Changes induced by osmotic stress in the morphology, biochemistry, physiology, anatomy and stomatal parameters of almond species (Prunus L. spp.) grown in vitro
2014-04-20ShakibaRajabpoorSoghraKianiKarimSorkhehFarahnazTavakoli
Shakiba Rajabpoor · Soghra Kiani · Karim Sorkheh Farahnaz Tavakoli
Introduction
Wild almond (Prunus L. spp.) is an osmotic stress-tolerant species (Rouhi et al. 2007; Sorkheh et al. 2011). Fruit trees have different morpho-biochemical, physiological and anatomical adaptations that allow them to survive osmotic stress situations (Save et al. 1995; Torrecillas et al. 1996; 1999; Sorkheh et al. 2011). Rouhi et al. (2007) found important differences between three almond species in their ecophysiological behaviour in response to osmotic stress. Adaptation to osmotic stress can vary considerably between species and even within a species (Rouhi et al. 2007; Sorkheh et al. 2011). Plants living in arid and semiarid regions use different mechanisms to survive in osmotic stress affected soils. The mechanisms involved in the control of water loss include stomatal closure, raised stomatal and cuticular resistance, changes in leaf area, orientation and anatomy (Escalona et al. 1999; Chaves et al. 2002; Lawlor 2002; Romero and Boita 2006; Vijayan et al. 2008). Liu and Zhao (2005) showed that the tolerant ramie (Boehmeria nivea L.) genotypes have higher relative water content, water potential and cell membrane stability, and accumulated more proline than the sensitive ones under 12–14 days of osmotic stress. The results of Sánchez et al. (1998) also indicated a potential role of proline in reducing the damage caused by dehydration. Lima et al. (2002) testified to increases in activity of superoxide dismutase, catalase and ascor-bate peroxidase to a greater extent in the drought tolerant clone of Coffea canephora Pierre ex A. Froehner than in the sensitive one. Malondialdehyde content has a close relation with osmotic stress tolerance of plants (Liu and Zhang 1994; Jiang and Huang 2001). Barbara et al. (1999) reported that the tolerant wheat (Triticum aestivium L.) cultivar could maintain higher photosynthetic activity than a sensitive cultivar under osmotic-stress conditions. Anatomical changes induced by water deficits in higher plants are better visual indicators (Shao et al. 2008). Responses of plant tissue to water stress depend on the anatomic characteristics that regulate the transmission brought by the cells (Matsuda and Rayan 1990; Olmos et al. 2007). Anatomical alterations might occur when a plant is subject to water deficit (Makbul et al. 2011). Tissues exposed to environments with low water availability showed reduction in cell size and increase in vascular tissue and cell wall thickness (Pitman et al. 1983; Guerfel et al. 2009; Makbul et al. 2011). Modifications to cell wall architecture and alterations of xylem/phloem ratios are involved in the resistance of plants to environmental stresses (Child et al. 2003).
Stomatal resistance in some trees such as almond (Prunus dulcis Mill.), peach (Prunus persica L.), olive (Olea europaea L.) and other trees increased with osmotic stress (Torrecillas et al. 1996; Giorio et al. 1999; Mahhou et al. 2005; Rouhi et al. 2006). In some plants, such as in Nerium oleander L., stomata are partly covered with outer epidermis or can even be crytoporus. The adaptations increase the distance that water vapour has to travel during transpiration. They also increase boundary layer resistance and therefore decrease transpiration. In some species, stomata lay in grooves that can be covered by scales, raphides and trichomes (Karschon 1974; Ehleringer 1980; Van Damme 1991). The anatomical features restrict water losses while simultaneously reducing daily carbon assimilation at the leaf level and decreasing the long-term net carbon gain by the whole plant (Romero and Boite 2006).
Rouhi et al. (2007) and Sorkheh et al. (2009) demonstrated that non-domesticated Prunus L. species have a lower sensitivity to water stress than cultivated genotypes because of morphological and physiological characteristics, like lower leaf area, stomata density and size, and lower leaf water potential. The selection of osmotic-resistant Prunus rootstocks from nondomesticated germplasm is the focus of several almond genetic research projects (Gradziel and Kester 1998; Camposeo et al. 2010). Even so, there is little information on stomatal characteristics of Prunus L. spp. (Fanizza and Reina 1990; Guirguis et al. 1995; Rouhi et al. 2007; Sorkheh et al. 2009; Camposeo et al. 2010).
The present investigation aimed to understand the influence of osmotic stress on morphological, biochemical, physiological, anatomical and stomatal characters of eight Iranian wild almond species. Different osmotic stress conditions representative of natural conditions in Iran were induced by Polyethylene Glycol 6000 (PEG 6000). The results of the study are expected to help almond breeders to select proper breeding stocks, parameters and thresholds of osmotic stress to develop osmotic-tolerant highyield genotypes in almond.
Material and methods
Wild almond species
We studied eight wild almond species (Prunus L. spp.) including three osmotic-tolerant species (Prunus arabica (Olivier) Neikle, Prunus glauca (Browicz) A.E. Murray, Prunus scoparia Spach) in the section labeled ‘Spartioides’, two moderately tolerant species (Prunus lycioides Spach, Prunus reuteri Bossi. et Bushe) in the section labeled ‘Lycioides’, and three osmotic stresssusceptible species (Prunus communis (L.) Archang, Prunus eleagnifolia (Spach) Fritsch, Prunus orientalis Mill. (Syn. Prunus argenta Lam.)) in the section labeled ‘Euamygdalus’. These eight species were categorized into the above three sections based on their responses to osmotic stress in vitro cultures as reported by Sorkheh et al. (2010).
Morphological studies
The morphological characters of 8-9 month-old nursery-raised saplings were investigated at the Faculty of Agriculture of Shahrekord University during 2010-2012. Single saplings were planted in earthen pots containing mixed soil (white sphagnum (45%), peat (40%) and perlite (15%)) (Rouhi et al. 2007). Saplings were grown in a greenhouse at 27±5°C, and 65±10% relative humidity (RH) under normal daylight and well-watered conditions from May 2010 until the end of January 2011. Pots were then arranged in a greenhouse-based gutter system described by Ranjbarfardooei et al. (2000) and Ranjbarfardooei et al. (2002). The plants were continuously irrigated using a circulating system consisting of a water pump, gutter and a reservoir containing a standard Hoagland nutrient solution.
Osmotic stress treatments according to Rouhi et al. (2007) consisted of a control treatment (osmotic potential of the nutrient solution (Ψs) = -0.1 MPa), and five osmotic stress levels (Mild osmotic stress (Ψs = -0.8, -1.0 MPa), Moderate osmotic stress (Ψs =-1.1, -1.2 MPa) and Severe osmotic stress (Ψs = -1.3 MPa). Osmotic stress levels were induced by adding non-penetrating polymers of PEG 6000 (Chazen et al. 1995) to the nutrient solution following the methods of Ranjbarfardooei et al. (2000) and Rouhi et al. (2007). The concentration of PEG 6000 in the nutrient solution was determined following Burlyn and Merrill (1973). Osmotic stress treatments started on April 5, 2012, by daily and linearly adding PEG 6000 until final osmotic stress levels were gained for all treatments after two weeks, following the method of Ashraf and Leary (1996). This meant that the osmotic potential of the nutrient solution decreased at a rate of -0.057, -0.071, -0.079, -0.086 and -0.093 MPa·day-1during the two weeks to achieve a final osmotic potential of -0.8, -1.0, -1.1, -1.2 and -1.3 MPa, respectively. Once the osmotic stress levels reached the levels desired for unification in the assessment, the plants were pruned 10 cm above ground level. The osmotic stress levels were kept constant by regularly checking the electric conductivity (EC) (HI 9933, Hanna Instruments Inc., Woonsocket, RI, USA) of the nutrient solution and adding distilled water when needed until the original EC value was reached again.
