Floristic diversity and regeneration status of woody plants in Zengena Forest, a remnant montane forest patch in northwestern Ethiopia
2014-04-20DesalegnTadeleErmiasLulekalDestawDamtieAdaneAssefa
Desalegn Tadele • Ermias Lulekal • Destaw Damtie • Adane Assefa
Introduction
Most of the forests in Ethiopia have disappeared and fragmented into small patches due to continuous deforestation, which is going on at a very alarming rate with an annual loss of about 141,000 ha (FAO 2007). Statistical figures regarding Ethiopian forests indicate a continuous decline from the original 35% forest cover in 1950 to 2.4% in 1992 (Sayer et al. 1992). During 1990 –2010, 2.65% (2.91 million ha) of the forest cover of the country was deforested (FAO 2010). In the northern highlands of Ethiopia, forest loss is more intensified, and, as a result, patches of natural forests are almost found only around churches and in areas which are not accessible for use by humans and livestock (EFAP 1994; Wassie et al. 2005). High population growth and the associated ever-increasing demand on natural forests for various forest products and agricultural land has put the remnant forest patches on the verge of disappearance (Bekele 1994).
Patches of natural forests in the highlands of Ethiopia can serve as seed sources for restoration of degraded areas, as points of reference for restoration activities, and for biodiversity conservation (Wassie et al. 2005; Wassie and Teketay 2006). However, the persistence of the remnant forest patches and their indigenous species in many areas are threatened. Fragmentation and habitat loss could influence the structure and regeneration of these forests (Cabin et al. 2002). Human-induced disturbances strongly influence the regeneration success of woody species and, in turn, determine the vegetation structure and composition of forests (Cotler and Ortega-Larrocea 2006). Tesfaye et al. (2002) noted significant pressure from disturbances such as intensive tree removal and grazing on forest regeneration in the Ethiopian highlands. Thus, the potential use of remnant forest patches in restoration and conservation activities is absolutely dependant on their sustainability (Wassie and Teketay 2006).
Zengena forest is a remnant evergreen Afromontane forest in the northwestern part of Ethiopia. The forest, which is a type of transition vegetations between the humid and dry Afromontane forests, was under pressure for long period of time due to agricultural expansion, settlement and overgrazing. This forest, which once covered a very large area, is currently restricted around the edge of Lake Zengena. According to comments given by some of the elders in the community living around the lake, the area that is currently used for crop production, grazing, and settlement was covered by vegetation, mainly Acacia abyssinica, Allophylus abyssinicus, Terminalia brownii, Prunus africana, Hagenia abyssinica, Apodytes dimidiata, and Schefflera abyssinica. The forest around the lake is maintained through traditional land management system by the local community, the presence of the lake, a major tourist destination in the district, where the forest around it provides additional services for the tourists and the topography of the area (steepness especially close to the edge of the lake which make part of the forest not easily accessible for use by humans and domestic animals). The forest can play significant role in maintaining the present volume and quality of the lake through its effect on the hydrological cycle and surface runoff. Thus, the sustainable use of the lake clearly depends on the management of this forest patch. However, there is no well-documented scientific work on the vegetation of Zengena forest except for description of some highland trees that were considered important by the local communities (SIM 2001).
In this study we aimed to investigate diversity and regeneration status of woody plants in a fragmented, remnant forest patch around Lake Zengena. With this objective we hypothesized the following: (1) Zengena forest can serve for biodiversity conservation by hosting several woody plant species that are almost absent in other areas; (2) Zengena forest, one of the several remnant forest patches in the country, has diverse woody plant species that are under serious anthropogenic pressure. The results from the present study will provide baseline information for the sustainability of Zengena forest and restoration of indigenous vegetation in the degraded areas, for the forest can serve as seed source and point of reference for restoration activities.
Materials and methods
Study area
The study was conducted in a fragmented remnant evergreen Afromontane forest patch, Zengena forest, located around Lake Zengena at 10°54′50″ N 36°58′00″ E in the Awi administrative zone, Banja District of the Amhara National Regional State, northwestern Ethiopia at 120 km south of the region’s capital, Bahir Dar (Fig. 1). Zengena forest surrounds Lake Zengena, a crater lake with an area of about 25 ha and maximum depth of 160 m (Endalew et al. 2004). With an altitude of between 2,470–2,585 m in the study sites, the forest has a tropical highland climate, which is seasonal and dominated by the dry season. The main rainy season extends from mid-June to mid-October with maximum rainfall occurring between July and August and mean annual rainfall ranging between 1,300 and 1,800 mm. The mean annual temperature ranges between 16 and 20 °C (Endalew et al. 2004).
