Responses of plant diversity and species composition to the cessation of fertilization in a sandy grassland
2014-04-20ShengnanShiZhanyuanYuQiongZhao
Sheng-nan Shi • Zhan-yuan Yu • Qiong Zhao
Introduction
Nitrogen is recognized as the limiting nutrient of net primary production in many terrestrial ecosystems (Vitousek et al. 1991; LeBauer et al. 2008). Therefore, nitrogen fertilization has been widely used to increase plant growth and productivity in terrestrial N-limited ecosystems (Frink et al. 1999). Increased nitrogen input increases soil N availability, which leads to changes in species composition and reduces species diversity (Wedin et al. 1996; Bobbink et al. 1998).
Biodiversity plays an important role in ecosystem functioning, sustainability and stability (Tilman et al. 1994; Tilman et al. 1996). The effect of nitrogen addition/deposition on species composition and diversity has been demonstrated in numerous studies (Willems et al. 1993; Stevens et al. 2004; Lu et al. 2010; Dupré et al. 2010). In comparison, studies of the resilience of vegetation species composition after fertilization was ceased or reduced are fewer (Boeye et al. 1997; Güsewell et al. 2002; Clark et al. 2010; Edmondson et al. 2013). Resilience is defined as the ability of ecosystems to recover after termination of fertilizer addition in our study (Lepš et al. 1982). To date, the reversibility of N-induced shifts in vegetation is largely unknown (Clark et al. 2009). The studies at the Schynige Platte showed that short-term and small-scale fertilization perturbation has long-lasting effect on subalpine acid grassland, and the ecosystem can recover to its original state with time (Spiegelberger et al. 2006). Similarly, Boxman et al. (1998) reported that nitrogen enrichment was a fully reversible process. In contrast, Semelová et al. (2008) reported no signs of plant recovery several years after fertilization was terminated. Research conducted in a large grassland suggested that recovery of species richness was relatively rapid compared with most other studies (Hegg et al. 1992; Walker et al. 2004; Josef et al. 2009). In alluvial grassland a duration of 16 years was adequate to restore species richness, but not plant species composition or N/P ratios in plant biomass (Hrevušová et al. 2009). O'Sullivan et al. (2011) concluded that the recovery of pre-fertilization plant species composition re-quired more time than that recovery of soil chemical characters in limestone and acidic grasslands. This conclusion applied as well to boreal forest ecosystems (Strengbom et al. 2011). These studies suggested that the resilience of ecosystems differs not only between ecosystems, but also between processes within the same ecosystem (Lavorel et al. 1999). The studies were mainly conducted in Europe and America where the rate of nitrogen deposition had been reduced or stabilized (NADP 2000), on farm fields that were previously fertilized on an experimental basis (Hejcman et al. 2007) and in artificial roof experiments (Boxman et al. 1998). The fertilization/deposition histories of these sites differed and this might explain the sometimes contradictory results. In addition, ecosystem recovery might be influenced by factors other than fertilization such as hysteresis, soil seed banks, nearby seed sources and other factors. Thus, the studies investigating the vegetation recovery need to be carried out on a global scale and over long time periods.
Keerqin Sandy Lands are the largest of China′s four major sandy lands. A previous fertilization experiment reported by Yu et al. (2006) demonstrated that the limiting factor of the semi-arid sandy grassland was nitrogen. The present study was conducted on this experimental fertilization site from 2009 to 2011 after anthropogenic nitrogen inputs ceased in 2008. The aim of our research was to test the reversion rate and pattern of semi-arid sandy grassland in terms of plant diversity and community composition after cessation of fertilization.
Materials and methods
Experimental site
The experimental site was located at Daqinggou Ecological Station (latitude 42°58′ N, longitude 122°21′ E, 260 m.a.s.l.) of the Institute of Applied Ecology, Chinese Academy of Sciences, in the southeastern Keerqin Sandy Lands of northeast China. The area has a typical continental monsoon climate. The mean annual temperature is 6.4 °C with minimum and maximum mean monthly temperatures of -12.5 °C and 23.8 °C, respectively. Mean annual precipitation is 450 mm, with more than 60% from June to August. The soil is classified as Typic Ustipsamment with a texture of 90.9% sand, 5.0% silt, and 4.1% clay (Zeng et al. 2009). The growing season lasts from late April to early September.