Mean day and night temperature in the greenhouse were 32°C and 20°C, and humidity was 65% and 85%, respectively. Daily maximum natural photosynthetic active radiation (PAR) intensity (PAR Quantum Sensor SKP 215, Skye Instruments Ltd., Powys, UK) at plant level varied between 870 and 250 µmol·m-2·s-1. Treatments were arranged as a factorial set-up with complete random design and with three replications. In total 8×18 almond plants were used. Data on the number of primary branches sprouted, height of the longest shoot, number leaves in the longest shoot, inter-nodal length, single leaf size and weight, were recorded as reported earlier (Sorkheh et al. 2007; Sorkheh et al. 2009). Leaf dimensions (length, width) of the 6th through 11th leaf counting from the top were measured with a ruler at the onset of the experiment. Leaf area for each plant was determined by a planimeter (Li-3000, LI-COR, Lincoln, NE, USA).
Biochemical studies
Biochemical assays were carried out on the 5th leaf from the top of each twig on the 30th day after pruning to unify the rate of growth, which is most important for biochemical assessment in wild almond species. Chlorophyll concentration of the leaf was determined following the method of Arnon (1949) with slight modification according to Sorkheh et al. (2012). Total soluble sugar, protein, proline and phenol concentrations were determined by the methods of Morris (1948), Lowry et al. (1951), Bray and Thrope (1954) and Bates et al. (1973), respectively, with a slight modification for wild almond species according to Sorkheh et al. (2012). In vivo activity of the enzyme nitrate reductase was measured by the method of Hageman and Hucklesby (1972). Mineral concentrations (Na+and K+) in the leaf were estimated after digesting with the tri-acid method. In this method leaves from the 9th to 11th positions from the top of the twig according to Vijayan et al. (2008) were dried at 80°C for 48 h in a hot air oven, as in most of the plants the 5th leaves were not sufficient for mineral analysis due to their use in biochemical assays. The dried leaves were then powdered with a grinder. Leaf powder (1 g) was later digested with 10 mL of tri-acid digestion mixture (nitric acid, perchloric acid and sulphuric acid in 10: 4: 1 ratio) by boiling until the solution became colourless. After cooling, the digestion mixture was diluted with distilled water, fltered through Whattman flter paper No.1 and the volume made up to 100 mL. Na+and K+were determined by fame ionisation with a fame photometer (Systronics, Naroda Industrial Estate, Gujarat, India).
Physiological measurements
Physiological measurements were made on the 5th leaf from the top of plants growing in either osmotic-stressed or well-watered conditions. All analyses were done according to Zhang (1989) and with a modification updated for wild almond species according to Liu et al. (2005) and Sorkheh et al. (2011, 2012).
Relative permeability (REC) of protoplast membranes
REC was measured by using a conductimeter (HI 9933, Hanna Instruments Inc., Woonsocket, RI, USA) according to Liu et al. (2005) with some modifications based on Sorkheh et al. (2011). Leaf samples were cut into equal sized pieces (0.3 g for each treatment) and placed in 25 mm × 150 mm culture vessels containing 15 mL of distilled water, and shaken on an orbital shaker (100 rpm) for 24 h at room temperature. The first conductance of the bathing solution was measured using a conductivity meter (ECa). The tubes were then autoclaved at 115°C for 10 min and final readings were taken following autoclaving and incubated for 24 h at room temperature (ECb).

Malondialdehyde (MDA) content
1 g of fresh leaves (W) was cut into small pieces, put into a mortar with an amount of liquid nitrogen and ground into slurry. The slurry was transferred into a centrifuge tube and made up to 10 mL (V1), and centrifuged at 4000 rpm for 15 min. 1.5 mL clean solution (V2) was then collected into a cuvette from the centrifuge tube, 2.5 mL of 0.5% thiobarbituric acid (V3) was added, the cuvette boiled for 15 min and cooled quickly in cold water. Finally, the solution was centrifuged at 1800 rpm for 10 min, and optical density (OD) of the clean solution was tested under 532 and 600 nm.

where 0.155 is the extinction coefficient in ml nmol-1.
Catalase activity
One gram of fresh leaf (W) was cut into small pieces, put into a mortar containing an amount of phosphoric acid buffer (pH = 7), and ground into a slurry. The slurry was transferred into a centrifuge tube and made up to 10 mL (V1), centrifuged at 4000 rpm for 15 min and the clean solution was stored. 2 mL extraction solution (V2) was collected into flask 1, and 2 mL boiled extraction solution into flask 2 (as a control), 5 mL of 1% H2O2was added to each flask, and the flasks were kept in water at 30°C for 10 min (T), then 5 ml of 1% H2SO4was added to each flask to stop enzymic reaction. The left H2O2was titrated with 0.1 mol L-1KMnO4to keep the pink color for 30 s.

where X1is the volume of 0.1 mol L-1KMnO4used for flask 1, X2is for flask 2, while 1.7 is the equivalent of H2O2(mg) for 1 mL of 0.1 mol L-1KMnO4.

Net photosynthetic rate (Pn)
Net photosynthetic rate (Pn) was measured by using half a leaf (main leaf vein left), trichloroacetic acid 5% was applied on the petiole to stop transport of photosynthetic products and the petiole was wrapped with tinfoil to support the leaf in an erect position. We then oven-dried and weighed a certain area of leaf (leaf discs from the cut half-leaf, leaf area (A), leaf weight (W1). Four hours (T) later, the left half-leaf was cut off, oven-dried and weighed to the same size of leaf (W2).

where 1.5 is the index for converting assimilated dry matter into CO2according to Liu et al. (2005).
Leaf anatomical studies
Leaf samples were fixed in formalin-acetic acid-alcohol (1:1:18 ratio) for 16 h. Samples were dehydrated by passing through alcohol grades of 30, 50, 70, 80, 90 and 95% for 1 h each and finally transferred to absolute alcohol for 16 h. Dehydrated samples were transferred to alcohol-chloroform mixtures (3:1; 1:1; 1:3) and finally to pure chloroform. Thin slices of paraffin (56°C melting point) were added to the chloroform at 10 min intervals until full saturation was gained. Later, the leaf-chloroformparaffin mixture was kept on a hot plate at 37°C for 48 h and shifted to 45°C for 16 h. The samples were then transferred to 60°C bath, the molten paraffin was poured out and fresh paraffin slices were added. This was repeated 3-4 times at 30 min intervals, until no smell of chloroform remained in the leaf sample. After adding fresh paraffin, the molten paraffin along with the leaf samples were transferred to paper boats. Proper orientation of the material was carried out with a hot needle. After proper solidification, sections were prepared from the samples with a rotary microtome and stained with Delafield’s Haematoxylin, counter stained with 1.00% methylene blue and mounted with euparol. Thickness of epidermis, palisade and spongy layers were recorded under a microscope (OLYMPUS BX51, Japan).
Stomatal studies
Imprints of epidermal cells and stomata of both sides of the leaf were taken from the 8th leaf from the top by using clear nail polish (Deborah 77600, Italy) at the end of the second stress week. A thin layer of polish was applied with a small brush onto the leaf surface so that it was distributed on a rectangular area of about 1 cm2. A transparent film with a replica print of the epidermis and stomata was formed after evaporation of the solvent. Shrinkage of the replicas might have occurred during drying, so that the surface area of the reproduced structure might have been smaller than the original on the leaf. This phenomenon was avoided by using adhesive tape to fix the replica on a microscope slide (Weyers and Meidner 1990; Jones 1992; Elagoz et al. 2006). In this study, a fully expanded leaf (upper and lower sides) of each plant from each treatment was used for measurement of stomatal density. Replicas were looked at under a light microscope (Olympus GX 31, Japan) with magnification of 40 × 10. To make stomatal counting easier, the image from the microscope was transferred onto a TV screen by a video camera (JVC TK - 860 E). Stomata were directly counted on the TV screen and converted to stomatal density (the number of stomata for each mm2) by means of a calibration plate (Graticules LTD, England, 200 × 0.01= 2 mm). The minimum number of microscopic fields needed for determination of stomatal density was determined according to the method of Zaid and Hughes (1995). Based on this method, for each treatment, the stomata of 96 fields were counted (3 replications in each treatment × 16 areas and imprint × 2 counts for each area).