Vegetation sampling
Systematic sampling method was employed for inventory of woody vegetation in the study site. Line transects, as described by Bullock (1996), were laid down the altitudinal gradient, following eight aspects of the site that run from the shore of the lake to the forest edge. A total of 27 plots of 20 m × 20 m were established along the line transect at 25 m altitudinal intervals. At each quadrat, altitude was recorded using a “Pretel” digital altimeter, and GPS readings were also taken. In each plot, a complete list of woody species was made and floristic data were collected. The number of individuals of each species was counted and diameter at breast height (DBH) of woody species2 cm was measured using a measuring tape following Martin (1995) and Cunningham (2001). Individuals of those species with DBH <2 cm were recorded and considered for regeneration status assessment as seedlings. Voucher specimens of each species were collected and taken to the National Herbarium, Addis Ababa University, Addis Ababa, Ethiopia for identification. Plant nomenclature in this study follows Friis (1992), Edwards et al. (1995, 2000) and Hedberg et al. (2003).

Fig. 1: Map of Ethiopia showing the study site
Data analysis
The diversity of woody species was determined using the Shannon-Wiener Diversity Index (H') and Evenness or Equitability Index (E) (Barnes et al. 1998; Krebs 1999). Frequency of a species was computed as the proportion of samples within which a species is found, and density was computed by converting the count from the total quadrats into a hectare basis as indicated in Kent and Coker (1992).The density of individuals with DBH > 10 cm and DBH > 20 cm was computed and the ratio of these two was taken as a measure of the proportion of small- and large-sized individuals (Grubb et al. 1963). The patterns of species population structure were established based on density of species in different DBH classes and interpreted as indication of variation in population dynamics.
The importance value index (IVI) of a species (Mueller-Dombois and Ellenberg 1974) was calculated by summing up relative density, relative frequency, and relative dominance, where density is the total number of individuals of a species per total area sampled, frequency is the number of plots where a species occurs per total number of plots sampled, and dominance is total basal area of a species (sum of basal area of each individual plant) per area sampled. The basal area (m2) of each plant is calculated as (DBH/200)2× 3.14, where DBH is in cm.
Results and discussion
Floristic composition and species diversity
A total of 50 woody plant species (Appendix 1) belonging to 31 families were recorded in the study area. Out of the total species recorded 17 (34%) were trees, 7 (14%) trees or shrubs, 23 (46%) shrubs and 3 (6%) lianas (Appendix 2). The overall Shannon-Wiener diversity and evenness of woody species in Zengena forest was 2.74 and 0.72, respectively. The overall diversity of the forest falls within the normal range of Shannon-Wiener diversity, which lies between 1.5 and 3.5 and rarely exceeds 4.5 (Kent and Coker 1992). The families with highest number of species were Fabaceae (5 species) and Asteraceae (4 species). Twenty families (40%) were found each represented by one species.
Floristically, Zengena forest had fewer number of woody plant species than some other Afromontane forests in Ethiopia like Zegie peninsula (Alelign et al. 2007), Masha-Anderacha (Yeshitila and Bekele 2003), Harenna forest (Bussmann 1997), Dindin forest (Shibru and Balcha 2004), Belete forest (Hundera and Gadissa 2008) and Tara Gedam and Abebaye forests (Zegeye et al. 2011). On the other hand, Zengena forest had a higher number of woody species compared with other Afromontane forests like Menagesha-Suba (Demissew 1988), Jirren forest (Tesfaye and Berhanu 2006) and Adelle and Boditi forests (Yineger et al. 2008).
The forest had relatively high Shannon-Wiener diversity and evenness index scores compared with that of Chilimo forest (Woldemariam et al. 2000), Jirren forest (Tesfaye and Berhanu 2006), islands of Lake Ziway (Zegeye et al. 2006) and Abebaye forest (Zegeye et al. 2011). The evenness value 0.72 showed almost an equitable distribution of individuals among various species. The relatively high diversity and evenness values of Zengena forest could be attributed to low disturbance, site conditions and species characteristics (Wassie et al. 2005; Zegeye et al. 2006, 2011). Zengena forest is not such exposed to disturbance by humans and livestock. The forest, especially close to the shore of the lake, is not easily accessible for use by humans and livestock due to its steepness. Moreover, the various forest management activities, mainly for biodiversity conservation and maintaining volume and quality of the lake, may contribute for the low disturbance in the forest.
Fabaceae (5 species) and Asteraceae (4 species) were the most dominant families in Zengena forest. Asteraceae has been reported as a dominant plant family in Afromontane forests in Ethiopia, most likely due to its successful dispersal capacities and wide range of ecological adaptations (Tadesse 2004; Yineger et al. 2008). The woody species of Zengena forest contains plants characteristic of forests in south and southwestern parts of the country where most of the remaining natural forests are found (Hedberg 1989; Friis 1992; Senbeta and Teketay 2003; Yeshitela and Bekele 2003). Some of these species include Prunus africana, Ritchiea albersii, Apodytes dimidiata, Erythrina brucei and Allophyllus abyssinicus.