The site was used as cropland in 1997 and then naturally restored to grassland in 2000. Afterwards, the herbaceous vegetation recovered rapidly to coverage of 80%. The dominant plant species were Pennisetum flaccidum, Chenopodium acuminatum, and Cleistogenes chinensis.
Experimental design
In 2004, a nitrogen addition experiment was conducted on flat sandy grassland that was fenced to exclude livestock grazing from 2003. We sampled six replicate plots on two treatments (nitrogen addition and control). The area of each plot was 16 m2(4 m × 4 m), and there was a 2-m buffer strip between any two adjacent plots. From 2004 to 2008, N (NH4NO3) was added at the rate of 20 g N·m-2·a-1. From 2009 to 2011, fertilizer addition was stopped to document vegetation recovery.
Measurement of plant responses
Annual vegetation surveys were carried out in mid-August, the time of peak biomass, in the period 2008-2011. In each 16 m2plot, four randomly located 1 m × 1 m quadrats were sampled to investigate plant abundance and cover. Plant abundance was visually estimated according to the Braun-Blanguet scale. The cover of each species and the total cover of each plot were estimated directly in percentages. The height of vegetation in each plot was estimated using a tape measure.
Data processing and analysis
Data processing
We used plant species richness, the Shannon-Wiener index (H) and the Simpson index (D) to describe species diversity. Plant species richness was the number of different species per square meter. The Shannon-Wiener index was calculated as:
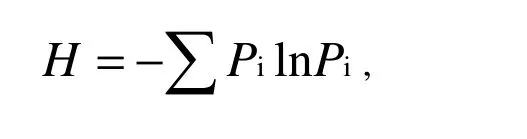
The Simpson index was calculated as:
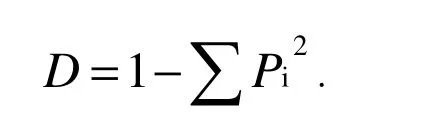
Where Piis the relative importance values of species i, the species relative importance value is the mean of relative cover, relative frequency and relative density. Because climatic variation and other factors also affect species diversity, when we analyzed differences between years we calculated relative species richness by dividing the plant species richness in a given year by the mean value in the control plots for that year (Clark 2008). The relative Shannon-Wiener index, relative Simpson index, the relative height and coverage were calculated in the same way.
Data analysis
Species richness and species diversity in former nitrogen addition treatment plots and control plots were analyzed using Student′s t-test. The changes of ecosystem composition after cessation of fertilization between years were tested using one-way ANOVA. LSD method was used for multiple comparisons if the data were homogeneous, otherwise Tamhane analysis was used. All analyses were performed using SPSS 13.0 and the accepted significance level was α = 0.05.
Results
Plant diversity
Species richness, diversity and evenness on formerly fertilized plots were significantly lower than on control plots during all years 2009-2011 (p <0.05; Table 1). Relative species richness showed an increasing trend over time since cessation of fertilizing. Relative species richness increased by 40% (from 0.55 in 2008 to 0.77 in 2011) and was significantly greater in 2011 than in 2010 (p <0.05; Fig. 1c). Relative diversity was from 0.75 in 2008 to 0.89 in 2011. Relative evenness was from 0.87 in 2008 to 0.94 in 2011. The differences in relative diversity and relative evenness between years were not significant (p >0.05).

Fig. 1: Changes of relative diversity, relative evenness and relative species richness from 2008 to 2011 (mean±SE, n =6). Different small letters represented the significant difference of mean values among years (p <0.05). The same below.

Table 1: Responses in 2009-2011 of diversity, evenness and species richness to cessation of nitrogen addition in 2008.