Data analysis
Analysis of variance (ANOVA) was carried out to assess differences between Prunus species, osmotic stress levels, and their interactions on measured characters. Data were statistically analyzed using a factorial design using SAS program (SAS Institute 2000). The mean separations were assessed with a Duncan multiple range test at the 1% statistical level of significance.
Results
Morphological characters
Mean length and width of fully expanded leaves of the eight investigated species are shown in Fig. 1. Leaf size differed significantly (p <0.01) between all species, with the largest and smallest leaves for Prunus communis L. and Prunus lycioides L., respectively. The most striking symptoms visually observed in osmotic-stressed Prunus species were yellowing of younger leaves at the shoot top and between leaf veins, and retardation of growth. In susceptible species the influence was so severe that burnt lesions appeared on younger leaves. Older leaves, however, remained green but senescence of the leaf was rapid for susceptible species. Shoot growth inhibition caused by osmotic stress was visible in all Prunus species, though it varied signifcantly between the ‘osmotic tolerant’ and ‘susceptible groups’. The susceptible species like ‘Euamygdalus’ showed reduced growth (71.5%) caused by drought stress at -1.2 Mpa as compared to that of the control. Plant height was also signifcantly reduced by osmotic stress at -1.2 Mpa from 35.9 to 15.5 cm. The decline in plant height in response to osmotic stress was lowest in ‘Spartoidies’ and highest in ‘Euamygdalus’. Correspondingly, there was also a reduction in shoot length and leaf number for each branch as compared to the control. For ‘Euamygdalus’ the mean number of leaves was reduced by 64.3% at -1.2 Mpa osmotic stress level. Leaf size declined from 50.2 cm per leaf in the controls to 22.2 cm at -1.2 Mpa osmotic stress level for the ‘Euamygdalus’ section and from 32.4 cm in the controls to 15.5 cm at -1.2 Mpa in the ‘Lycioiedes’ section. Individual leaf fresh and dry weights also differed signifcantly by species and osmotic stress levels. For Prunus species in the ‘Euamygdalus’ section, leaf fresh weight decreased from 638 mg for the controls to 272 mg at -1.2 Mpa, and for Prunus species in ‘Spartioides’ section the decrease was from 504 mg in controls to 198 mg at -1.2 Mpa.
The weight loss in dry leaf was greatest at higher osmotic stress level, demonstrating higher leaf water content for plants grown at higher osmotic stress level. Shoot fresh and dry weights declined with increasing osmotic stress level. On average, there was an 80% decrease in shoot dry weight at -1.2 Mpa. The reduction in shoot weight was more in drought-susceptible species, that is, species in the ‘Euamygdalus’ section.
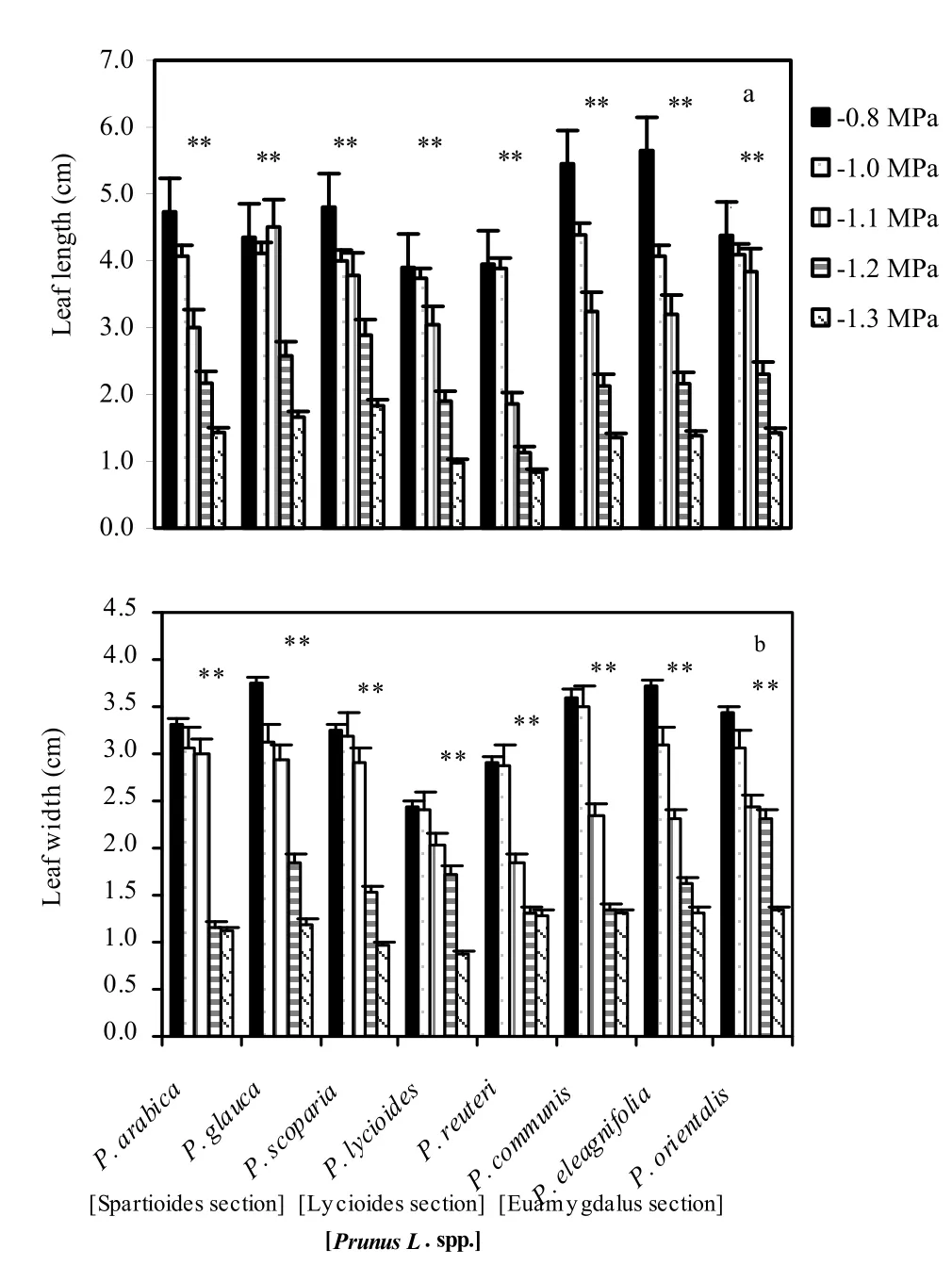
Fig. 1: Leaf length (a) and width (b) of fully expanded leaves for the eight investigated wild almond species induced by drought stress at different osmotic stress levels. Mean values are given with one standard error of the mean. **significant at 0.01 level. The values shown in the figure were means of studied traits and denote statistically differences at the 1% level between species for a certain osmotic stress level.