Species-area curve
The species-area curve—which reveals the pattern of species diversity with increasing area (Rosenzweig 1995)—for the study area is indicated in Fig. 2. The curve clearly showed an initial sharp increase in the number of species with increasing area and tendency towards flattening. Similar patterns were observed in the two islands of Lake Ziway (Zegeye et al. 2006).
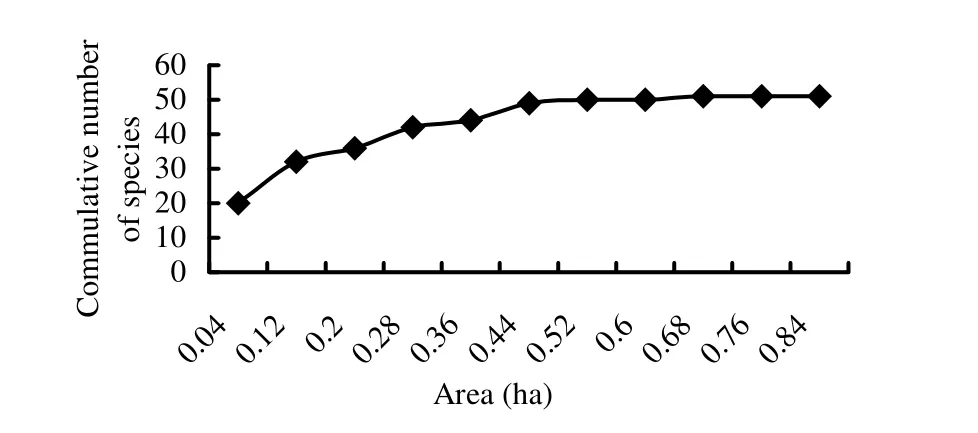
Fig. 2: Species-area curve for Zengena forest
Density and frequency of woody species
The density and frequency of woody plant species found in the sampled plots have shown differences among species (Appendix 1). The total density of woody species in Zengena forest was 2,202 individuals ha-1. The density was low compared to some other Afromontane forests like Tara Gedam and Abebaye forests (Zegeye et al. 2011), Zegie peninsula (Alelign et al. 2007), Kimphee forest (Senbeta and Teketay 2003) and Jirren forest (Tesfaye and Berhanu 2006). At DBH >10 cm, the density of species was 206 individuals·ha-1. The corresponding value at DBH >20 cm was 96 individuals·ha-1. The ratio of density of individuals with DBH >10 cm to DBH >20 cm was 2.15, implying the dominance of small-sized individuals in Zenegena forest.
The value of the aforementioned ratio was 2.4 for Masha Andracha forest (Yeshitila and Bekele 2003), 2.52 for Adelle forest (Yineger et al. 2008), both having higher proportions of small-sized individuals than Zengena forest. On the other hand, the value obtained from the above ratio for Zengena forest is comparable to the ratio obtained for Boditi forest (Yineger et al. 2008), Dindin forest (Shibru and Balcha 2004) and Belete forest (Hundera and Gadissa 2008). Clausena anisata had the largest contribution for the predominance of small-sized individuals in Zengena forest.
The species with the highest density was Clausena anisata, followed by Rapanea rhododendroides, Bersama abyssinica, Vernonia leopoldi, and Maytenus arbutifolia. These five most abundant species (10% of all species) contributed about 64 % of the total density. Clausena anisata and Rapanea rhododendroides alone accounted 41% of all individuals. Alelign et al. (2007) reported five species (about 6% of all species) contributing more than half of all individuals recorded in Zegie peninsula. Similarly, the five most abundant species in Tara Gedam (4.5% of all species) and Abebaye forest (5.7% of all species) contributed about 32% and 51% of the total density, respectively (Zegeye et al. 2011).
