emission and environmental degradation has emphasized the need for the development of forest plantations. As a result, a surge in areas of new forest plantations has been observed over the past decades in Brazil, China and India (Singh 2013). Particularly, short-rotation trees (e.g. Eucalyptus) have long been recognized as sources of raw material for bioenergy, pulp and paper industries, and they sequester carbon (Shepherd et al. 2011). Eucalypts are fast-growing tree species used in short rotation plantations in many parts of the world owing to their wide range of environmental tolerance and multiple uses. China has the second largest (after Brazil) area of eucalypt plantations in the world and their impact is increasingly important in ecological, social, economic, and other issues (Wang et al. 2004). Most eucalypt plantations are concentrated in southern China (Liu et al. 1994), where the soils are widely deficient in plant-available P(Xu et al. 2001), although the total P level in the soils is generally high (Chen et al. 1996).
Currently, many small-scale farmers in southern China widely plant Eucalyptus species or hybrids without knowledge of their ability to tolerate low P stress. P deficiency is one of the major limitations for plant growth and productivity, particularly in the tropics, where the soil is highly weathered and acidic (Raghothama 1999; Shenoy and Kalagudi 2005). Generally, plants that receive sub-optimal levels of P grow slowly, with a yield loss up to 5%–15% (Shenoy and Kalaguid 2005). Further, P starvation reduces photosynthetic rate, stomatal conductance, photosynthetic phosphorylation, ATP production, the production and export of triose-P and ribulose-1, 5-bisphosphate regeneration (Jacob and Lawlor 1991; Thomas et al. 2006b; Warren 2011). Although application of P-containing fertilizers is usually recommended to enhance P availability, its high cost and sustained availability coupled with eutrophication and hypoxia of lakes and marine estuaries (Scholz et al. 2013) are limiting its wider application in short-rotation plantations. Therefore, judicious selection of species that can tolerate low P stress is a reliable option.
Research on responses of eucalypt species to low P stress is generally scanty (Kirschbaum and Tompkins 1990; Warren 2011; Thomas et al. 2006a); particularly there is a lack of knowledge about P-efficient eucalypt species suitable for planting. As a result, small-scale farmers can receive little guidance in their selection of eucalypt species. This study, therefore, aims at providing scientific evidence for selection of eucalypt species suitable for short-rotation planting as well as those tolerant to low phosphorus. We chose seven major eucalypt species and hybrids currently used in short-rotation plantation in southern China as experimental materials. We measured their photosynthetic rate, chlorophyll content, growth, and dry matter accumulation under different levels of phosphorus supply.
Materials and methods
Plant materials and growth conditions
In this study, seven species/hybrids of eucalypt (E. dunnii, E. grandis, E. grandis × E. camaldulensis, E. urophylla × E. camaldulensis, E. urophylla × E. tereticornis, E. grandis × E. tereticornis, E. urophylla × E. grandis) were used as experimental materials. Seedlings of E. dunnii were raised in the nursery from seeds, while seedlings of the other species/hybrids were raised from tissue culture by the Seedling Centre of Yong'an Forestry Group, Fujian Province, China. All seedlings were four months old, and they were kept in a glasshouse at the Forestry College, Fujian Agriculture and Forestry University for one month to avoid shock due to changes in environmental conditions and to become established. Thereafter, the root system of each seedling was washed with water and transferred to a growing medium, which was a mixture of yellow soil (loess) and sand, with the following chemical properties: organic matter content (0.312 g·kg-1), total nitrogen (0.058 g·kg-1) total phosphorus (0.106 g·kg-1), total potassium (2.618 g·kg-1), and a trace amount of available phosphorus. The growing condition in the greenhouse was 29.3/23°C (day/night); a photon flux density of about 21 mol quanta m-2·d-1and relative humidity of ca. 42.7% and 67.7% during the light and dark period of the experiment, respectively. The seedlings were left to grow in these conditions for two weeks to reduce internal phosphorus concentrations to appropriate lower levels.
Experimental design
We established a factorial experiment involving seven eucalypt species and four P concentrations. The P treatments applied were 18 mg·kg-1KH2PO4(normal P supply), 12 mg·kg-1KH2PO4(mild P deficiency), 6 mg·kg-1KH2PO4(moderate P deficiency), and 0 mg·kg-1KH2PO4(severe P deficiency), following the procedure described by Xu (1997). Each treatment had three replicates, thus the total number of seedlings was 84. The P stress treatments lasted for 4 months from mid-July to November 2006. Throughout the experiment period, nitrogen and potassium were added to the different treatments in non-limiting quantities in the form of solution at a rate of 100 mL per pot every 2 days.