Leaf physiological response
There were significant differences in malondialdehyde content, catalase activity, and relative permeability of protoplast membrane, proline production, and net photosynthetic rate between the tolerant and sensitive almond species when the plants were grown in osmotic-stressed environments (Table 1). Under wellwatered conditions, all the wild almond species showed similar malondialdehyde concentrations. However, under osmotic stress, malondialdehyde concentrations changed significantly less in the osmotic tolerant section labeled ‘Spartioides’ species, suggesting better maintained membrane-bound activities (Table 1). When placed under osmotic stress, all Prunus species increased catalase activity (Table 1). Under well-watered conditions, osmotictolerant species showed greater calatase activity than osmoticsensitive species, inferring that osmotic tolerant Prunus L. spp. might better scavenge active oxygen under normal growing conditions. Under osmotic-stressed conditions, osmotic-tolerant and drought-sensitive Prunus species scavenged active oxygen at different rates. All the wild almond species showed increased permeability of protoplast membranes under osmotic-stress environments (Table 1). However, rises in relative permeability for osmotic-tolerant species were significantly less than for osmoticsensitive species, meaning that osmotic-tolerant species maintained better membrane integrity under osmotic stress. Under well-watered conditions, the osmotic-tolerant almond species assimilated carbon faster than osmotic-sensitive species (Table 2), meaning that osmotic tolerant wild almond species maintained higher rates of carbon assimilation under osmotic stress.
Leaf chemical constituents
The chemical constituents of leaf differed signifcantly between tolerant and susceptible Prunus species at higher osmotic stress levels (Figs. 2, 3 and 4). Leaf chlorophyll concentration rose at low levels of osmotic stress in osmotic-tolerant species such as Prunus arabica, 2.31 to 2.56 mg·g Fw-1, Prunus glauca, 1.94 to 2.00 mg·g Fw-1and Prunus scoparia, 1.37 to 2.16 mg·g Fw-1from control to -1.0 MPa osmotic stress level; at higher osmotic stress levels (0.75 to 1.2 MPa), however, it declined signifcantly. In susceptible species such as the ‘Euamygdalus’ section of Prunus, chlorophyll concentration started to decline even at a moderate osmotic stress of -1.0 MPa (Fig. 2).
Leaf alkali soluble proteins declined signifcantly at higher drought stress. The decline in susceptible Prunus species was much greater than that in the tolerant species. In the three species of the ‘Euamygdalus’ section, leaf protein decline averaged 41% at -1.2 MPa compared to the control. In osmotic-tolerant species like Prunus arabica it only declined by 21–25%. But, in Prunus glauca protein concentration was greater at all osmotic levels compared to the controls (Fig.3). Proline also increased with osmotic stress, particularly in the osmotic tolerant species. The proline concentration in Prunus glauca increased 9.5-fold compared to the controls, though in ‘Euamygdalus’ the proline rose only 2-fold at -1.2 MPa, relative to the controls (Fig. 3).
Soluble sugars increased at low osmotic stress but decreased at -1.0 and -1.2 MPa. In tolerant species, the rise in sugar concentration was much higher than for sensitive species. However, under higher osmotic stress levels, sugar decreased even in tolerant species (Fig. 3).
Concentration of Na+in the leaf was higher with increasing osmotic stress although the increase was greater in the osmoticsensitive species. In tolerant species such as Prunus glauca, Na+accumulation at -1.2 MPa was 2.4 times greater than in the control. However, in susceptible Prunus species like ‘Euamygdalus’the accumulation of Na+was 5.0-fold greater than in the control. K+in the leaves increased with higher osmotic stress level but the increase was not as pronounced as that of Na+.

Table 1: Responses of malondialdehyde, catalase, relative permeability of protoplast membranes and proline in fresh leaf samples of in vitro selected almond species in control and stressed treatments
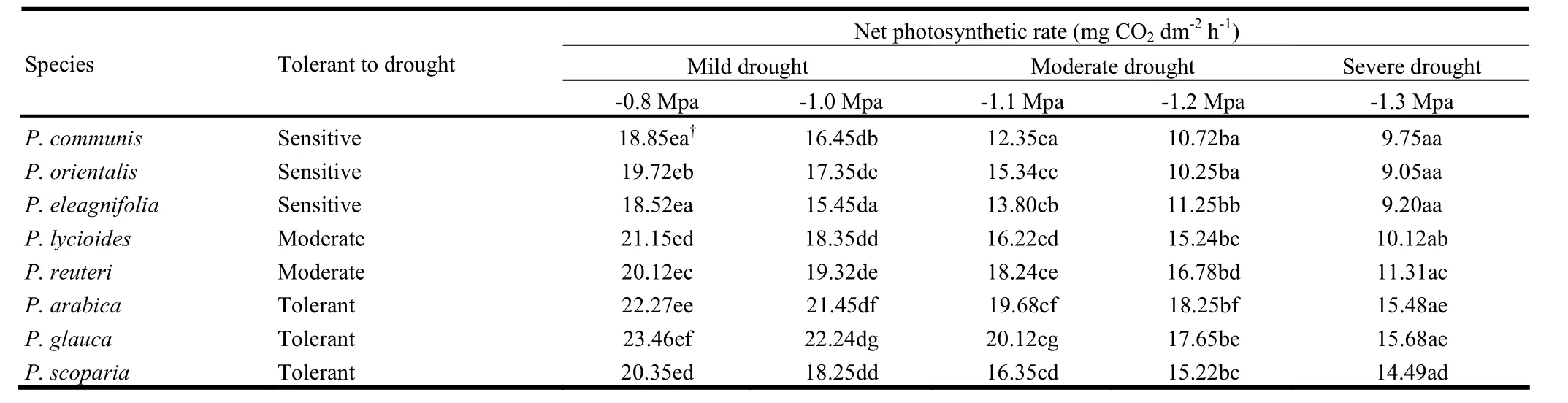
Table 2: Net photosynthetic rate of in vitro selected almond species in control and stressed treatments.
