The effects of understory vegetation on P availability in Pinus radiata forest stands: A review
2014-04-20ArivinRivaie
A. Arivin Rivaie
Introduction
Pinus radiata has been planted across a range of climatic and soil conditions on approximately 4.0 million hectares, with the largest plantations in Chile and New Zealand (about 1.5 million ha each), and Australia (0.77 million ha). P. radiata plantations are also found at moderate scales in Spain (0.29 million ha) and South Africa (57 000 ha) and at smaller scales in several other countries (Manley and Maclaren 2009; Afif-Khouri et al. 2010; Watt et al. 2010), in which almost 80% of plantations are grown primarily for wood production (Evans 2009). Recently, in P. radiata forest plantations of several countries, there has been a steady trend towards wider tree spacing with lower initial stocking of P. radiata and also an increased rate of application of phosphorus (P) fertiliser (Payn et al. 2000; Burkes et al. 2003). The reasons for wider tree spacing are availability of better-quality nursery stock and improved establishment practices (MacLaren 1993). These silvicultural practices are expected to increase the potential for understory growth due to increased light and greater nutrient resources (Gadgil et al. 1988; Brockerhoff et al. 2008). Under wider-spaced trees, understory vegetation could become a more significant component of P cycling in P. radiata forests than under closely-spaced stands, especially before canopy closure.
Understory vegetation frequently causes harmful effects due to competition (antagonisms). However, interactions between some understory species and P. radiata have been shown to provide beneficial effects to the tree (Richardson et al. 1996; 2006; Mead 2010). Richardson et al. (1996) demonstrated a positive interaction between P. radiata and understory vegetation on P uptake by P. radiata in a field study. He reported that some species of grass, herbaceous broadleaves and buddleia significantly increased P concentration in needles of 3-year-old radiata pine trees, while broom, gorse, lotus and pampas had no effect on needle P concentration.
Phosphorus is an important nutrient in the radiata pine forest plantations, for example in New Zealand, Australia, and Spain, as most of the soils are P deficient or marginally deficient (Hunter et al. 1991; Payn et al. 1998; 2000; Ouro et al. 2001; Snowdon 2002; Zas and Serrada 2003; Romanyà and Vallejo 2004; Mead 2005a; Zabowski et al. 2007; May et al. 2009). However, most of the information available on the P fertiliser requirements of radiata pine was obtained from trials on first rotation forests that were managed under silvicultural regimes which were quite different from those in use today. Nowadays, radiata pine forests in these countries are mostly planted with wider tree spacing and lower initial stocking of trees compared to those prior to the 1990s. As noted earlier, such forest regimes have the potential for increased understory growth with a greater influence on the tree response to P fertiliser than has been the case in the past.
In this paper I review literature on the significance of understory vegetation in radiata pine forest plantations, especially the role of the understory in enhancing or reducing P availability to forest trees.
Growth rate of P. radiata and understory vegetation
Growth rate of P. radiata
The above-ground biomass of P. radiata has been reported to increase at a slow rate from 1 to 3 years of age, followed by faster growth from 3 to 12 years and again at a slow rate from the age of canopy closure at around 13 years to 29 years (Madgwick et al. 1977; Knight 1978; Madgwick 1985; Madgwick and Oliver 1985; Madgwick et al. 1988; Bi et al. 2010) (Fig. 1). An average production rate of 15 t·ha-1·a-1for above-ground biomass of P. radiata from 2 to 22 years of age at a final-crop stocking rate of 540 stems ha-1was reported by Madgwick et al. (1977) for first rotation plantations on pumice soils at Kaingaroa forest, central North Island, New Zealand. Madgwick and Oliver (1985) estimated annual above-ground biomass production of first rotation P. radiata stands on pumice soils at the Long Mile area of Rotorua, New Zealand, from 5 to 13 years of age at 35.9 t·ha-1·a-1. The growth rate at the Long Mile area was approximately twice that at Kaingaroa forest because of the higher final-crop stocking rate in the forest at Long Mile (5190 stems·ha-1) compared to that at Kaingaroa forest (540 stem·ha-1).