Measurements
After one month of evoking stress, seedling height, root collar diameter, photosynthetic rate and chlorophyll content of Eucalyptus species/hybrids were determined monthly for three consecutive months to gain insight into the dynamics of growth and physiological responses over extended stress periods. To reduce sampling error, leaves were taken at the same location from the new offshoot each time and measurements were done three times on each sample to obtain an average value. Chlorophyll content was measured following the procedure described by Zhang (Zhang 1986) using a 1:1 mixture of acetone and ethanol. Photosynthetic rate was measured with the ECA-PB0402 photosynthesis analyzer. At the end of the phosphorus stress experiment, destructive harvests of the replicates were made, and fresh biomass of leaves, stems, and washed roots were determined. Dry weight of each component was determined after drying at 70oC until constant weight.
Statistical analysis
Prior to statistical analysis, relative increments (%) in seedling height and root collar diameter were computed as the ratio of difference between post- and pre-stress values divided by pre-stress values. The total dry mass and dry mass of leaves, stems and roots were log-transformed to meet the homoscedasticity assumption for the analysis of variance (Zar 1996). Two-Way ANOVA was performed to examine differences in growth and dry matter production among eucalypt seedlings and P treatments. When the interaction effect was significant, one-way ANOVA was run for each species separately to examine responses of each species to P treatments. Means that differed significantly were compared using Tukey’s HSD test. For photosynthetic rate and chlorophyll content data sets, repeated measures-ANOVA was performed to examine differences among eucalypt seedlings, phosphorus treatments and stress period using the following linear model:

where Yijkis the photosynthetic rate and chlorophyll content, µ is the overall mean, βiis the effect of the between-subject factors, i, P treatment and species, λjis the effect of the within-subject factor, j, stress period, (βλ)ijis the interaction of the betweenand within-subject factors, and εj(i), εj(k)are random errors of the between- and the within-subjects factor, respectively with k number of replicates. Mauchly’s test of sphericity was used to test the homogeneity of variance assumption, when violated, the degrees of freedom for testing the significance of the within-subject factor were adjusted using Huynh–Feldt correction factor, which is less biased than other correction factors (Davis 2002). The Bonferonni adjustment for multiple comparisons (p = 0.02) was employed to control the inflation of α. All statistical analyses were done using the SPSS 17 software package (SPSS 17 for Windows, Release 2009 Chicago:SPSS, Inc.).
Results
Seedling growth
Height growth of seedlings varied significantly among eucalypt species (p <0.01) and for the interaction effect (p <0.01), but not among levels of P supply (p =0.190). Subsequent one-way ANOVA for each species separately revealed significant differences in height growth by level of P supply for E. dunnii (p =0.04) and E. urophylla × E. tereticornis (p <0.01). Severe P deficiency (0 mg·kg-1) resulted in slower height growth of E. dunnii seedlings than mild to moderate P deficiencies and normal P supply, whereas P deficiency resulted in slower height growth for E. urophylla × E. tereticornis compared to normal P supply (Table 1). For the remaining eucalypt species, differences in height increment were insignificant across all levels of P supply. Diameter growth of seedlings also showed significant differences among eucalypt species (p =0.005), but not among levels of P supply (p =0.188), or the interaction effect (p =0.682). Averaged over all levels of P supply, increment in diameter was higher for E. dunnii and E. grandis × E. tereticornis compared to E. grandis × E. camaldulensis (Table 2). For the remaining species diameter increments were similar.

Table 1: Relative height increment (%) of seven eucalypt seedlings in response to different levels of P supply (mean ± SE). Means across the rows followed by different letter are significantly different at 5 % level.