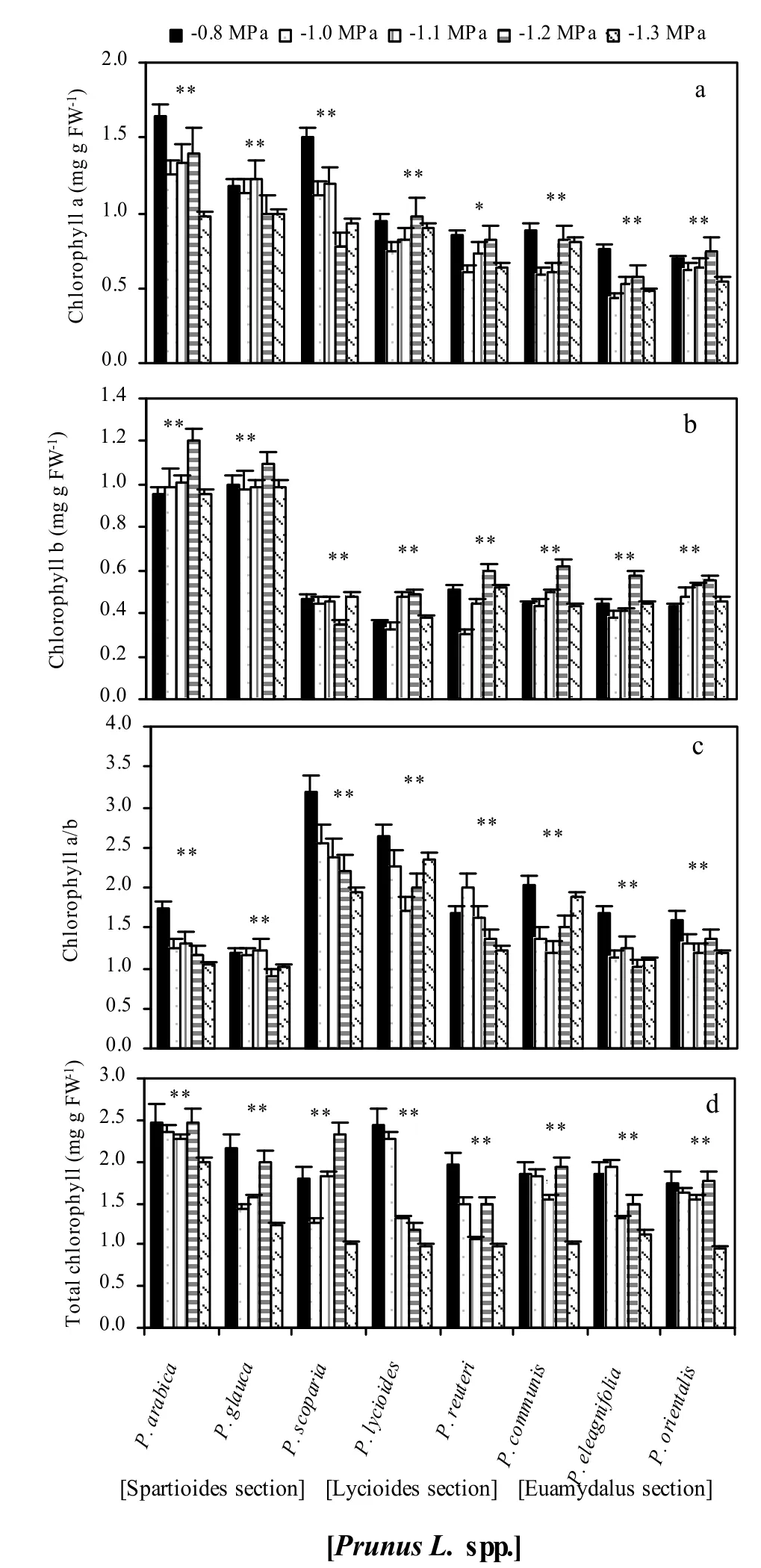
Fig. 2: Chlorophyll a (a), chlorophyll b (b), chlorophyll a/b ratio (c) and total chlorophyll (d) of fully expanded leaves for the eight investigated wild almond species induced by drought stress at different osmotic stress levels. Mean values are given with one standard error of the mean. **significant at 0.01 level. The values shown in the figure were means of studied traits and denote statistically differences at the 1% level between species for a certain osmotic stress level.
Leaf anatomical characters

Fig. 3: Protein (a), sugar (b), proline (c) content of fully expanded leaves for the eight investigated wild almond species induced by osmotic stress at different osmotic levels. Mean values are given with one standard error of the mean. **significant at 0.01 level. The values shown in the figure were means of studied traits and denote statistical differences at the 1% level between species for a certain osmotic stress level.
Osmotic stress had variable effects on leaf anatomy of wild almond species (Table 3). Leaf succulence remained almost unaffected in species of the section labeled Euamygdalus and Lycioides, but increased in all the other species. The extent of the rise was great at a low level of osmotic stress. In osmoticsensitive species leaf succulence increased at -0.8 MPa, then declined sharply. In osmotic-tolerant species leaf succulence increased at -1.2Mpa. The spongy cell layer was found to increase in all Prunus species with increasing osmotic stress. Although the thickness of the palisade layer declined or remained unaltered for all the Prunus species, in osmotic stress-tolerant species, it increased with increasing osmotic stress levels (Fig. 5).
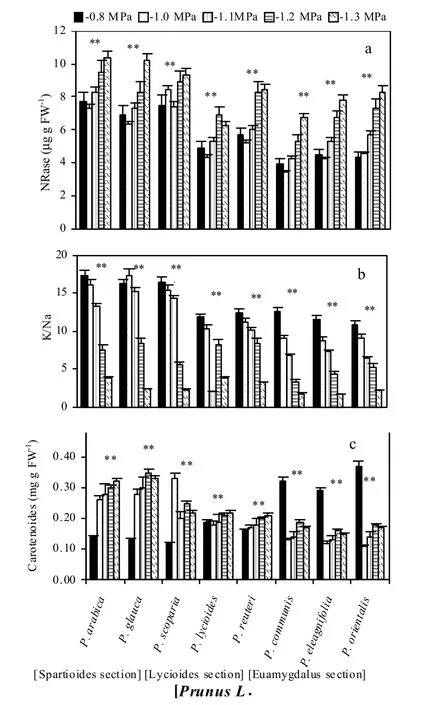

Fig. 5: Anatomical features of control and severe osmotic stress wild almond species of Prunus scoparia leaf. a (Control) and b (severe osmotic stress: 1.3 Mpa)- Cross sections of leaf. le: lower epiderma, ue: upper epiderma, pp: palisade parenchyma, sp: spongy parenchyma. Bare indicate 100 μm
Leaf stomatal parameters
Stomata for Prunus communis and P. scoparia were only observed on the abaxial side of the leaves, though in Prunus lycioides they were observed on both sides of the leaves (Fig. 6). However, stomatal density for all treatments was less in the adaxial compared to the abaxial surface of the leaves in the latter species. Stomatal density was similar for Prunus communis and P. lycioides. Stomatal density of the two species decreased with increasing osmotic stress level without significance (p >0.01) (Fig. 5 and 7). Stomatal density differed significantly between the control and osmotic-stressed Prunus communis and Prunus lycioides. Under well-watered condition, stomatal density was highest for Prunus scoparia and lowest for P. lycioides. While under osmotic stresses, P. lycioides and P. communis had the lowest and the highest stomatal densities, respectively. P. scoparia lost its leaves in all drought stress treatments.
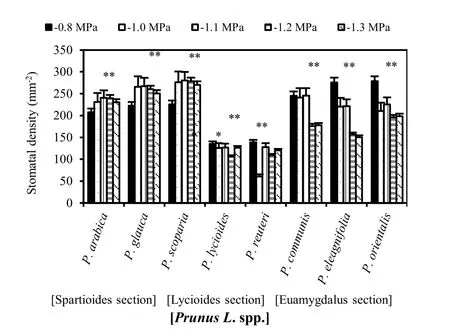
Fig. 6: Stomatal density of fully expanded leaves for the eight investigated wild almond species induced by drought stress at different osmotic levels. Mean values are given with one standard error of the mean. **significant at 0.01 level. The values shown in the figure were means of studied traits and denote statistically differences at the 1% level between species for a certain osmotic stress level.
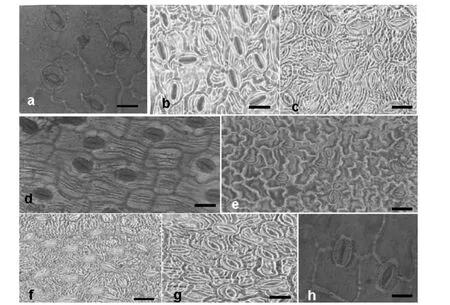
Fig. 7: Anatomical features of fully expanded leaves stomatal imprint for Prunus arabica (a), Prunus glauce (b), Prunus scoparia (c), Prunus lycioides (d), Prunus reuteri (e), Prunus communis (f), Prunus eleagnifolia (g) and Prunus orientalis (h) at control level. Bare indicate 100 μm.

Table 3: Total leaf thickness, palisade and spongy layers in fresh leaf samples of in vitro selected almond species in control and stressed treatments.