Fig. 1: The above-ground biomass of P. radiata with increasing age (1 year, Knight 1978; 2 to 4 years, Madgwick et al. 1977; Madgwick 1985; 5 to 13 years, Madgwick and Oliver 1985; Madgwick et al. 1988; 17 to 29 years, Madgwick et al. 1977; Madgwick 1985; Bi et al. 2010). Note that the data for different age groups are taken from sites with different agroecological conditions
Growth rate of understory vegetation
Understory biomass productivity in forest stands varies widely with tree species and age. Forrest and Ovington (1970) measured the above-ground biomass of understory species (native grasses, bracken and broadleaved plants) under P. radiata stands with increasing age of the stands on a sandy loam soil at Billapaloola plantation, Tumut, New South Wales, Australia (Fig. 2). They reported that following canopy closure (the ninth year) grasses were completely absent and were replaced by bracken. Their results showed that during the third and fifth year after planting of P. radiata the understory biomass ranged from 46% to 82% of total above-ground forest biomass. This percentage declined sharply beyond 5 to 8 years to 1.6-3.4% because of increased growth of P. radiata and decreased growth of understory vegetation (Fig. 2).
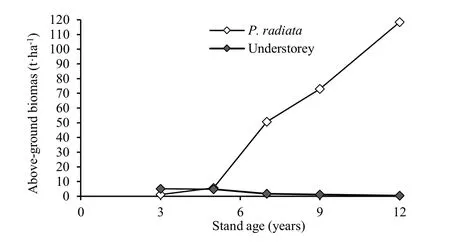
Fig. 2: Above-ground biomass of understorey vegetation and P. radiata with increasing stand age (adapted from Forrest and Ovington 1970)
Meanwhile, Long and Turner (1975) who worked in Douglas fir stands also reported that the above-ground biomass of understory decreased with increasing age of the stands. The understory biomass (8 t·ha-1) in younger stands (22-30 years) in this study was a significant fraction (12%) of the total above-ground forest biomass (65 t·ha-1) compared with the understory biomass in older stands (3%) (3 and 211 t·ha-1for understory vegetation and total biomass, respectively, when the trees were 73 years old). In Eucalyptus diversicolor stands, Grove and Malajczuk (1984) reported that understory vegetation represented approximately 50% of the above-ground biomass in 8- and 11-year-old stands, but 10% of biomass in the 4- and 36-year-old stands. The important component of understory vegetation in these stands was the legume Bossiaea laidwiana, which constituted 57% of the total understory biomass in the 8-year-old stand.
The above-ground understory biomass at Kaweka (allophanic soil) and Kinleith forests (pumice soil) in New Zealand under 4 to 5-year-old second rotation P. radiata was 8.4 and 12.2 t·ha-1, respectively (A. Rivaie unpublished data). The above-ground biomass of P. radiata was not measured in these forests. However, Beets and Pollock (1987) reported that the above-ground biomass of 4-year old P. radiata was 23.6 t·ha-1on a pumice soil at Puruki forest, New Zealand. Using these data the above-ground understory biomass at Kaweka and Kinleith forests can be estimated as 26% and 38 % of the total above-ground biomass, respectively.
All the studies reviewed above showed that the understory biomass under conifers up to the age of canopy closure is a significant component of the total above-ground biomass, and in some cases it can exceed the conifer biomass.
Phosphorus forms and its cycle in P. radiata forests
Phosphorus is a major essential nutrient that often controls plant growth and development in agricultural and forest ecosystems (Vance et al. 2003; Radersma and Grierson 2004). In soils, P is supplied by parent material, branch/litterfall and fertiliser input. Phosphorus supply to the plant is dependent on the total amount of P in the soil, as well on the forms in which it exists (Khanna and Ulrich, 1984).
Phosphorus forms
Soil P can be partitioned into two broad categories: inorganic (Al, Fe, and Ca-P) and organic P forms. The dynamics of P transformations in soils are controlled by chemical and biological processes (Khanna and Ulrich 1984; Condron and Newman 2011). Phosphorus from the soil solution is taken up by plants directly either as the primary orthophosphate ion, H2PO4-, or the secondary orthophosphate ion, HPO4=(Anderson 1980; Magid et al. 1996; Morgan et al. 2005).
In acid soils, inorganic P reacts with free Fe and Al to form insoluble precipitates (Haynes 1984). In addition, P is adsorbed onto the variably charged surfaces of oxides and hydrous oxides of iron and aluminium, and clay minerals (Syers et al. 1971; Hinsinger 2001). In calcareous soils, P usually accumulates as Ca bound P (Hedley et al. 1982; Tiessen et al. 1983).