Species composition
The species composition on formerly fertilized plots differed from that on control plots in all years after cessation of nitrogen fertilization. The dominant species shifted from Cannabis sativa, Phragmites communis and Chenopodium acuminatum in 2008 to Cannabis sativa, Phragmites communis and Artemisia scoparia in 2011. The dominant species in 2010 were the same as in 2011. The dominance of dominant species declined from 66.2% in 2008 to 57.5% in 2011. Although species composition differed by treatment in 2011, C. sativa and P. communis were dominant on formerly fertilized plots and on control plots.
Functional composition of species
The importance value of annual plants on formerly fertilized plots was higher than on control plots, the differences were significant in 2009 and 2010 (p <0.05 both years) but not in 2011 (p >0.05; Table 2). The yearly variation in biennial plants between treatments was not significant (p >0.05; Table 2). The importance value of perennial plants was significantly lower on formerly fertilized plots than on control plots in every year after nitrogen addition ceased (p <0.05; Table 2).

Table 2: Responses of functional groups to nitrogen addition ceased in 2009-2011.
Plant height and coverage
Vegetation height and coverage on formerly fertilized plots were greater than on control plots in 2009-2011 (Table 3). The differences in vegetation height between treatments were significant in 2009 and 2011 (p <0.05) but not in 2010 (p >0.05; Table 3). In contrast, the differences in coverage between treatments were significant in 2009 and 2010 (p <0.05) but not in 2011 (p >0.05; Table 3). The relative height values in 2010 and 2011 were significantly lower than in 2009 (Fig. 2a). Relative height declined by 44% in 2010 and 39% in 2011 compared to 2009. Relative coverage declined after fertilization ceased, and coverage was significantly greater in 2009 than in 2011 (p <0.05; Fig. 2b). The responses of coverage to cessation of nitrogen inputs varied among dominant species. The coverage of C. sativa declined from 36% in 2009 to 30% in 2011. However, the coverage of P. communis increased from 15% in 2009 to 29% in 2011. A. scoparia became a dominant species in 2010 and 2011 when its coverage reached 12% and 7%, respectively.

Table 3: Responses during 2009-2011 of vegetation height and coverage to cessation of nitrogen addition in 2008

Fig. 2: Changes of relative vegetation height and coverage by year in 2009-2011 (mean±SE, n =6).
Discussion
Species richness, diversity and evenness were dissimilar on formerly fertilized plots and control plots. These results showed that the residual effect of fertilization was still observed three years after fertilization ceased. Inorganic nitrogen concentration in soils was significant higher on formerly fertilized plots than on control plots in 2009 (Li et al. 2012), but was similar between treatments in 2012. We don’t know whether or not the differences of soil available nitrogen were significant between treatments in 2010-2011 (no data). Therefore, these results might be explained by nitrogen accumulated in soils during 5-years of annual fertilization, so that the available nitrogen was still higher than on control plots after nitrogen addition ceased. In a similar study in a pine forest, soil NH4+gross mineralization rates on fertilized plots remained higher than on control plots, fourteen years after nitrogen addition ceased (Chen et al. 2006).
A second explanation is that soil nitrogen availability was in accordance with the controls, but hysteresis or limitation of soil seed banks delayed the recovery of plant diversity. Shifts in resource availabilities to match those of control plots might not be sufficient for recovery of the original community when hysteresis affects communities characterized by resource-ratio competitive interactions (Suding et al. 2004). One study in a formerly fertilized field showed that short-term seed limitations were the dominant factors preventing recovery of plant diversity (Clark et al. 2010).
Relative species richness, relative diversity and relative evenness increased annually after cessation of fertilization. This demonstrates that the effects of fertilization declined and plant species were able to recover with each year after fertilization was stopped. However, because our study ended in 2011, we cannot estimate the time span required for species diversity to recover to levels recorded on control plots. Long-term experiments are needed to observe the dynamics of species diversity of sandy grasslands. A study with fertilization history similar to that of our study area estimated that grassland vegetation would need nine years to revert to the state of control plots after five years of nitrogen fertilization at 20 g N·m-2·a-1(Mountford et al. 1996).