Discussion
Leaf morphological traits related to osmotic tolerance include density of fine hairs on the leaf surface, thickness of the cuticle, darker lamina color and more erect leaf architecture (Li 1993; He et al. 1995; Adam et al. 2002). The present study showed that wild almond (Prunus L. spp.) species responded differently to osmotic stress levels. The morphological changes observed in this study agreed with the fndings of Sorkheh et al. (2010). However, in contrast to the results of Sorkheh et al. (2010) on biochemical changes, in the present study we found drastic changes in leaf biochemical constituents. Accumulation of soluble sugars is a common phenomenon for plants growing at high salinity (Liu and Zhao 2005). Sucrose and other simple sugars are effective in stabilizing proteins and in the adjustment of cellular osmotic potential (Garg et al. 2002; Taji et al. 2002). The decline in a sugar concentration observed in this study at a greater drought stress level (-1.2 MPa) might have been caused by excessive use of carbohydrate substrates in energy production to counteract the adverse effect of osmotic stress (Shalhevet et al. 1974). Abiotic stress, for example, osmotic stress and salinity, is known to influence protein synthesis through inhibition of amino acid incorporation (Garg et al. 1997) and proteolysis (Uprety and Sarin 1976). Our results showed that, at higher osmotic stress levels, leaf protein concentration declined signifcantly in osmotic-susceptible species but rose in osmotic stress-tolerant species, particularly in wild almond species of the ‘Spartioides’section.
The increased leaf chlorophyll concentration under high osmotic stress levels in osmotic stress-tolerant species suggests the possibility of using the chlorophyll concentration as a preliminary selection criterion in Prunus spp. for osmotic stress tolerance (Sorkheh et al. 2011; Sorkheh et al. 2012). The increase in proline concentrations observed at all osmotic stress levels can be explained, as proline is one of the most prominent osmolytes that accumulate during osmotic adjustment under abiotic stress. Proline is also involved in stabilizing sub-cellular structures, scavenging free radicals, and buffering the cellular redox potential under stress conditions. It can also act as a protein compatible hydrotrope (Srinivas and Balasubramanian 1995) to alleviate cytoplasmic acidosis, and to maintain proper NADP+/NADPH ratios (Hare and Cress 1997). Rapid break-down of proline on relief of stress could also provide enough reducing agents to support mitochondrial oxidative phosphorylation and generation of ATP for recovery from stress and repair of stress-induced damage (Hare and Cress 1997; Hare et al. 1998). The increase in proline concentration was greater in osmotic-tolerant species such as Prunus species of the ‘Spartioides’ section. This species-specific difference in proline accumulation under stress has been reported for several other crops (Ahmad et al. 1981; Petrusa and Winicov 1997; Sorkheh et al. 2012). The increased activity of NRase, observed in this study, agrees with Levitt (1980). Higher NRase activity in response to abiotic stress such as salinity was also reported in barley, tomato and many other crops (Arad and Richmond 1976; Tal 1997). Our results showed increased accumulation of K+in tolerant species. In contrast, Agastian and Vivekananda (1997) showed decline in K+concentration in some mulberry varieties planted on saline soils. Under salt stress, plants maintain high concentrations of K+and low concentrations of Na+in the cytosol, a high cytosolic K+/Na+ratio is regarded as a key requirement for growth on high salinity soils (Weinberg et al. 1982). This is achieved by regulating the expression and activity of K+and Na+transporters along with H+pumps that generate the driving force for transport (Zhu et al. 1993).
Malondialdehyde is the last product in membrane liposome peroxidation, its content is a measure of the peroxidation of the membrane bound liposome: the higher the malondialdehyde content, the greater the membrane damage (Liu and Zhang 1994; Jiang and Huang 2001; Liu et al. 2005). Catalase activity is associated with elimination of active oxygen caused by stress factors such as osmotic stress. Catalase, along with superoxide dismutase, protects plant cells by scavenging superoxide radicals, H2O2, and other superoxide compounds, and prevents or reduces production of free hydroxyl radicals (Liu and Zhang 1994; Sorkheh et al. 2011). Permeability of the protoplast membrane assists in governing electrolyte leakage from plant cells, so less increase in permeability under stress conditions implies greater integrity of plant cell membranes (Liu and Zhang 1994; Sorkheh et al. 2011). Osmotic-tolerant Prunus species produced smaller changes in biochemical responses than did osmotic-sensitive cultivars for malondialdehyde content, catalase activity, relative permeability of protoplast membranes, and net photosynthetic rate. These results imply that osmotic-tolerant species maintain cell integrity better than osmotic-sensitive species when almond plants are subjected to osmotic stress. Net photosynthetic rate and carbon deposition culminate all these biological responses and chemical processes (Liu et al. 2005).
Increased in stomatal density might reduce the moisture retention capacity of harvested wild almond species leaves if stomata remain open on harvesting (Vijayan et al. 1997b). The increased stomatal density at higher osmotic stress levels agrees with the reports for barley (Belkhodja et al. 1999) and bread wheat (Bhagwat and Bhatia 1993). The increase in leaf thickness under high osmotic stress conditions was mainly due to a rise in the spongy layers, while the length of palisade cells remained unaffected. Wignarajah et al. (1975) reported similar results for beans. The highest stomatal density (380 mm-2) was reported by Ranjbarfardooei et al. (2002) for Pistacia khinjuk stocks, which is higher than the highest stomatal density (257 mm-2) recorded in our study. Zamani et al. (2002) reported stomatal density in plum (600 mm-2) to be three times higher than that in almond, peach and apricot, the latter three species had the same stomatal density of 200 mm-2. Also, Ferdinand et al. (2000) reported a range of stomatal density between 233-305 mm-2for Prunus serotina Ehrh. But stomatal density, which might influence stomatal resistance, did not differ consistently by osmotic stresses levels in our experiment. Thus, differences in stomatal resistance between all three species including almond, peach and apricot at control and all osmotic stress levels can be mainly attributed to differences in stomatal closure. In arid and semi-arid regions where osmotic stress is the major growth limiting factor, increasing stomatal resistance and early closing of stomata could be advantageous (Sean et al. 1998).
In conclusion, Prunus species are moderately tolerant to osmotic stress, but signifcant differences exist in the osmotic tolerance of wild almond species. Our results confrmed the fndings of Sorkheh et al. (2010) that in vitro culture of seed is an easy and efficient means to screen large numbers of Prunus L. spp. for osmotic tolerance. Osmotic tolerant species used in this study can now be used for breeding osmotic stress tolerant rootstocks in almond.
Acknowledgement
The authors would like to thank the ANRR Center of Shahrekord for material assistance. We are also thankful to Ms. Kh. Chenaneh-Hanoni for helping in arranging the facilities and for their kind help in undertaking this study. Thanks are also due to Dr. Dario Kremer, University of Zagreb and Prof. Craig Ledbetter, USDA, for their helpful comments on an earlier draft of the manuscript and improvement of English grammar.
Ahmad I, Wainwright SJ, Stewart GR. 1981. The solute and water relations of Agrostis stolonifera ecotypes differing in their salt tolerance. New Phytology, 87: 615–629.
Adam HP, Cairns, JE, Horton, P, Jones, HG, Griffiths, H. 2002. Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. Journal of Experimental and Botany, 53: 989–1004.
Agastian STP, Vivekananda M. 1997. Effect of induced salt stress on growth and uptake of mineral nutrients in mulberry (Morus alba) genotypes. Indian Journal of Agricultural Science, 67: 469–472.
Arad S, Richmond AE. 1976. Leaf cell water and enzyme activity. Plant Physiology, 57: 656–658.
Arnon DI. 1949. Copper enzyme in isolated chloroplast polyphenol oxidase in Beeta vulgaris. Plant Physiology, 24: 1–15.
Ashraf M, Leary JWO. 1996. Effect of drought stress on growth, water relations, and gas exchange of two lines of sunflower differing in degree of salt tolerance. International Journal of Plant Science, 157: 729–732.
Barbara L, Scartazza A, Brugnoli E, Navari-Izzo F. 1999. Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiology, 119: 1091–1100.