In forest stands, labile organic P (Po) fractions have been reported to be an important source for tree P nutrition (Turner and Lambert 1985, 1986; Adams et al. 1989; Parfitt et al. 1994; Condron et al. 2005). The concentration of Poin the soil is controlled by the processes of immobilisation and mineralisation. In New Zealand, it has been reported that Poin the topsoil (0-10 cm) under conifer stands ranged from 25% to 63% of total P (Davis and Lang 1991; Condron et al. 1996; Chen et al. 2000). Orthophosphate monoesters (accounting for 24-64% of the total P and 80-97% of the total organic P) were the predominant species of organic P in a soil under P. radiata, followed by much smaller quantities of orthophosphate diesters (<19% of organic P) and traces of phosphonates (Condron et al. 1996; Chen et al. 2004).
Phosphorus turnover through microbial biomass and Pomineralisation are important biological mechanisms that influence Pirelease into the soil solution (Tarafdar and Claassen 1988; Frossard et al. 2000). In forest soils the release of Pifrom Poduring decomposition of soil organic matter on the forest floor is an essential process in maintaining P supply. Biological and biochemical processes are involved in Pomineralisation. The demand for energy of the soil microbial population facilitates mineralisation of Poassociated with soil C as a consequence of biological C mineralisation, whereas P-esters (independent of the main bulk of organic matter) are biochemically mineralised by extracellular enzymes, such as phosphatases (McGill and Cole 1981; Magid et al. 1996). These biological and biochemical processes take place predominantly in the rhizosphere soil where the microbial activity is higher than in the bulk soil. In addition, other biological mechanisms can influence the availability of P in soil, such as root-induced pH changes and release of organic anions, and the development of specialised root structures (Bowen and Rovira 1991; Junk et al. 1993; Toal et al. 2000; Chen et al. 2002).
Phosphorus cycle
The P status of a young undeveloped virgin soil is dependent primarily on the P content of the parent material, consisting of predominantly apatite, with some iron and aluminium phosphates in acid soils (Norrish and Rosser 1983). This primary P is weathered through the action of climate and vegetation to give secondary inorganic mineral P and inorganic P in soil solution. The inorganic P in soil solution is taken up by plants and soil microbes, or sorbed to become iron and aluminium phosphate minerals in acidic soils and calcium phosphate in calcareous soils (Adams and Walker 1975; Walker and Syers 1976; Crews et al. 1995; Condron and Newman 2011) (Fig. 3).
In P. radiata plantations in New Zealand, values of plant-available P measured by Bray-2 P soil test in the topsoil (0-10 cm depth) varies widely, ranging from 1.0 μg·g-1(a podzol soil of Auckland) to 46.5 μg·g-1(a pumice soil of Rotorua) (Skinner et al. 1991) and total P ranges from 466 to 1300 μg·g-1soil (Hunter and Hunter 1991).
Phosphorus in soil solution is taken up by the tree, understory vegetation and soil microorganisms, or leached below the root zone. The P taken up by the tree is distributed into the various plant components depending on their requirements (Fig. 3).
Small amounts of P taken up by the tree are returned to the soil through litterfall/branchfall (3.4 kg P ha-1a-1) (Will 1959) and from thinning/pruning or logging (40-55 kg P ha-1) (Will 1968). In addition, a portion of the P taken up by the understory vegetation is returned to the soil as litterfall or after weed control prior to planting and post-planting, or after canopy closure (1-4 kg·ha-1) (Parfitt et al. 1994). Some P is removed from the cycle in the main stem during production thinning or logging (33 kg P ha-1) (Will 1968).
After decomposition, the litter from radiata trees and understory vegetation releases P to soil solution. Meanwhile, P also enters the cycle through P fertiliser application, a routine silvicultural operation in commercial forests managed intensively for high production (Fig. 3). Fertilisers are commonly applied during planting (15 g P per seedling; 100 kg P ha-1) and prior to canopy closure (100 to 110 kg P ha-1), when the tree canopy is developing (Will 1968; Madgwick et al. 1977; Mead and Gadgil 1978; Ballard 1980; Skinner and Payn 1993). Canopy closure occurs at 4 to 7 years, depending mainly on the stocking rate (Skinner and Payn 1993).
Phosphorus content of above-ground biomass
In P. radiata plantations, P can exist in trees and in understory vegetation. The relative proportion of P in the trees and understory vegetation depends mainly on the age and spacing of the t rees.