Species composition changed on our study area after 3 years without addition of nitrogen. We attribute this to lower concentrations of nitrogen in soils that altered interspecific competitive interactions after nitrogen addition ceased. Although the dominant species differed by treatment, the dominance of C. sativa and P. communis decreased from 59% in 2008 to 45% in 2011. This was probably because these species had higher nitrogen use efficiency under the nutrient enrichment conditions in 2008, but had reduced competitiveness when nitrogen availability declined after fertilization ceased. Community recovery is a complex process and is affected by many factors. Spiegelberger et al. (2006) studied mountain ecosystems and reported that resilience can be low in response to perturbations that substantially alter soil pH. Josef et al. (2009) hypothesized that the restoration of grasslands after cessation of fertilization was more rapid in productive grasslands than in unproductive grasslands. On our study area nitrogen is the main nutrient limiting net primary production (Yu et al. 2006). Inorganic nitrogen concentration in soils was significantly increased and soil pH was significantly decreased after five consecutive years of nitrogen addition (Zeng et al. 2010; Li et al. 2011). We predict that the recovery of plant community will require long periods of time in N-poor sandy grasslands.
The response of plant composition to cessation of nitrogen inputs differed by functional group. The important value of annual plants declined and treatments were similar in 2011. This was probably due to the lower dominance of annual-C4plants. Salsola collina and Digitaria sanguinalis disappeared in 2011. The annual-C4plants composition on formerly fertilized plots were consistent with levels on control plots in 2011. Biennial plants were the composits Artemisia scoparia and Artemisia sieversiana. The differences of biennial plant importance were not significant between treatments in 2008-2011. But the importance values of these species increased after nitrogen addition ceased. Compared to annual plants, perennial plants showed fewer signs of recovery. The importance values of perennial plants differed significantly between treatments