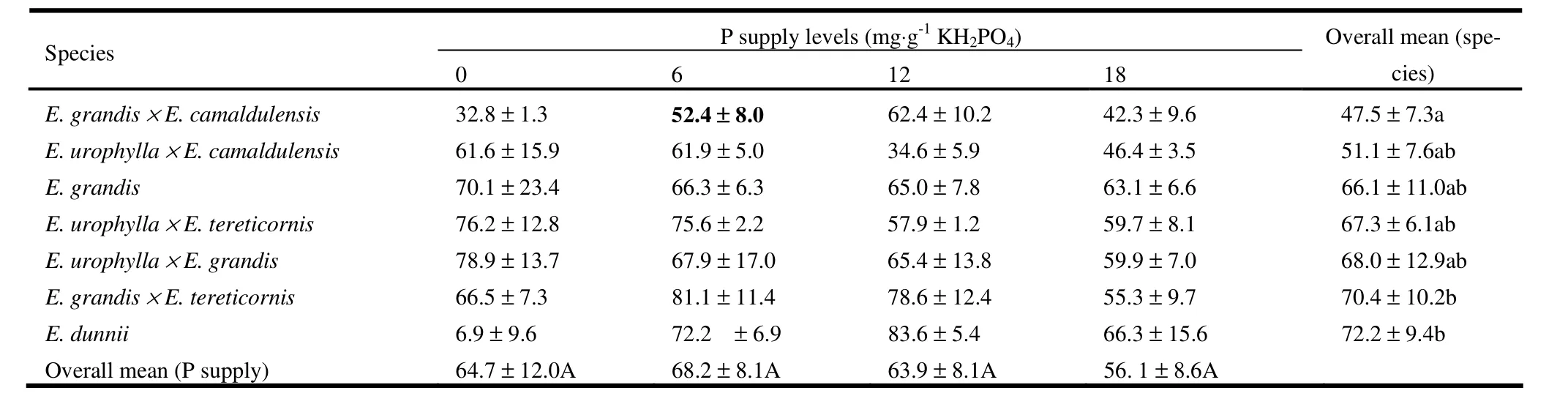
Table 2: Relative increment of root collar diameter (%) of seven eucalypt seedlings in response to different levels of P supply (mean ± SE). Overall means for P supply across the row and for species across the column followed by the same letter (S) are not significantly different.
Dry matter accumulation and distribution
Dry matter of root, shoots and whole plant varied significantly among eucalypt species (p <0.001), but not among levels of P supply (p >0.05). Significant interaction effect also was detected for shoot dry matter (p =0.013) and whole plant dry matter (p =0.006). Average over all levels of P supply, E. grandis and E. dunnii had the highest root dry mass compared to E. urophylla × E. grandis, E. grandis × E. tereticornis and E. urophylla × E. camaldulensis (Table 3). For shoot dry mass, species-wise ANOVA revealed significant differences among levels of P supply for E. urophylla × E. camaldulensis (p =0.008) and E. urophylla × E. tereticornis (p =0.015). E. urophylla × E. camaldulensis seedlings exposed to mild and severe P deficiencies produced significantly lower shoot dry mass than seedlings grown under normal P supply, whereas E. urophylla × E. tereticornis seedlings grown under mild P deficiency produced more shoot dry mass than seedlings grown under conditions of moderate and severe P deficiency and normal P supply (Table 3). Whole plant dry matter was mainly distributed into the shoot (stems followed by leaves) rather than in the root systems. With regard to whole plant dry matter, three species groups were discernible: species that were insensitive to P deficiency (E. dunnii, E. grandis, E. grandis × E. camaldulensis, and E. urophylla × E. grandis); species sensitive to severe P deficiency (E. grandis × E. tereticornis); and species tolerant of P deficiency (E. urophylla × E. camaldulensis); the latter group accumulated significantly higher dry matter under severe and moderate P deficient condition than under mild P stress (Table 3).