P. radiata biomass P content
The total P content of the above-ground tree biomass increases from 1 to 13 years of age. The rate of increase is greater in early years (2-6 years) than in later years (7-13 years) (Madgwick et al. 1977; Knight 1978; Madgwick 1985; Madgwick and Oliver 1985; Madgwick et al. 1988) (Fig. 4). The increasing P content of the tree is mainly due to increasing dry matter content of the tree (Madgwick et al. 1977) (Fig. 1).

Fig. 3: The P cycle in a P. radiata forest stand (adapted from Chauhan et al. 1981; Mitchell 2000)
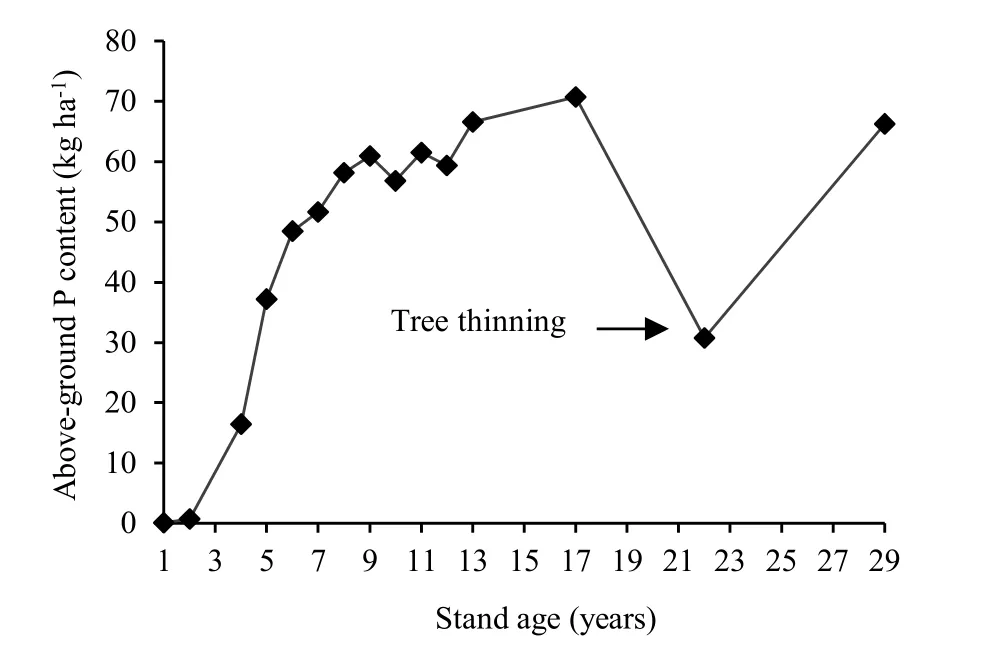
Fig. 4: The total P content of P. radiata with increasing stand age (1 year, Knight 1978; 2 to 4 years, Madgwick et al. 1977; Madgwick 1985; 5 to 13 years, Madgwick and Oliver 1985; Madgwick et al. 1988; 17 to 29 years, Madgwick et al. 1977; Madgwick 1985). Note that the data for different age groups are taken from sites with different agroecological conditions.
Will (1968) estimated that P. radiata stands on a pumice soil in Kaingaroa forest, on the central North Island of New Zealand, removed approximately 41 kg P ha-1from the soil during the first 10 years of growth. After this phase, during the next 25 years, the amount of P taken up by the trees was only 11 kg·ha-1. This led Will (1968) to suggest that the largest demand for P by the trees occurs prior to canopy closure.
The P uptake pattern of P. radiata trees from the time of planting was also reported by Madgwick et al. (1977). They reported that during the first 2 years after establishment the net annual P uptake of intensively managed P. radiata plantations on a good site in the northeastern corner of the Kaingaroa forest was relatively low (1.5 kg·ha-1·a-1), while between the second and fourth years after establishment the net annual P uptake was much higher (7.9 kg·ha-1·a-1). They also reported that between the second and fourth years, the stand was rapidly closing its canopy. Therefore, the net annual P uptake decreases towards the end of this phase due to a steady-state nutrient cycle developing under closed canopy conditions. Madgwick et al. (1988) suggested that at around 6 years of age, the maximum mean annual increment of P was attained. Thereafter, needle production decreased with stand age (from 7 years), and this decrease reduced the annual gross uptake of nutrients. Madgwick et al. (1977) also reported that in young stands, total P content increased at a relatively higher rate than the total biomass.