Bates LS, Waldren RP, Teare LD. 1973. Rapid determination of free proline for water stress studies. Plant Soil, 39: 205–207.
Belkhodja R, Morales F, Abadia A, Medrano H, Abadia J. 1999. Effect of salinity on chlorophyll fuorescence and photosynthesis of barley (Hordeum vulgare L) grown under a triple-line source sprinkler system in the feld. Photosynthetica, 36:375–387.
Bhagwat SG, Bhatia CR. 1993. Selection for fag leaf stomatal frequency in bread-wheat. Plant Breeding, 110: 129–136.
Bray H, Thrope WV. 1954. Analysis of phenolic components of interest in metabolism. Methods Biochemical Analysis, 1: 27–52.
Burlyn EM, Merrill RK. 1973. The osmotic potential of polyethylene glycol 6000. Plant Physiology, 51: 914–916.
Camposeo S, Palasciano M, Vivaldi G A, Godini A. 2010. Effect of increasing climatic water deficit on some leaf and stomatal parameters of wild and cultivated almonds under Mediterranean conditions. Scientia Horticulture, doi:101016/jscienta201009022.
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osorio ML, Carvalho I, Faria T, Pinheiro C. 2002. How plants cope with water stress in the field? Photosynthesis and growth. Annual Botany, 89: 907–916.
Chazen O, Hartung W, Neumann PM. 1995. The different effects of PEG 6000 and NaCl on leaf development are associated with differential inhibition of root water transport. Plant Cell Environmental, 18: 727–735.
Child RD, Summers JE, Babij J, Farrent JW, Bruce DM. 2003. Increased resistance to pod chatter is associated with changes in the vascular structure in pods of a resynthesized Brassica napus line. Journal of Experimental Botany, 54: 1919–1930.
Ehleringer J. 1980. Leaf morphology and reflectance in relation to water and temperature stress. In: Turner N C and Kramer PJ (eds), Adaptation of plants to water and high temperature stress. New York: Willey, pp. 293-309.
Elagoz V, Han SS, Manning WJ. 2006. Acquired changes in stomatal characteristics in response to ozone during plant growth and leaf development of bush beans (Phaseolus vulgaris L) indicate phenotypic plasticity. Environmental Pollution, 140: 395–405.
Escalona JM, Flexas J, Medrano H. 1999. Stomatal and non-stomatal limitations of photosynthesis under water stress in field-grown grapevines. Australian Journal of Plant Physiology, 26: 421–433.
Fanizza G, Reina A. 1990. Response of Amygdalus communis and Amygdalus webbii seedlings to water stresses. In: XXIII International Horticultural Congress, p.474.
Ferdinand JA, Fredericksen TS, Kouterick KB, Skelly JM. 2000. Leaf morphology and ozone sensitivity of two open pollinated genotypes of black cherry (Prunus serotina) seedlings. Environmental Pollution, 108: 297–302.
Garg AK, Kim JK, Owens TG, Ranwala AP, Do Choi Y, Kochian LV, Wu RJ. 2002. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proceeding National Academic Science USA, 99: 15898–15903.
Garg BK, Kathju S, Vyas SP, Lahri AN. 1997. Sensitivity of cluster bean to salt stress at various growth stage. Indian Journal Plant Physiology, 2: 49–53.
Giorio P, Sorrentino G, d́Andria R. 1999. Stomatal behaviour, leaf water status and photosynthetic response in field-grown olive trees under water deficit. Environmental and Experimental Botany, 42: 95–104.
Gradziel TM, Kester DE. 1998. Breeding for self fertility in California almond cultivars. Acta Horticulture, 470: 109–117.
Guerfel M, Baccouri O, Boujnah D, Chaibi W, Zarrouk M. 2009. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L) cultivars. Science Horticulture, 119: 257–263.
Guirguis NS, Soubhy I, Khalil MA, Stino GR. 1995. Leaf stomata and stem lenticels as a means of identification of some fruits stocks. Acta Horticulture, 409: 229–239.
Hageman RH, Hucklesby DP. 1972. Nitrate reductase from higher plants. Methods Enzymology, 23: 491–503.
Hare PD, Cress WA. 1997. Metabolic implications of stress- induced proline accumulation in plants. Plant Growth Regulation, 21: 79–102.
Hare PD, Cress WA, Staden, JV. 1998. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environmental, 21: 535–553.
He SP, Yan QJ, Song GY, Xu ML. 1995. Progress in morphological and physiological and biochemical indexes of drought resistance identification of maize. Agricultural Research in the Arid Areas, 13: 67–73.
Jiang YW, Huang BR. 2001. Drought and heat stress injury to two coolseason turf grasses in relation to antioxidant metabolism and lipid peroxidation. Crop Science, 41: 436– 442.
Karschon R. 1974. The relation of seed origin to growth of Eucalyptus camaldulensis Dehn In Israel. Israel Journal Agricultural Research, 23: 159-173.
Lawlor DW. 2002. Limitation to photosynthesis in water stressed leaves: stomata vs metabolism and the role of ATP. Annual Botany, 89: 1–15.
Levitt, J. 1980. Responses of Plants to Environmental Stresses: Water, Radiation, Salt and Other Stresses, Academic Press, New York, 2nd Ed., Vol. 2. pp. 365–488.
Li Y. 1993. Assessment method and indexes for drought resistance of crops. Agricultural Research in the Arid Areas, 11: 91–100.
Lima ALS, DaMatta FM, Pinheiro HA, Totola MR, Loureiro ME. 2002. Photochemical responses and oxidative stress in two clones of Coffea canephora under water deficit conditions. Environmental and Experimental Botany, 47: 239–347.
Liu AR, Zhao KF. 2005. Osmotica accumulation and its role in osmotic adjustment in Thellungiella halophila under salt stress. Journal Plant Physiology and Molecular Biology China, 31: 389–395.
Liu ZQ, Zhang SC. 1994. Physiology of Drought Resistance in Plants. Beijing: Agricultural Press of China, p.398.
Lowry OH, Rosebrough NJ, Farr AL, Randall R. 1951. Protein measurements with folin–phenol reagent. Journal of Biology and Chemistry, 193: 265–275.
Mahhou A, DeJong TM, Cao T, Shackel KS. 2005. Water stress and crop load effects on vegetative and fruit growth of 'Elegant Lady' peach [Prunus persica (L) Batch] trees Fruits, 60: 55–68.
Makbul S, Saruhan Güler N, Durmuş N, Güven S. 2011. Changes in anatomical and physiological parameters of soybean under drought stress. Turkish Journal Botany, 35:1–9.
Makbul S, Türkmen Z, Coşkunçelebi K, Beyazoğlu O. 2008. Anatomical and pollen characters in the genus Epilobium L (Onagraceae) from northeast. Anatolia Acta Biologia Cracov Botany, 50: 57–67.
Matsuda K, Rayan A. 1990. Anatomy: A key factor regulating plant tissue response to water stress. In: Kaft ernan F (ed), Environment Injury to Plants. San Diego: Academic Press, p 290.
Morris DL. 1948. Quantitative determination of carbohydrates with Dreywood anthrone reagent. Science, 107: 254–255.
Olmos E, Sanchez-Blanco MJ, Fernandez T, Alarcon JJ. 2007. Subcellular effects of drought stress in Rosmarinus officinalis. Plant Biology, 9: 77–84.
Petrusa LM, Winicov I. 1997. Proline status in salt-tolerant and salt sensitive alfalfa cell lines and plants in response to NaCl. Plant Physiology and Biochemistry, 35: 303–310.
Pitman WD, Holte C, Conrad BE, Bashaw EC. 1983. Histological differences in moisture stressed and non-stressed Klein grass forage. Crop Science, 23: 793–795.