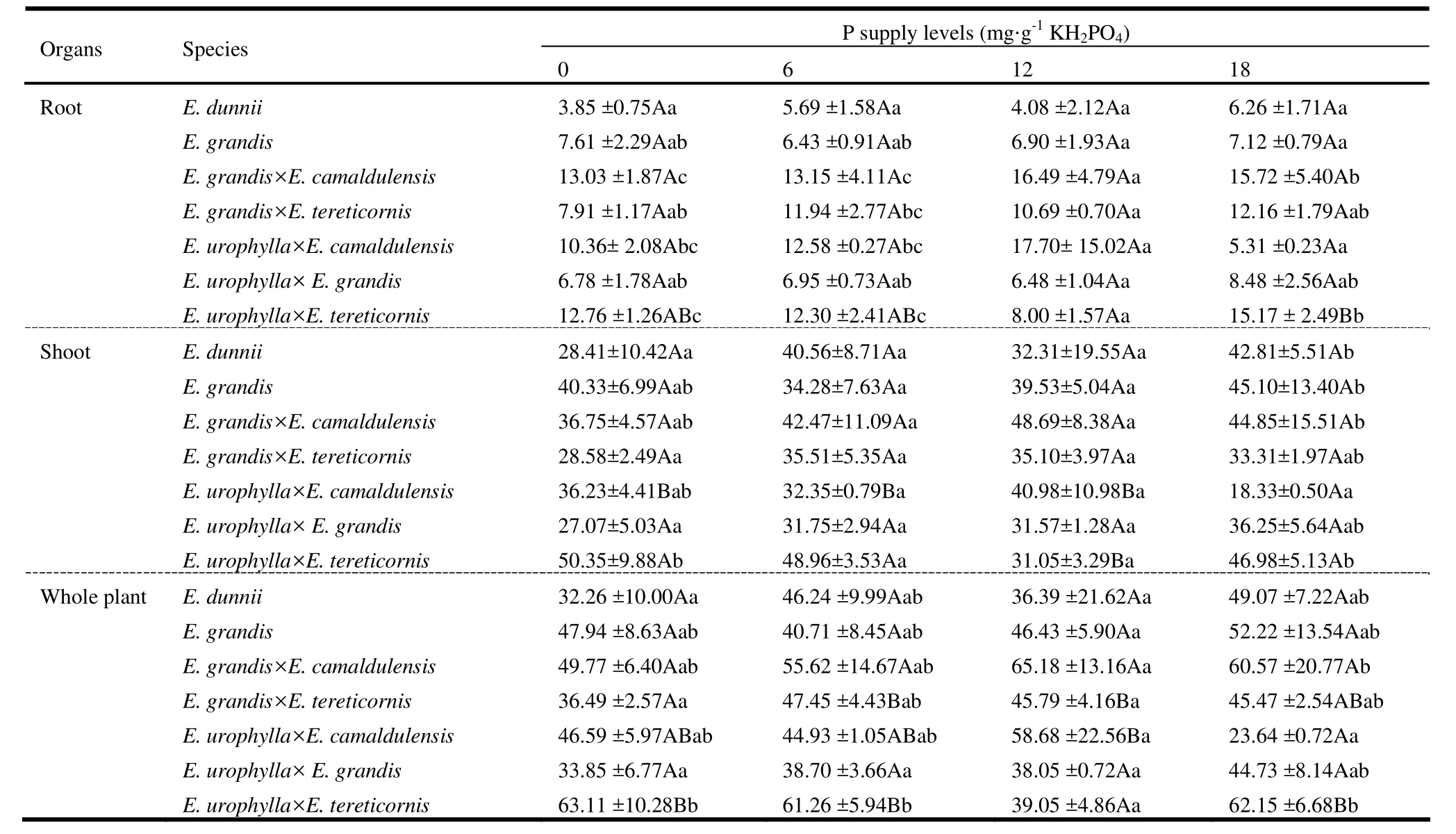
Table 3: Dry matter accumulation (g) and distribution of seven eucalypts in response to different levels of P supply (mean ± SE). Means for P supply across the row followed by the same capital letter (s) and for species across the column followed by the same small letter (s) are not significantly different.
Chlorophyll content
The chlorophyll content of leaves differed significantly with respect to within-subject factors (stress period and its first order interaction with P treatment and species; p <0.0001) as well as between-subject factors (species, treatments and their interaction; p <0.0001). For all species, the average chlorophyll content decreased with increasing duration of stress period across all treatments (Table 4). The chlorophyll content also decreased consistently for all species with severe P deficiency across all durations of stress period; except E. urophylla × E. tereticornis that had a similar level of chlorophyll content across P treatments as the stress period extended to three months. During the first stress period (1 month post-stress), chlorophyll content of E. grandis, E. urophylla × E. tereticornis, and E. grandis × E. tereticornis was insensitive to moderate P deficiency; that of E. urophylla × E. camaldulensis, E. urophylla × E. grandis, and E. dunnii was insensitive to mild P deficiency; while that of E. grandis × E. camaldulensis was sensitive to either moderate or mild P deficiency.