The relative quantities of P in the various components of the above-ground P. radiata trees changes with increasing age of the trees. Smith et al. (1994) studied the distribution of P in various components of above-ground 5-year-old second-rotation P. radiata trees on sand dunes in the Woodhill forest on the west coast of the North Island, New Zealand, where P was not limiting tree growth (needle P 0.14%). They reported that the largest percentage of P (50.7%) within the aboveground components of the tree was in the foliage.
Frederick et al. (1985) also studied the distribution of P in various components of 8-year-old first-rotation P. radiata trees grown on a pumice soil at Poukani North Block in the central North Island of New Zealand when P supply was not limiting growth. They also showed that the largest percentage (47.9%) of P within the above-ground biomass of the tree was in the foliage. This is because the P concentration in the foliage is higher than in other tree components, even though the foliage is only the third largest component of tree biomass after stem wood and branches. The second largest portion of P (22.9%) was in the stem wood, as this component had the highest dry matter weight. Branches constitute a considerable portion (16.7%) of total P within the tree as well.
Madgwick et al. (1988) obtained similar results to Frederick et al. (1985) for P partitioning within the aboveground components of 8-year-old first-rotation P. radiata trees. They reported that the largest percentage (40%) of P was in the foliage. The next largest portion of P (25%) was in the stem wood, while the third largest portion (19.1%) of the P was in the branches. However, unlike in the 5-year-old trees studied by Smith et al. (1994), in the 8-year-old trees studied by Frederick et al. (1985) and Madgwick et al. (1988), the second largest percentage of P was in the stem wood (23-25%). This is probably because in the older trees (8-year old trees) the stem wood would have had a relatively higher dry matter weight than that in the 5-year-old trees. In the much older trees, stem wood appears to contain the largest P content in the above-ground tree biomass. Webber and Madgwick (1983) reported that the above-ground biomass P content of a 29-year-old P. radiata stand in Kaingaroa State Forest, which contained 426 tonnes dry matter per hectare, was 66.3 kg·ha-1. Of this, 20 kg·ha-1(30.1%) was in the foliage, 9.5 kg·ha-1(14.3%) was in the branches, 5.5 kg·ha-1(8.3%) was in the cones, 7.4 kg·ha-1(11.1%) was in the stem bark, and 24 kg·ha-1(36.1%) was in the stem wood. These results show that approximately half the above ground P (47.2%) is removed from the ecosystem through stem harvest.
Under wider-spaced second-rotation P. radiata plantations, understory vegetation might also become a factor influencing P uptake. Richardson et al. (1996) reported that the presence of understory vegetation increased or decreased P concentration in the needles of P. radiata, depending on the species beneath the trees. Therefore, the weed species might have differentially affected the distribution of P within the trees, although Richardson et al. (1996) did not report on this aspect. In addition to tree age and spacing, soil P fertility influences the amount of P taken up by the tree. For example, Parfitt et al. (1994) reported that P uptake rates of 7-year-old closely-spaced P. radiata treated with 4 and 125 kg P ha-1were 3.5 and 5.1 kg·ha-1·a-1, respectively. In older trees, the P uptake rates of 11-year-old P. radiata treated with 25 and 100 kg P ha-1were 6.0 and 8.1 kg·ha-1·a-1, respectively.
Understory biomass P content
The importance of understory P in P. radiata plantations depends on a number of factors, including tree age and spacing. Parfitt et al. (1994) estimated that the P content of understory vegetation under an 11-year-old radiata pine plantation on a Te Kopuru sand at Shenstone forest, South Island, New Zealand was 1 kg·ha-1, whereas the P content of P. radiata was 27 kg·ha-1, and the total P content of the ecosystem pools (understory, pine tree and 0-15 cm soil depth containing LFH horizons) was 162 kg P ha-1. Therefore, the P content of the understory was only 3.6% of the P content in the above-ground biomass and 0.6% of the P content in the ecosystem pool. From the study of Parfitt et al. (1994), it appears that the understory vegetation does not significantly contribute to P cycling in the forest plantation when trees are 11 years old. However, from the biomass data during the first 3-8 years of tree growth, it appears that the P content of understory vegetation could well be a significant proportion of the total above-ground P content, but no data on the relative P contents of the understory vegetation and P. radiata trees for the first few years of tree growth are available in the literature.