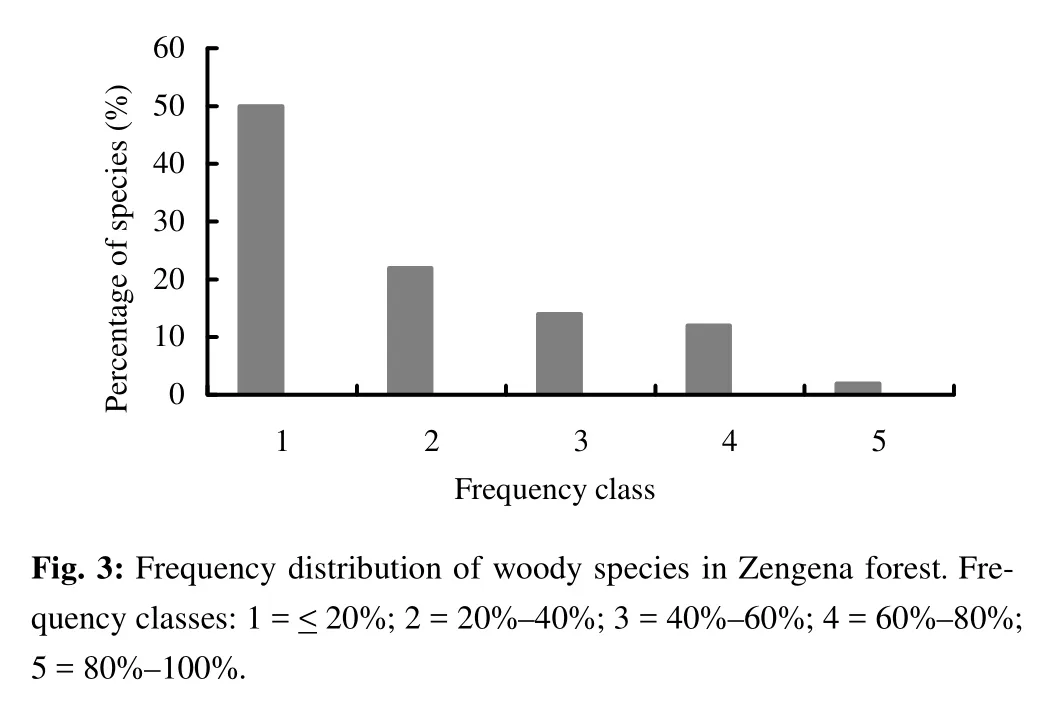
The species with the highest frequency value was Rapanea rhododendroides (90%), followed by Maytenus arbutifolia (78%), Bersama abyssinica (73%) and Brucea antidysentrica, Clausena anisata, Dombeya torrida, Prunus africana and Vernonia leopoldi (62% each) (Appendix 1). A high frequency value represents a wider distribution of the species in the forest. The frequency distribution of species (Fig. 3) showed high percentage of species at lower frequency classes with more than 70% of the species having a frequency value less than 40% indicating high floristic heterogeneity of the study area. The variation in density and frequency among species may be attributed to differences in site conditions, species characteristics, economic importance of species and disturbance (Tesfaye et al. 2002; Dalle and Fetene 2004; Shibru and Balcha 2004; Tesfaye and Teketay 2005; Hundera and Gadissa 2008).
Basal area and IVI of woody species
The total basal area of woody species in Zengena forest was 22.3 m2ha-1(Appendix 1). The basal area of the forest is small compared to many other Afromontane forests in the country, such as Adelle forest (Yineger et al. 2008), Tara Gedam and Abebaye forests (Zegeye et al. 2011), Dindin forest (Shibru and Balcha 2004), Belete forest (Hundera and Gadissa 2008) and Mana Angetu forest (Lulekal et al. 2008). The species with the highest basal area was Prunus africana, followed by Acacia abyssinica, Dombeya torrida, Rapanea rhododendroides, and Erythrina brucei. These five species accounted about 78.2% of the basal area, while Prunus africana alone made up about 45% of the basal area in the forest. The basal area contributed by Prunus africana was due to more large individual trees than the remaining species and showed its highest ecological importance in the forest (Cain and Castro 1959). Erythrina brucei had high basal area due to its large size, though it had low density. Fifteen species each had an input of less than 0.1 % to the total basal area in the forest because of their low density and small size.
Prunus africana exhibited the highest IVI (51.6), followed by Rapanea rhododendroides (31.7), Clausena anisata (28.7), Bersama abyssinica (17.1), and Dombeya torrida (15.5). These five species contributed about 49.2% of the total IVI. The highest IVI values of Clausena anisata and Bersama abyssinica were due to their high relative density and relative frequency although they had low relative dominance or basal area. The IVI values showed the relatively highest ecological significance (dominance) of Prunus africana and Rapanea rhododendroides in the Zengena forest (Curtis and McIntosh 1950). Species with low IVI values—such as Croton macrostachyus, Hypericum revolutum, Calpurnia aurea, Carissa spinarum, and Dovyalis cafrra—need prioritization for conservation measures (Shibru and Balcha 2004).