Photosynthetic rate
The photosynthetic rate differed significantly with respect to within-subject factors (stress period and its first and second order interactions with P treatment and species; p <0.0001) as well as between-subject factors (species, treatments and their interaction; p <0.0001). The average photosynthetic rate increased consistently with increasing P supply across all stress periods for all species; except E. urophylla × E. tereticornis (Table 5). For this hybrid, the photosynthetic rate was significantly lower under normal P supply than under mild P deficiency but much higher compared to severe and moderate P deficiencies during the second stress period. The temporal variation in photosynthetic rate was vivid for most of the species; except E. dunnii that had similar photosynthetic rate under P deficient conditions in the second and third stress periods. As a whole, E. urophylla × E. camaldulensis had the highest photosynthetic rate consistently across all P treatments, while E. urophylla × E. grandis and E. urophylla × E. tereticornis had the lowest photosynthetic rate in mild to severe P deficient conditions.
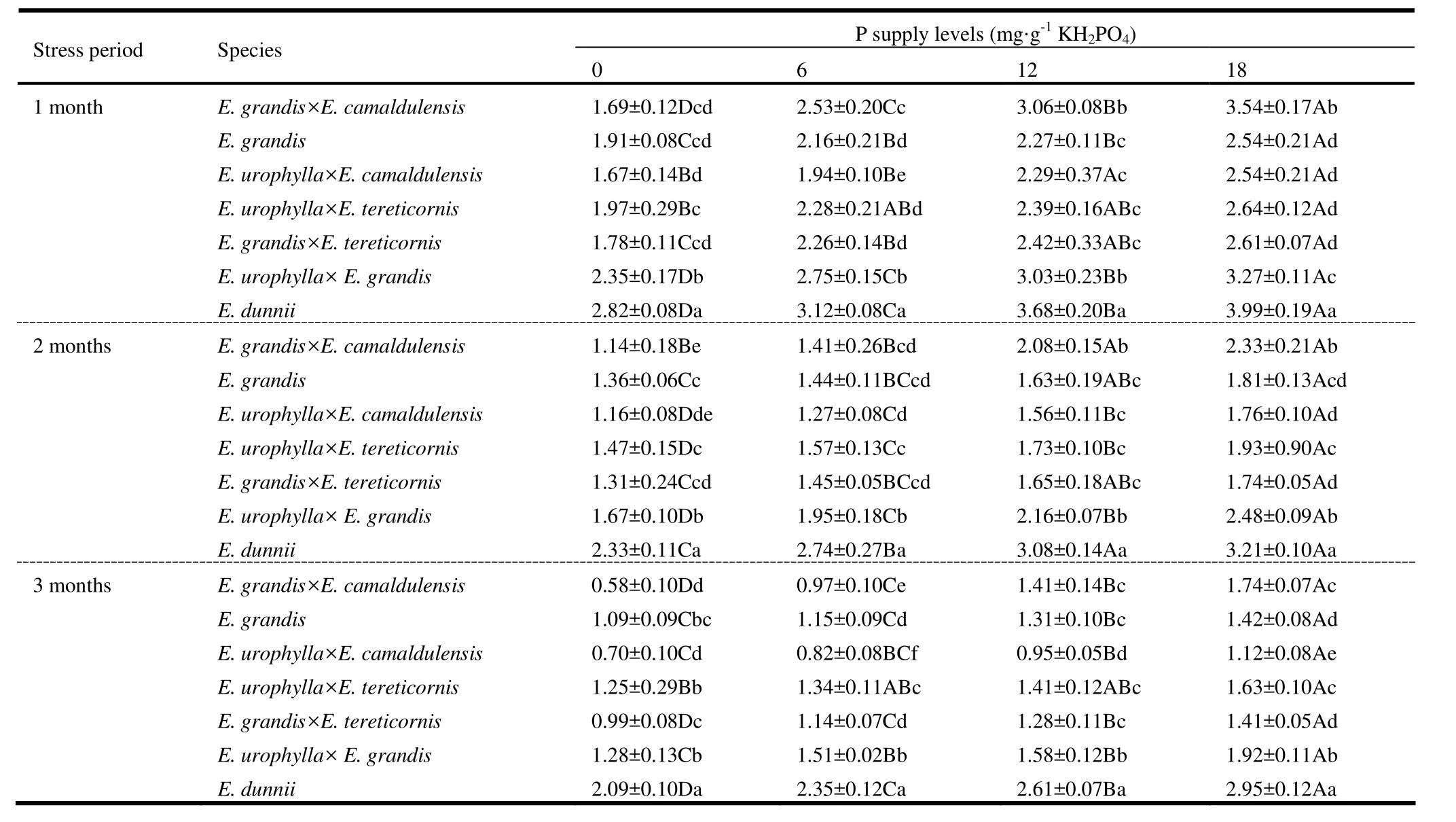
Table 4: Chlorophyll content (mg·g-1 fresh weight of needles) of seven eucalypts in response to different levels of P supply (mean ± SE). During the first stress period, means for P supply across the row followed by the same capital letter (s) and for species across the column followed by the same small letter (s) are not significantly different. The mean comparison for second and third stress periods is similar with that of the first stress period.