In second-rotation P. radiata forest plantations in New Zealand there has recently been a steady trend towards wider tree spacing and increased rates of P fertiliser application (Payn et al. 2000; Mead 2005a; Zabowski et al. 2007). These changes in silvicultural practice are expected to increase the potential for understory growth due to increased light and greater nutrient resources (Gadgil et al. 1988; Brockerhoff et al. 2008). In such conditions, understory vegetation would become a more significant component of P cycling in P. radiata forests than under closely-spaced stands, especially prior to canopy closure. For example, the P content of understory vegetation under a 4-year-old second-rotation P. radiata stand on an allophanic soil at Kaweka forest ranged from 3.4 to 7.8 kg·ha-1(at the stocking rate of 1000 stems·ha-1) (A. Rivaie unpublished data) and the P content in the 4-year-old P. radiata was 16.5 kg·ha-1(Madgwick 1985). Therefore, the P content of understory as a percentage of the total P content in above-ground biomass was 17% to 32%. These percentages are much higher than the 3.6% reported for understory under an 11-year-old radiata pine stand (at the stocking rate of 1600 stems·ha-1) by Parfitt et al. (1994) and Hunter and Graham (1983).
The role of understory vegetation in nutrient cycling
Understory vegetation control by the application of herbicides is common in the establishment of P. radiata plantations in New Zealand and this usually results in a considerable increase in tree growth (Richardson et al. 1993; 1996; Wagner et al. 2006; Mead 2005b; Rubilar et al. 2008). This practice is recommended because many studies have shown that growth rates and survival of trees is reduced in the presence of understory vegetation. This is probably due to the competition of understory vegetation with P. radiata for water, nutrients and light (Nambiar and Zed 1980; Gadgil et al. 1992; Clinton et al. 1994; Richardson et al. 1996; Mason and Milne 1999; Watt et al. 2003ab; Rubilar et al. 2008; Mead 2010).
On the other hand, several studies have suggested that understory vegetation enhances nutrient cycling and conservation within forest stands, depending on the type of understory species (Tappeiner and Alm 1975; Zou et al. 1995; Condron et al. 1996; Bao et al. 1996; O’Connell and Grove 1996; Binkley et al. 2000; Jiang and Zhai 2000; Richardson et al. 1996; 2006; Mead 2010; Rivaie 2011).
Nitrogen supply by understory legumes
Leguminous understory vegetation may have a significant role in supplying N to forest ecosystems. Watt et al. (2003b) suggested that the presence of broom (Cytisus scoparius L.), a leguminous understory, under P. radiata may enhance long-term growth of the tree on wet sites through N released from the death of the broom after radiata canopy closure. In a dry site he found that broom competed for soil N with radiata trees.
Binkley et al. (1984) studied the effects of Sitka alder (Alnus sinuata (Regel) Rydb.), a leguminous shrubby species, in a 23-year-old Douglas-fir stand in a gravely clay loam Typic Haplorthod, in British Columbia, Canada. They reported that when Sitka alder was present in the forest, the above-ground ecosystem biomass was 55% greater than when it was absent. The above-ground biomass of Sitka alder was 21% of the total aboveground ecosystem biomass. In the presence of Sitka alder, total aboveground biomass and stem biomass of Douglas-fir were higher than those in the absence of the Sitka alder (by 20% and 30%, respectively). The needle N concentration of the Douglas-fir stand in the presence of Sitka alder was significantly higher than that in the absence of this understory. The total N content in the aboveground biomass and forest floor was 558 kg·ha-1in the presence of Sitka alder and 208 kg·ha-1in its absence. Of these quantities of total N, the N content in the aboveground biomass of Douglas-fir was 155 kg·ha-1in the presence of this understory and 129 kg ha-1in the absence of this understory.
Klemmedson (1994) reported that the presence of New Mexican locust (Robinia neomexicana Gray), an N-fixing spiny shrub under ponderosa pine (P. ponderosa Douglas), significantly increased N concentration (0.314% for pine-locust stands and 0.173% for pine-alone stands) in the upper soil layer (0-5 cm) under the pines, as well as pine needle N concentration (1.07% for pine-locust stands vs 0.90% for pine-alone stands) in a forest on montmorillonitic Typic Agriborolls at the Coconino National Forest, Arizona, USA.