during 2009-2011. Two species that were present during the fertilization period, Lespedeza hedysaroides and Astragalus melilotoides, were not recorded in 2011, the third year after fertilization stopped. The recovery rate of species composition was slower than that of species richness, similar to the situation on prairie grassland, where relative species numbers, but not species composition, recovered to control plot levels 13 years after nitrogen addition ceased (Clark et al. 2008).
Vegetation height and coverage of formerly fertilized plots were greater than on control plots in 2011. Relative height and coverage declined over time. The recovery rates for coverage differed by functional group after nitrogen addition stopped. The relative coverage of annual plants was significant lower and the relative coverage of perennial plants was significant higher in 2011 than in 2009. In contrast, the differences of relative coverage of biennial plants between years were not significant (Fig. 3).
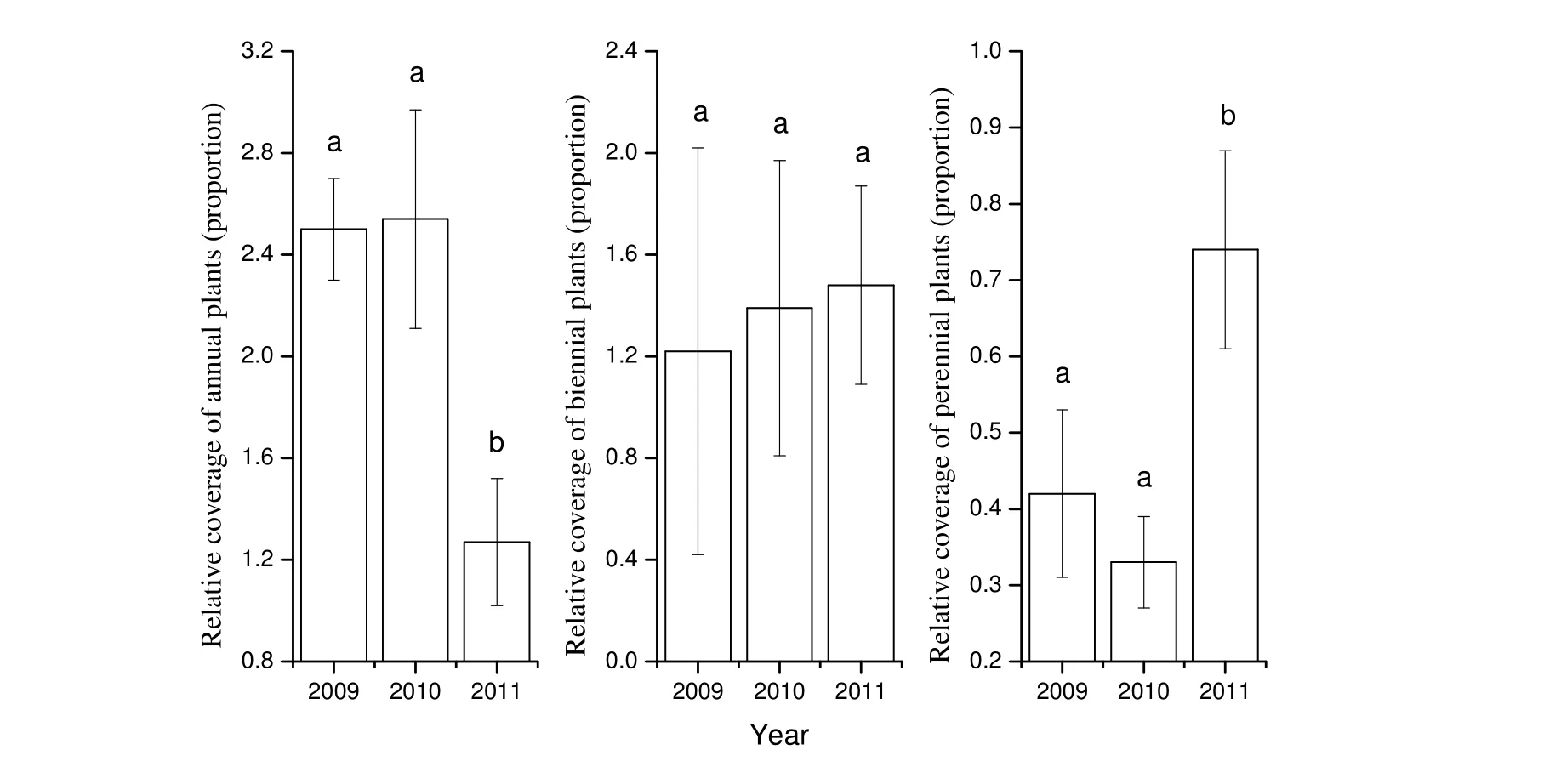
Fig. 3: Changes of relative coverage among functional groups between treatments in 2009-2011.
Conclusion
Species richness recovered faster than species composition in Keerqin Sandy Lands after cessation of nitrogen inputs. Annual plants were more sensitive than perennial plants to the cessation of nitrogen inputs. We conclude that the recovery rate differed not only between community characteristics (species diversity and species composition), but also between functional groups within the same community characteristic. The different responses were probably due to differences in N acquisition strategies and sensitivities to the cessation of nitrogen addition between short-lived plants and long-lived perennial plants.
Bobbink R, Hornung M, Roelofs JG. 1998. The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. Journal of Ecology, 86(5): 717-738.
Boeye D, Verhagen B, Van Haesebroeck V, Verheyen RF. 1997. Nutrient limitation in species-rich lowland fens. Journal of Vegetation Science, 8(3): 415-424.
Boxman AW, van der Ven PJM, Roelofs JGM. 1998. Ecosystem recovery after a decrease in nitrogen input to a Scots pine stand at Ysselsteyn, the Netherlands. Forest Ecology and Management, 101:155-163.