Table 5: Photosynthetic rate (µmol·m-2·s-1) of seven eucalypts in response to different levels of P supply (mean ± SE). During the first stress period, means for P supply across the row followed by the same capital letter (s) and for species across the column followed by the same small letter (s) are not significantly different. The mean comparison for second and third stress periods is similar with that of the first stress period.
Discussion
It is well known that P plays a key role in various plant metabolic processes and is one of the most important growth-limiting nutrients. For most species investigated in the present study, P deficiency had insignificant impact on seedling growth, except E. dunnii and E. urophylla × E. tereticornis that had low height increment under severe P deficiency. Eucalypts are efficient in translocating P within the plant, for example, translocating P from wood during heartwood formation (Grove et al. 1996; Laclau et al. 2000) in a form that is readily mobilized (Mulligan and Sands 1988). Furthermore, a recent metabolite profiling study shows small changes in most amino acids, carbohydrates and organic acids of the tricarboxylic acid (TCA) cycle in response to P supply for E. globulus (Warren 2011). This small effect of P on carbohydrates, organic acids and amino acids is believed to reflect a functional homeostasis among C metabolism, rates of photosynthesis and growth, which in turn, may reflect a conservative, long-term growth and metabolic strategy of eucalypts. This internal homeostasis might explain the lack of significant differences in growth and whole plant dry matter accumulation among different levels of P supply for most of the species in the present study. The insensitivity of dry matter accumulation to P deficiency for most of the species could also be attributed to their ability to adapt to soil with low nutrient status, and certain eucalypt species produce considerable biomass on extremely deficient sites (Mulligan and Sands 1988). As a whole, the results from the present study are consistent with previous studies made on E. globulus (Warren 2011) and E. grandis (Kirschbaum and Tompkins 1990; Thomas et al. 2006a).
P deficiency substantially reduced the photosynthetic rate and chlorophyll content of all eucalypts investigated in the present study, which is consistent with previous studies. For example, a substantial reduction in photosynthesis was observed for E. globulus (Warren 2011) and E. Grandis (Kirschbaum and Tompkins 1990), for Fraxinus mandshurica seedlings(Wu et al. 2005), and for larch seedlings (Guo et al. 2005) as was a constant decline in total chlorophyll content of Fraxinus mandshurica seedlings (Xu et al. 2001). The mechanisms by which P deficiency affects photosynthesis have been variously contemplated. For example, Warren (2011) demonstrated that the increase in photosynthesis of E. globulus with P supply is correlated with the maximum rate of CO2-limited carboxylation, and amounts of P, phosphate and fructose 6-phosphate. P deficiency decreases the maximum rate of CO2-limited carboxylation, and triose phosphate utilization (Turnbull et al. 2007; Lewis et al. 1994), as well as limitations in RuBP regeneration and/or the amount or activity of Rubisco (Jacob and Lawlor 1991; Thomas et al. 2006b; Warren 2011). Others suggest that P-starved plants have slower photosynthesis compared with P-replete controls due to reduced stomatal conductance and concentrations of CO2in sub-stomatal cavities (Warren 2011; Kirschbaum and Tompkins 1990). The reduction in chlorophyll content and photosynthetic rates with plant age regardless of P supply might be due to mutual shading and increased ratio of older to younger leaves, as well as increased rates of dark respiration and translocation of assimilates.