Population structure
The density distribution of individuals of all woody species in the various diameter classes was not uniform in Zengena forest, but showed a progressive trend of decline (Fig. 4). The number of individuals decreased with the increasing diameter classes, suggesting a more or less inverted J-shaped population structure, an indication of stable population structure or healthy regeneration status (Shibru and Balcha 2004; Zegeye et al. 2006, 2011; Alelign et al. 2007). However, it needs to be noted that some species are not in healthy regeneration status. For example, Allophylus abyssinicus, Dombeya torrida and Prunus africana had individuals missed in the middle or larger size classes, indicating selective removal of individuals of preferred size (Shibru and Balcha 2004; Alelign et al. 2007; Hundera and Gadissa 2008). About 87% of the individuals had DBH value of less than 10 cm, which indicates the dominance of small-sized individuals in the forest. In Zegie peninsula, about 89% of the individuals in the forest had a DBH value of less than 7.5 cm (Alelign et al. 2007).
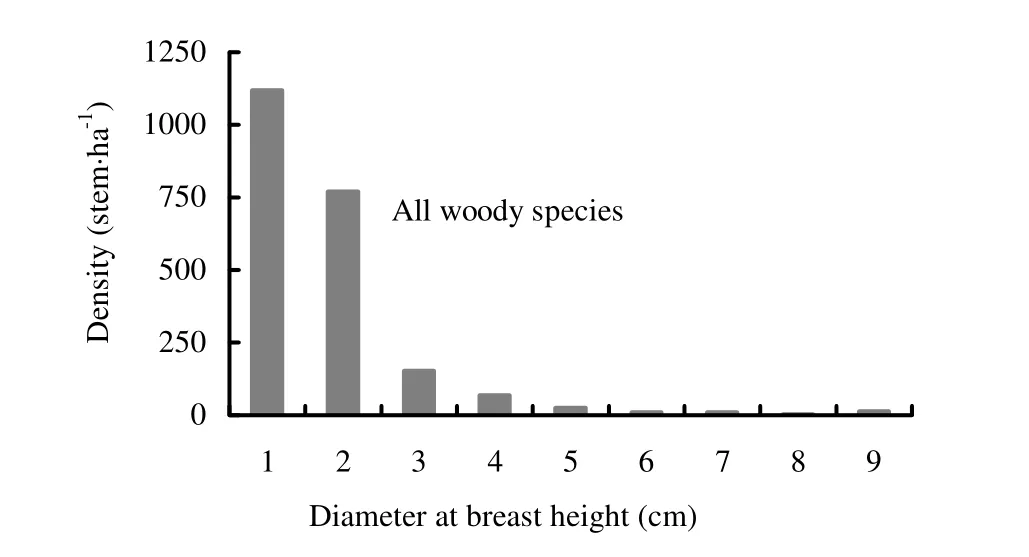
Fig. 4: Diameter class distribution of all woody species recorded in Zengena forest. Diameter classes: 1= 0-2 cm; 2=2-10 cm; 3=10-20 cm; 4=20-30 cm; 5=30-40 cm; 6=40-50 cm; 7= 50-60 cm; 8=60-70 cm; 9= >70 cm.
Analysis of population of woody plants in Zengena forest revealed four major regeneration patterns (Fig. 5). The first pattern was an inverted J-curve formed by the species with the highest number of individuals in the lower DBH classes and a gradual decline in number of individuals toward higher DBH classes. This pattern was represented by Buddleja davidii and suggested good reproduction and recruitment potential of the species in the forest. The second pattern was well represented by Prunus africana with missed or few individuals at some diameter classes, showing selective cutting of medium-sized individuals. These species had good reproduction potential but recruitment was hampered.

Fig. 5: Diameter class distribution of selected woody species recorded in Zengena forest. Diameter classes: 1= 0-2 cm; 2=2-10 cm; 3=10-20 cm; 4=20-30 cm; 5=30-40 cm; 6=40-50 cm; 7= 50-60 cm; 8=60-70 cm; 9= >70 cm.
The third pattern, exemplified by Acacia abyssinica, showed missed or few individuals in the lower and higher size classes and increasing number of individuals around the middle classes. This population pattern showed hampered reproduction caused by poor recruitment and selective removal of the species at higher diameter classes leading to scarcity of mature individuals that would serve as seed sources. The fourth pattern, represented by Erythrina brucei, showed individuals only presented at higher DBH classes, suggesting hampered reproduction and recruitment of the species in the forest. Therefore, all species in the forth regeneration pattern, which also includes Ficus vasta, Ficus sur, Croton macrostachyus, Apodytes dimidiata and Schefflera abyssinica, should be prioritized for conservation measures.