Ramanjulu S, Veeranjulu K, Sudhakar C. 1993. Sodium, potassium and nitrogen status of some mulberry (Morus alba L) cultivars under NaCl salinity. Indian Journal Plant Physiology and Biochemistry, 19: 103–106.
Ranjbarfardooei A, Samson R, Van Damme P, Lemeur R. 2000. Effects of drought stress induced by polyethylene glycol on pigment content and photosynthetic gas exchange of Pistacia khinjuk and P mutica. Photosynthetica, 38: 443–447.
Ranjbarfardooei A, Samson R, Lemeur R, Van Damme P. 2002. Effects of osmotic drought stress induced by a combination of NaCl and polyethylene glycol on leaf water status, photosynthetic gas exchange, and water use efficiency of Pistachia khinjuk and P mutica. Photosynthetica, 40: 165–169.
Romero P, Botia P. 2006. Daily and seasonal patterns of leaf water relations and gas exchange of regulated deficit-irrigated almond trees under semiarid conditions. Environmental and Experimental Botany, 56: 158–173.
Rouhi V, Samson R, Van Damme P, Lemeur R. 2006. Stem photosynthesis in three different almond species during drought and subsequent recovery. 27th International Horticulture Congress, August 13-19, Seoul, South Korea.
Rouhi V, Samson R, Lemeur R, van Damme P. 2007. Photosynthetic gas exchange characteristics in three different almond species during drought stress and subsequent recovery. Environmental and Experimental Botany, 59: 117–129.
Sanchez FJ, Manzanares M, de Andres EF, Tenorio JL, Ayerbe L. 1998. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Research, 59: 225–235.
SAS Institute Inc. 2000. SAS/STAT user’s guide version 6 vol 2 4th edn SAS Institute Cary NC, USA.
Sharma SB, Ashokkumar P, McDonald D. 1991. A greenhouse technique to screen pigeon pea for resistance to Heterode racajani. Annual and Applied Biology, 118: 351–356.
Save R, Bill C, Domingo R, Ruiz-Sanchez MC, Torrecillas A. 1995. Some physiological and morphological characteristics of citrus plants for drought resistance. Plant Science, 110: 167–172.
Sean CC, Stefan KA, Janet EC, Sangeeta J, Narendra S, Marianne P, Hamlyn GJ. 1998. The role of solute accumulation, osmotic adjustment and changes in cell wall elasticity in drought tolerance in Ziziphus mauritiana (Lamk). Journal of Experimental Botany, 49: 967–977.
Shalhevet J, Yaron D, Horowitz U. 1974. Salinity and citrus yield: an analysis of results from a salinity survey. Journal of Horticultural Science, 49: 15–27.
Shao HB, Chu LY, Jaleel CA, Zhao CX. 2008. Water deficit stress induced anatomical changes in higher plants. CR Biology, 331: 215–225.
Sorkheh K, Shiran B, Gradziel TM, Epperson NK, Martínez-Gómez P, Asadi E. 2007. Amplified fragment length polymorphism as a tool for molecular characterization of almond germplasm: genetic diversity among cultivated genotypes and related wild species of almond, and relationships with agronomic traits. Euphytica, 156: 327–344.
Sorkheh K, Shiran B, Rouhi V, Asadi E, Jahanbazi H, Moradi H, Gradziel TM, Martínez-Gómez P. 2009. Phenotypic diversity within native Iranian almond (Prunus spp) species and their breeding potential. Genetic Resources and Crop Evolution, 56: 947–961.
Sorkheh K, Shiran B, Khodambashi M, Rouhi V, Ercisli S. 2010. In vitro assay of native Iranian almond species (Prunus L spp) for drought tolerance. Plant Cell Tissue and Organ Culture, 105: 395–404.
Sorkheh K, Shiran B, Rouhi V, Khodambashi M, Sofo A. 2011. Regulation of the ascorbate–glutathione cycle in wild almond during drought stress. Russian Journal Plant Physiology, 58: 76–84.
Sorkheh K, Shiran B, Khodambashi M, Rouhi V, Mosavei S, Sofo A. 2012. Exogenous proline alleviates the effects of H2O2induced oxidative stress in wild almond species. Russian Journal Plant Physiology, 59: 788–798.
Srinivas V, Balasubramanian D. 1995. Proline is a protein- compatible hydrotrope. Langmuir, 11: 2830–2833.
Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K. 2002. Important roles of drought- and coldinducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant Journal, 29: 417–426.
Tal M. 1997. Physiology of polyploidy plants DNA, RNA, protein and abscisic acid in autotetraploid and diploid tomato under low and high salinity. Botany Gaz, 138: 119–122.
Torrecillas A, Alarcón JJ, Domingo R, Planes J, Sánchez-Blanco MJ. 1996. Strategies for drought resistance in leaves of two almond cultivars. Plant Science, 118: 135–143.
Torrecillas A, Galego R, Perez-Astor A, Ruiz-Sanchez MC. 1999. Gas exchange and water relations of young apricot plants under drought conditions. Journal of Agricultural Science, 132: 445–452.
Uprety DC, Sarin B. 1976. Physiological studies on salt tolerance in Pisum sativum L Tonic composition and nitrogen metabolism. Acta Agronomy Academic Science, 25: 455–460.
Van Damme P. 1991. Adaptation to drought stress in plants II: Morphological adaptations. Med Fac Landbouww Rijks uninv Gent, 52: 1–8.
Vijayan K, Raghunath MK, Das KK, Tikader A, Chakraborti SP, Roy BN. 1997. Studies on leaf moisture of mulberry germplasm varieties. Indian Journal Seric, 36: 155–157.
Vijayan K, Chakraborti SP, Ercisli S, Ghosh PD. 2008. NaCl induced morpho-biochemical and anatomical changes in mulberey (Morus spp) Plant Growth Regulation, 56: 61–69.
Weinberg R, Lerner HR, Pojkoff-Mayber A. 1982. A relationship between potassium and proline accumulation in salt-stressed Sorghum bicolor. Physiologia Plantarum, 55:5–11.
Weyers J, Meidner H. 1990. Methods in Stomatal Research, Longman Scientific and Technical, London.
Wignarajah K, Jennings DH, Handley JF. 1975. The effect salinity on growth of Phaseolus vulgaris L Anatomical Changes in the frst trifoliate leaf. Annual Botany, 39: 1029–1038.
Zaid A, Hughes H. 1995. A comparison of stomatal function and frequency of in vitro polyethylene glycol treated and greenhouse grown plants of date palm, Phoenix dactylifera L. Tropical Agriculture (Trinidad), 72: 130–134.
Zamani Z, Taheri A, Vezvaei A, Poustini K. 2002. Proline content and stomatal resistance of almond seedlings as affected by irrigation intervals. Acta Horticulture, 491: 411–416.
Zhang XZ. 1989. Investigation Methods for Crop Physiology. Beijing: Agric Press of China, p.259.
Zhu JK, Shi J, Singh U, Wyatt SE, Bressan RA, Hasegawa PM, Capita NC. 1993. Enrichment of vitronectin and fbronectin like proteins in NaCladapted plant cells and evidence for their involvement in plasma membrane-cell wall adhesion. Plant Journal, 3: 637–646.
杂志排行
Journal of Forestry Research的其它文章
- The effects of understory vegetation on P availability in Pinus radiata forest stands: A review
- Phytosociology, structure and dynamics of Pinus roxburghii associations from Northern Pakistan
- Characterization of expressed genes in the establishment of arbuscular mycorrhiza between Amorpha fruticosa and Glomus mosseae
- Dependences of longleaf pine (Pinus palustris) natural reproduction on environments above ground
- Effects of gaps on regeneration of woody plants: a meta-analysis
- A modified Murashige and Skoog media for efficient multipleshoot induction in G. arborea Roxb.