Eucalypt species/hybrids investigated in the present study exhibited differential sensitivity to P supply, which could be due to significant genotypic variation in tolerance to P deficiency, as observed in other species (Wu et al. 2011a, b). It is well known that a cascade of morpho-physiological adaptive mechanisms is switched on to cope with P deficiency and improve P acquisition from the soil and its subsequent utilization (Shenoy and Kalagudi 2005; Raghothama 1999). Plants tolerant to P deficiency produce and release an increased amount of organic acids (Yu et al. 2008) and phosphatases (Baldwin et al. 2001; Chen 2003) to mobilize fixed P from organic and inorganic sources. Fixed P from internal RNA pools can be mobilized through increased production of ribonucleases during low P stress condition (Bariola et al. 1994). Plants growing in P deficient environments display an enhanced expression of high affinity P transporters to optimize P uptake from the rhizosphere (Raghothama 1999). Several genes are expressed and involved in the P starvation rescue system (Li et al. 2009). The high dry matter accumulation exhibited by some of the eucalypt species (e.g. E. urophylla × E. tereticornis and E. urophylla × E. camaldulensis) in mild to moderate P deficient conditions might be attributed to one or more of the above mechanisms, which calls for further research to elucidate the underlying mechanisms for low P tolerance.
Conclusions
The study shows that photosynthetic rate and chlorophyll contents of eucalypt species/hybrids are the most sensitive physiological processes to even mild P deficiency compared with seedling growth and biomass production. This is likely due to functional homeostasis, which in turn, may enable them to metabolically adjust for a conservative and long-term growth strategy. This study provides evidence about site matching with appropriate species/hybrids to maximize growth and productivity of short rotation plantations. Based on our glasshouse experiment, E. grandis × E. camaldulensis and E. urophylla × E. tereticornis are candidate species for planting on slightly P deficient sites in southern China. However, field studies are necessary to further verify these results. On plantation sites where severe P deficiency exists, P fertilization needs to be considered because some species/hybrids (e.g. E. grandis × E. tereticornis) produced less biomass.
Acknowledgement
We thank Professor Aiqin Liu and Shanshan Zheng for chemical analysis in the laboratory.
Baldwin JC, Athikkattuvalasu SK, Kashchandra GR. 2001. LEPS2, a phosphorus starvation-induced novel acid phosphatase from tomato. Plant Physiology, 125: 728-737.
Bariola PA, Christie JH, Crispin BT, Michael TV, Vanita DJ, Pamela JG. 1994. The Arabidopsis ribonuclease gene RNS1 is tightly controlled in response to phosphate limitation. The Plant Journal, 6: 673-685.
Chen HJ, Li YQ, Chen DD, Zhang Y, Wu LM, Ji JS. 1996. Soil phosphorus fractions and their availability in Chinese fir plantations in south China, Forest Research, 9: 121-126.
Chen HJ. 2003. Phosphatase activity and P fractions in soils of an 18-year-old Chinese fir (Cunninghamia lanceolata) plantation. Forest Ecology and Management, 178: 301-310.
Davis CS. 2002. Statistical methods for the analysis of repeated measurements. London: Springer, p. 203-298.
Grove TS, Thomson BD, Malajczuk N. 1996. Nutritional physiology of eucalypts: uptake, distribution and utilization. Nutrition of eucalypts. Australia: CSIRO, pp. 77-108.
Guo SL, Yan XF, Bai B, Yu S. 2005. Responses of larch seedling’s photosynthetic characteristics to nitrogen and phosphorus deficiency. Chinese Journal of Applied Ecology, 16: 589-594.
Jacob J, Lawlor DW. 1991. Stomatal and mesophyll limitations of photosynthesis in phosphate deficient sunflower, maize and wheat plants. Journal of Experimental Botany, 42(8): 1003-1011.
Kirschbaum MUF, Tompkins D. 1990. Photosynthetic responses to phosphorus nutrition in Eucalyptus grandis seedlings. Functional Plant Biology, 17: 527-535.
Laclau JP, Bouillet JP, Ranger J. 2000. Dynamics of biomass and nutrient accumulation in a clonal plantation of Eucalyptus in Congo. Forest Ecology and Management, 128: 181-196.
Lewis JD, Griffin KL, Thomas RB, Strain BR. 1994. Phosphorus supply affects the photosynthetic capacity of loblolly pine grown in elevated carbon dioxide. Tree physiology, 14: 1229-1244.
Li LH, Qiu XH, Li XH, Wang SP, Lian XM. 2009. The expression profile of genes in rice roots under low phosphorus stress. Science in China Series C: Life Sciences, 52: 1055-1064.
Liu JZ, Li ZS, Li JY. 1994. Utilization of plant potentialities to enhance the bio-efficiency of phosphorus in soil. Chinese Journal of Eco-Agriculture, 1: 18-25.