Conclusions and recommendations
Zengena forest is a fragmented, small-sized remnant forest patch formed around Lake Zengena. The forest harbors 50 woody plant species suggesting that the remnant forest patches in the highlands of northern Ethiopia can serve as treasure houses of several woody plant species that are almost disappearing in other areas (Wassie et al. 2005). Zengena forest contains woody plants—such as Allophylus abyssinicus, Terminalia brownii, Prunus africana, Apodytes dimidiata and Schefflera abyssinica—which were once common in the adjacent land currently used for crop production, grazing, and settlement. The forest also contains plants characteristic of forests in south and southwestern parts of the country where most of the remaining natural forests are found. However, further forest fragmentation will affect successional processes in this remnant forest patch by reducing its potential to maintain the original biodiversity and ecological processes. Several previous works have shown that most of the dominant tree species in remnant forest patches will not form soil seed banks following the removal of mature individuals in the standing vegetation (Teketay 1997, 1998; Senbeta and Teketay 2002; Wassie and Teketay 2006). Sustainability of forest patches, including Zengena forest, should be given due attention for the existence of many woody plant species, especially in the central and northern highlands of Ethiopia where the original forests have almost disappeared (EFPA, 1994). Furthermore, species showing poor regeneration or with low IVI values—such as Hypericum revolutum, Croton macrostachyus, Apodytes dimidiata and Schefflera abyssinica—should be prioritized for conservation measures.
The work was financed by Bahir Dar University Research and Community Service as a study grant (BDU/RCS/Sc/03/06) to the first author. The authors are grateful to the staff members of The National Herbarium, Ethiopia for their support during species identification. We would like to thank Tsegaye Sewnet for generating map of Ethiopia that shows the study area and the anonymous reviewers for useful comments.
Alelign A, Teketay D, Yemshaw Y, Edwards S. 2007. Diversity and status of regeneration of woody plants on the peninsula of Zegie, northwestern Ethiopia. Tropical Ecology, 48(1): 37-49.
Barnes BV, Zak DR, Denton SR, Spurr SH. 1998. Forest Ecology. 4th. Ed. New York: John Wiley and Sons, pp. 10-74.
Bekele T. 1994. Phytosociology and ecology of a humid Afromontane forest on the central plateau of Ethiopia. Journal of Vegetation Science, 5: 87-98.
Bullock J. 1996. Plants. In: WJ Sutherland (ed), Ecological Census Techniques: A Handbook. Cambridge: Cambridge University Press, pp.111–138.
Bussmann RW. 1997. The forest vegetation of the Harenna Escarpment (Bale Province, Ethiopia)-Syntaxonomy and phytogeographical affinities. Phytocoenologia, 27(1): 1-23.
Cabin RJ, Weller SG, Lorence DH, Cordell S, Hadway LJ. 2002. Effects of microsite, water, weeding, and direct seeding on the regeneration of native and alien species within a Hawaiian dry forest preserve. Biological Conservation, 104: 181-190.
Cain SA, Castro GMO. 1959. Manual of vegetation analyses. New York: Harper and Brothers, pp. 325.
Cotler H, Ortega-Larrocea MP. 2006. Effects of land use on soil erosion in a tropical dry forest ecosystem, Chamela watershed, Mexico. Catena, 65: 107-117.
Cunningham AB. 2001. Applied Ethnobotany: People, Wild Plant Use and Conservation. London: Earthscan, pp. 96-115.
Curtis JT, Mclntosh RP. 1950. The interrelationship of certain analytic and synthetic phytosociological characters. Ecology, 31: 434-455.
Dalle G, Fetene M. 2004. Gap-fillers in Munessa-Shashemene forest. Ethiopian Journal Biological Sciences, 3: 1–14.
Demissew S. 1988. The floristic composition of Menagesha State Forest and the need to conserve such forests in Ethiopia. Mountain Research and Development, 8: 243-247.
Edwards S, Tadesse M, Hedberg I. 1995. Flora of Ethiopia and Eritrea, Vol. 2, Part 2: Canellaceae to Euphorbiaceae. The National Herbarium, Addis Ababa and Department of Systematic Botany, Uppsala: EMPDA, pp. 165-380.
Edwards S, Tadesse M, Demissew S, Hedberg I. 2000. Flora of Ethiopia and Eritrea, Vol. 2, Part 1: Magnoliaceae to Flacourtiaceae. The National Herbarium, Addis Ababa and Department of Systematic Botany, Uppsala: EMPDA, pp.1-532.
EFAP. 1994. Ethiopian Forestry Action Program. Addis Ababa, Ethiopia, pp. 14.
Endalew M, Goshu G, Zelalem W. 2004. Exploratory fishery and basic limnology of Lake Zengena, Ethiopia. Field report. Bahir Dar fish and other aquatic life research center, Bahir Dar, Ethiopia, pp. 1-5.
FAO 2007. The state of the world’s forests report. Rome: FAO, pp. 144.