Chen Yu, Högberg P. 2006. Gross nitrogen mineralization rates still high 14 years after suspension of N input to a N-saturated forest. Soil Biology & Biochemistry, 38(7): 2001-2003.
Clark CM, Hobbie SE, Venterea R, Tilman D. 2009. Long-lasting effects on nitrogen cycling 12 years after treatments cease despite minimal long-term nitrogen retention. Global Change Biology, 15(7): 1755-1766.
Clark CM, Tilman D. 2008. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature, 451: 712-715.
Clark CM, Tilman D. 2010. Recovery of plant diversity following N cessation: effects of recruitment, litter, and elevated N cycling. Ecology, 91(12): 3620-3630.
Dupré C, Stevens CJ, Ranke T, Bleeker A, Peppler-Lisbach C, Gowing DJG, Dise NB, Dorland E, Bobbink R, Diekmann M. 2010. Changes in species richness and composition in European acidic grasslands over the past 70 years: the contribution of cumulative atmospheric nitrogen deposition. Global Change Biology, 16(1): 344-357.
Edmondson J, Terribile E, Carroll JA, Price EAC, Caporn SJM. 2013. The legacy of nitrogen pollution in heather moorlands: Ecosystem response to simulated decline in nitrogen deposition over seven years. Science of the Total Environment, 444: 138-144.
Frink CR, Waggoner PE, Ausubel JH. 1999. Nitrogen fertilizer: retrospect and prospect. PNAS, 96(4): 1175-1180.
Güsewell S, Koerselman W, Verhoeven JTA. 2002. Time-dependent effects of fertilization on plant biomass in floating fens. Journal of Vegetation Science, 13(5): 705-718.
Hegg O, Feller U, Dähler W, Scherrer C. 1992. Long term influence of fertilization in a Nardetum. Vegetatio, 103(2): 151-158.
Hejcman M, Klaudisová M, Štursa J, Pavlů V, Schellbergd J, Hejcmanová P, Hakl J, Rauch O, Vacek S. 2007. Revisiting a 37 years abandoned fertilizer experiment on Nardus grassland in the Czech Republic. Agriculture, Ecosystems & Environment, 118(1-4): 231-236.
Hrevušová Z, Hejcman M, Pavlů VV, Hakla J, Klaudisovád M, Mrkvička J. 2009. Long-term dynamics of biomass production, soil chemical properties and plant species composition of alluvial grassland after the cessation of fertilizer application in the Czech Republic. Agriculture, Ecosystems and Environment, 130(3-4): 123-130.
Josef K, Lenka P, Magda J, Petr M, Karel P. 2009. Spontaneous recovery of an intensively used grassland after cessation of fertilizing. Applied Vegetation Science, 12: 391–397.
Lavorel S, Prieur-Richard AH, Grigulis K. 1999. Invasibility and diversity of plant communities: from pattern to processes. Diversity and Distributions, 5: 41-49.
LeBauer DS, Treseder KK. 2008. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology, 89(2): 371-379.
Lepš J, Osbornová-Kosinová J, Rejmánek M. 1982. Community stability, complexity and species life history strategies. Vegetatio, 50(1): 53-63.
Li LJ, Zeng DH, Yu ZY, Fan ZP, Yang D, Liu YX. 2011. Impact of litter quality and soil nutrient availability on leaf decomposition rate in a semi-arid grassland of Northeast China. Journal of Arid Environments, 75(9): 787-792.
Li LJ, Zeng DH, Mao R, Yu ZY. 2012. Nitrogen and phosphorus resorption of Artemisia scoparia, Chenopodium acuminatum, Cannabis sativa, and Phragmites communis under nitrogen and phosphorus additions in a semiarid grassland, China. Plant, Soil and Environment, 58(10): 446-451.
Lu XK, Mo JM, Gilliam FS, Zhou GY, Fang YT. 2010. Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Global Change Biology, 16(10): 2688-2700.
Mountford JO, Lakhani KH, Holland RJ. 1996. Reversion of grassland vegetation following the cessation of fertilizer application. Journal of Vegetation Science, 7(2): 219-228.