Mulligan DR, Sands R. 1988. Dry matter, phosphorus and nitrogen partitioning in three Eucalyptus species grown under a nutrient deficit. New phytologist, 109: 21-28.
Raghothama KG. 1999. Phosphate acquisition. Annual review of plant biology, 50: 665-693.
Scholz RW, Ulrich AE, Eilittä M, Roy A. 2013. Sustainable use of phosphorus: A finite resource. Science of the Total Environment, 461–462: 799-803.
Shenoy VV, Kalagudi GM. 2005. Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnology advances, 23: 501-513.
Shepherd M, Bartle J, Lee DJ, Brawner J, Bush D, Turnbull P, Macdonel P, Brown TR, Simmons B, Henry R. 2011. Eucalypts as a biofuel feedstock. Biofuels, 2: 639-657.
Singh KD. 2013. Global forest resources assessments. In: Capacity building for the planning, assessment and systematic observations of forests. Environmental Science and Engineering. Springer. p. 203-211.
Thomas DS, Montagu KD, Conroy JP. 2006a. Leaf inorganic phosphorus as a potential indicator of phosphorus status, photosynthesis and growth of Eucalyptus grandis seedlings, Forest Ecology and Management, 223: 267-274.
Thomas DS, Montagu KD, Conroy JP. 2006b. Why does phosphorus limitation increase wood density in Eucalyptus grandis seedlings? Tree physiology, 26: 35-42.
Turnbull TL, Warren CR, Adams MA. 2007. Novel mannose-sequestration technique reveals variation in subcellular orthophosphate pools do not explain the effects of phosphorus nutrition on photosynthesis in Eucalyptus globulus seedlings. New phytologist, 176: 849-861.
Wang CN, Cao FX, Huang YF, Li J. 2004. Poly-usage of eucalyptus and its sub-metabolized production. Non-wood Forest Research, 22(3): 57-59.
Warren CR. 2011. How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree physiology, 31: 727-739.
Wu C, Sun HL, Wang ZQ. 2005. Change of potential photosynthesis rates in Fraxinus mandshurica seedlings supplied with different phosphorus levels, Journal of Anhui Agricultural University, 33: 2028-2031.
Wu PF, Ma XQ, Tigabu M, Wang C, Liu AQ, Oden PC. 2011a. Root morphological plasticity and biomass production of two Chinese fir clones with high phosphorus efficiency under low phosphorus stress. Canadian Journal of Forest Research, 41: 228-234.
Wu PF, Tigabu M, Ma XQ, Oden PC, He YL, Yu XT, He ZY. 2011b. Variations in biomass, nutrient contents and nutrient use efficiency among Chinese fir provenances. Silvae Genetica, 60: 95-105.
Xu D, Dell B, Malajczuk N, Gong M. 2001. Effects of P fertilization and ectomycorrhizal fungal inoculation on early growth of eucalypt plantations in southern China. Plant and soil, 233: 47-57.
Xu D. 1997. Effects of different phosphate levels on growth and nutrient uptake in different provenances of Eucalyptus urophylla S. T. Blake, Tropical and Subtropical Soil Science, 6(2): 76-81.
Yu YC, Yu J, Shan QH, Fang L, Jiang DF. 2008. Organic acid exudation from the roots of Cunninghamia lanceolata and Pinus massoniana seedlings under low phosphorus stress. Frontiers of Forestry in China, 3: 117-120.
Zar Jh. 1996. Biostatistical analysis (3rd edition). London: Prentice-Hall, p. 123-178.
Zhang XZ. 1986. Determination of chlorophyll content in plant tissue. Liaoning Agricultural Science and Technology, 3: 19-21.
杂志排行
Journal of Forestry Research的其它文章
- Carbon sequestration in Chir-Pine (Pinus roxburghii Sarg.) forests under various disturbance levels in Kumaun Central Himalaya
- Regional differences of water conservation in Beijing’s forest ecosystem
- Spatial modeling of the carbon stock of forest trees in Heilongjiang Province, China
- Spatial heterogeneity of factors influencing forest fires size in northern Mexico
- Community ecology and spatial distribution of trees in a tropical wet evergreen forest in Kaptai national park in Chittagong Hill Tracts, Bangladesh
- Diversity, regeneration status and population structure of gum- and resin-bearing woody species in south Omo zone, southern Ethiopia