FAO 2010. Global forest resources assessment. Rome: FAO, pp. 340.
Friis I. 1992. Forest and forest trees of North East tropical Africa. Kew Bulletin Additional Series, 15: 1-396.
Grubb PJ, Lloyd JR, Pennington JD, Whitmore JC. 1963. A comparison of montane and lowland rainforest in Ecuador: The forest structure, physiognomy and floristics. Journal of Ecology, 51: 567–601.
Hedberg I, Edwards S, Nemomssa S. 2003. Flora of Ethiopia and Eritrea, Vol. 4, Part 1: Apiaceae to Dipsacaceae. The National Herbarium, Addis Ababa and Department of Systematic Botany, Uppsala: EMPDA, 1-283.
Hedberg O. 1989. Rosaceae. In: I Hedberg and S Edwards (eds), Flora of Ethiopia, Vol. 3, Pittosporaceae to Araliaceae. The National Herbarium, Addis Ababa and Department of Systematic Botany, Uppsala, pp. 31-44.
Hundera K, Gadissa T. 2008. Vegetation composition and structure of Belete forest, Jimma Zone, Southwestern Ethiopia. Ethiopian Journal of Biological Sciences, 7(1): 1–15.
Kent M, Coker A. 1992. Vegetation Description and Analysis: A Practical Approach. London: Belhaven Press, pp. 1-33.
Krebs CJ.1999. Ecological Methodology. New York: Jim Green, pp. 410-455.
Lulekal E, Kelbessa E, Bekele T, Yineger Y. 2008. Plant species composition and structure of the Mana Angetu Moist Montane Forest, South-Eastern Ethiopia. Journal of East African Natural History, 97(2): 165–185.
Martin GJ. 1995. Ethnobotany: A Methods Manual. London: Chapman and Hall, pp. 1-66.
Mueller-Dombois D, Ellenberg H. 1974. Aims and Methods of Vegetation Ecology. New York: Wiley and Sons, pp. 547.
Rosenzweig ML. 1995. Species diversity in space and time. Cambridge: Cambridge University Press, pp. 8-49.
Sayer JA, Harcourt CS, Collins NM. 1992. The Conservation Atlas of Tropical Forests, Africa. Basingstoke, Great Britain: Macmillan Publishers, pp. 1-160.
Senbeta F, Teketay D. 2002. Soil seed banks in plantations and adjacent dry Afromontane forests of central and southern Ethiopia. Tropical Ecology, 43: 229-242.
Senbeta F, Teketay D. 2003. Diversity, community types and population structure of woody plants in Kimphee Forest, a virgin nature reserve in southern Ethiopia. Ethiopian Journal of Biological Sciences, 2(2): 169-187.
Shibru S, Balcha G. 2004. Composition, structure and regeneration status of woody species in Dindin natural forest, Southeast Ethiopia: An implication for conservation. Ethiopian Journal of Biological Sciences, 3(1): 15-35.
SIM. 2001. Manual of highland Ethiopian trees. SIM forestry study project. Injibara: Banawee Printing Press, pp. 310.
Tadesse M. 2004. Asteraceae (Compositae). In: I Hedberg, I Friis and S Edwards (eds), Flora of Ethiopia and Eritrea, Vol. 4, Part 2. The National Herbarium, Addis Ababa and Department of Systematic Botany, Uppsala: EMPDA, pp. 1-408.
Teketay D. 1997. The impact of clearing and converting dry Afromontane forests into permanent arable land on the composition and density of soil seed banks. Acta Oecologia, 18: 557–573.
Teketay D. 1998. Soil seed bank at an abandoned Afromontane arable site. Feddes Repertorium, 109: 161–174.
Tesfaye G, Berhanu A. 2006. Regeneration of indigenous of woody species in the understories of exotic tree plantations in southwestern Ethiopia. Ethiopian Journal Biological Sciences, 5(1): 31–43.
Tesfaye G, Teketay D, Fetene M. 2002. Regeneration of fourteen tree species in Harenna forest, southeastern Ethiopia. Flora, 197: 461–474.
Tesfaye G, Teketay D. 2005. The influence of logging on natural regeneration of woody species in Harenna montane forest, Ethiopia. Ethiopian Journal of Biological Sciences, 4 (1): 59-73.
Wassie A, Teketay D, Powell N. 2005. Church forests in North Gonder Administrative Zone, northern Ethiopia. Forests, Trees and Livelihoods, 15: 349-373.
Wassie A, Teketay D. 2006. Soil seed banks in church forests of northern Ethiopia: Implications for the conservation of woody plants. Flora, 201: 32–43.