NADP (National Atmospheric Deposition Program). 2000. National Atmospheric Deposition Program Annual Data Summary: Precipitation Chemistry in the United States. NADP Program Office, Illinois State Water Survey, University of Illinois, Champaign.
O'Sullivan OS, Horswill P, Phoenix GK, Lee JA. Leake JR. 2011. Recovery of soil nitrogen pools in species-rich grasslands after 12 years of simulated pollutant nitrogen deposition: a 6-year experimental analysis. Global Change Biology, 17: 2615-2628.
Semelová V, Hejcman M, Pavlů V, Vacek S, Podrázský V. 2008. The Grass Garden in the Giant Mts. (Czech Republic): residual effect of long-term fertilization after 62 years. Agriculture, Ecosystems & Environment 123(4): 337-342.
Spiegelberger T, Hegg O, Matthies D, Hedlund K, Schaffner, U. 2006. Long-term effects of short-term perturbation in a subalpine grassland. Ecology, 87(8): 1939-1944.
Stevens CJ, Dise NB, Mountford JO, Gowing DJ. 2004. Impacts of nitrogen deposition on the species richness of grasslands. Science, 303: 1876-1879.
Strengbom J, Nordin A, Näsholm T, Ericson L. 2001. Slow recovery of boreal forest ecosystem following decreased nitrogen input. Functional Ecology, 15(4): 451-457.
Suding KN, Gross KL, Houseman GR. 2004. Alternative states and positive feedbacks in restoration ecology. Trends in Ecology & Evolution, 19(1): 46-53.
Tilman D, Downing JA. 1994. Biodiversity and stability in grasslands. Nature, 367: 363-365.
Tilman D, Wedin D, Knops J. 1996. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature, 379: 718-720.
Vitousek PM, Howarth RW. 1991. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry, 13(2): 87-115.
Walker KJ, Stevens PA, Mountford JO, Manchester SJ, Pywell RF. 2004. The restoration and re-creation of species-rich lowland grassland on land formerly managed for intensive agriculture in the UK. Biological Conservation, 119(1): 1-18.
Wedin DA, Tilman D. 1996. Influence of nitrogen loading and species composition on the carbon balance of grasslands. Science, 274: 1720-1723.
Willems JH, Peet RK, Bik L. 1993. Changes in chalk-grassland structure and species richness resulting from selective nutrient additions. Journal of Vegetation Science, 4(2): 203-212.
Yu ZY, Zeng DH, Jiang FQ, Fan ZP, Chen FS, Zhao Qiong. 2006. Responses of key carbon cycling processes to the addition of water and fertilizers to sandy grassland in semi-arid region. Journal of Beijing Forestry University, 28(4): 45-50. (in Chinese)
Zeng DH, Li LJ, Fahey TJ, Yu ZY, Fan ZP, Chen FS. 2010. Effects of nitrogen addition on vegetation and ecosystem carbon in a semi-arid grassland. Biogeochemistry, 98: 185-193.
Zeng DH, Hu YL, Chang SX, Fan ZP. 2009. Land cover change effects on soil chemical and biological properties after planting Mongolian pine (Pinus sylvestris var. mongolica) in sandy lands in Keerqin, northeastern China. Plant and Soil, 317: 121-133.
杂志排行
Journal of Forestry Research的其它文章
- Carbon sequestration in Chir-Pine (Pinus roxburghii Sarg.) forests under various disturbance levels in Kumaun Central Himalaya
- Regional differences of water conservation in Beijing’s forest ecosystem
- Spatial modeling of the carbon stock of forest trees in Heilongjiang Province, China
- Spatial heterogeneity of factors influencing forest fires size in northern Mexico
- Community ecology and spatial distribution of trees in a tropical wet evergreen forest in Kaptai national park in Chittagong Hill Tracts, Bangladesh
- Diversity, regeneration status and population structure of gum- and resin-bearing woody species in south Omo zone, southern Ethiopia