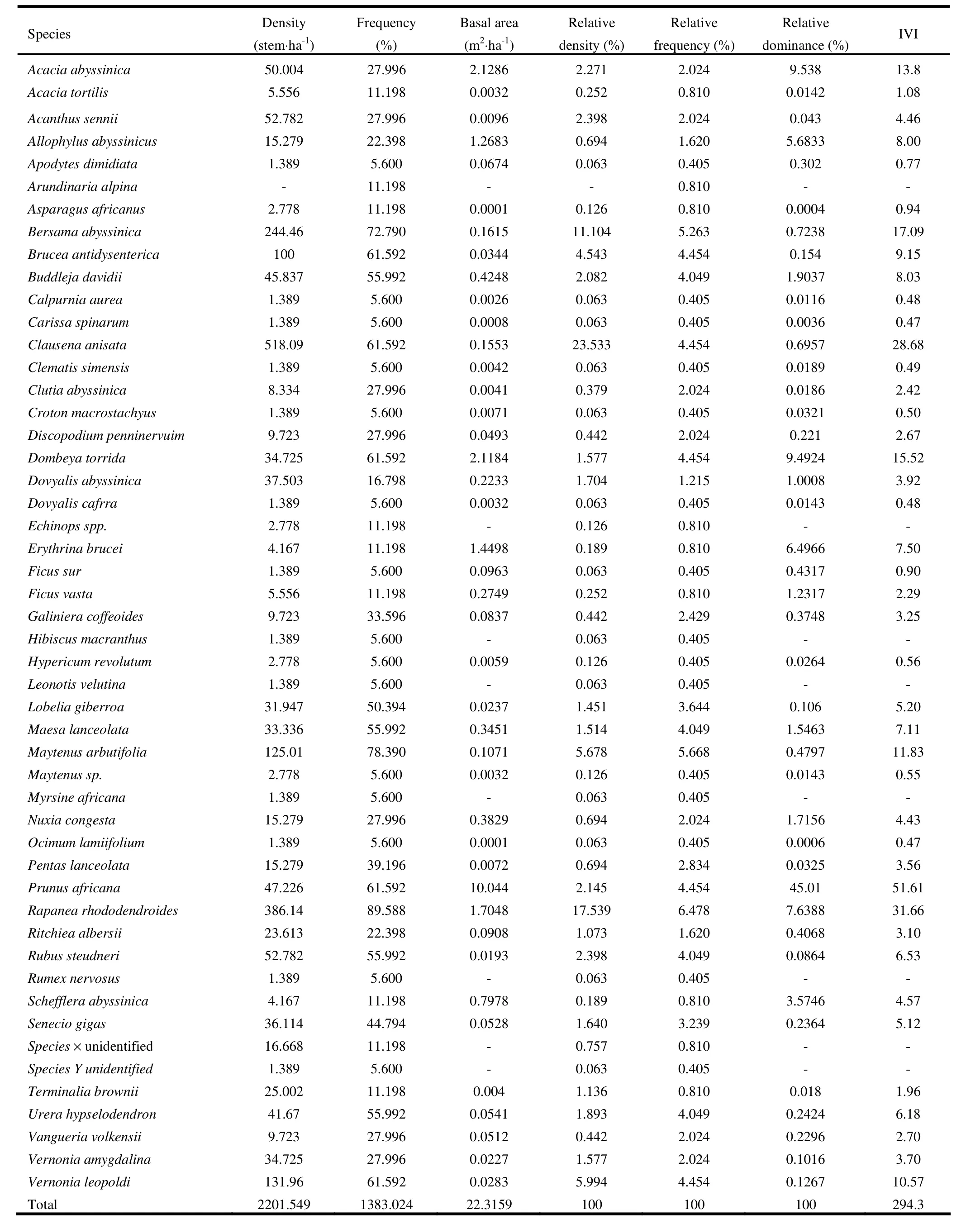
Appendix 1: Density, frequency, basal area and IVI of woody plants at Zengena forest.

Appendix 2: Family, local name and growth habit of woody plants recorded at Zengena forest.
杂志排行
Journal of Forestry Research的其它文章
- Carbon sequestration in Chir-Pine (Pinus roxburghii Sarg.) forests under various disturbance levels in Kumaun Central Himalaya
- Regional differences of water conservation in Beijing’s forest ecosystem
- Spatial modeling of the carbon stock of forest trees in Heilongjiang Province, China
- Spatial heterogeneity of factors influencing forest fires size in northern Mexico
- Community ecology and spatial distribution of trees in a tropical wet evergreen forest in Kaptai national park in Chittagong Hill Tracts, Bangladesh
- Diversity, regeneration status and population structure of gum- and resin-bearing woody species in south Omo zone, southern Ethiopia