Nutrient storage and release by understory
The nutrient contents in the understory litterfall may make a significant contribution to the total nutrient pool in the forest soil. Binkley et al. (1984) reported that in the presence of Sitka alder under Douglas-fir for over 23 years, total litterfall dry matter weight (litter from Douglas-fir + Sitka alder) was 3.6 times higher and the nutrient contents (N, P, K, Ca, and Mg) in the litterfall were 3 to 7 times higher than when Sitka alder was ab-sent. In addition, the available N, P, Ca, and Mg concentrations in the top 10 cm soil depth were significantly increased by the presence of the Sitka alder.
Similarly, Tappeiner and Alm (1975) reported that the presence of understory vegetation significantly increased Ca, N, K, Mg and P contents in the litterfall, compared with litterfall under pure red pine stands. Based on these results, they suggested that the understory vegetation accumulates, stores and releases nutrients. The understory litterfall also increased the total organic matter content in the litter layer of the forest. However, the rate of decomposition of this organic matter was faster in the presence of understory vegetation, especially hazel and herbs (Aster macrophyllum L., Pteridium aquilinum, Maianthenum canadense Desf., Diervilla lonicera Mill., Arctostaphylos uva-ursi (L.) Spreng.) compared to under red pine stands without understory vegetation. This suggests that the presence of certain understory species in forest stands might increase the tree growth through their role in accelerating nutrient cycling and enhancing the availability of nutrients in forest ecosystems. Maclean and Wein (1977) reported that understory species in a 13-16-year P. banksiana stand had significant quantities of nutrients in the above-ground biomass. They found that the understory species had 25% of the N and Ca, 30% of P, 40% of the Mg, and more than 65% of the K in the aboveground ecosystem.
Understory vegetation effect on P availability to tree crops
Plants may influence the chemical and biological properties within their rhizosphere, and in this way they may enhance the soil P availability and the P uptake of neighbouring plant species. Several mechanisms have been suggested for this increased soil P availability (Binkley et al. 1984; Horst et al. 2001; Li et al. 2003ab; Trolove et al. 2003; Zhang et al. 2004) and these are discussed below.
Binkley et al. (1984) reported that the presence of Sitka alder in Douglas fir forest significantly increased Bray-1 P concentration (21 μg·g-1) in the topsoil (0-10 cm) compared with that in the absence of this understory (8 μg·g-1). They considered this increase in Bray-1 P concentration to be related to the significant increase in P input through litterfall biomass in the presence of Sitka alder.
Sarno et al. (2004) reported that the presence of Paspalum conjugatum (introduced-understory) or native understory species (Chromolaena odorata, Clibadium surinamense, Clidemia hirta, Imperata cylindrica, Melastoma affine, Mikania micrantha, and P. conjugatum) in a coffee plantation in a vertic dystrudept at Lampung, Indonesia, increased available P compared with available P in the absence of these understory species in both the 0-10 cm (13.3 μg·g-1soil for understory-free plots, 14.9 μg·g-1soil for plots with P. conjugatum plots, and 32.5 μg·g-1soil for plots with native understory) and the 10-20 cm soil depths (3.9 μg·g-1soil, 6.6 μg·g-1soil, and 10.8 μg·g-1soil, respectively). In another study in Indonesia, Salam et al. (2001) reported that in a coffee plantation, soil phosphatase activities in bulk soils in the plots with P. conjugatum and with native understory (135 and 158 μg p-nitrophenol·g-1·h-1, respectively) were higher than those in the plots without understory (108 μg p-nitrophenol·g-1·h-1). In rhizosphere soils, the phosphatase activity difference between these plots might have been even higher (Trolove et al. 2003) but this was not measured in this study. Total P uptakes by the trees in plots with and without understory species were also not reported in these studies.
Many reports suggest that N-fixing plant species facilitated their neighbours by increasing soil P availability. Giardina et al. (1995) studied the effect of red alder (Alnus rubra Bong), an N-fixing understory species, in a 21-year old Douglas fir (Pseudotsuga menziesii (Mirb.) Franco) plantation on soil phosphate chemistry in a deep gravely clay loam soil at Oregon, USA. They reported that the soils (0-15 cm) under red alder + Douglas fir had significantly higher concentrations of anion resin-Pi, NaOH-Pi, and HCl-Pi(65-225% greater) than those under pure Douglas fir. The soil phosphatase activity under red alder + Douglas fir (29 μmol p-nitrophenol·g-1·h-1) was also higher than that under pure Douglas fir (10 μmol p-nitrophenol· g-1·h-1). They explained the increased Piavailability (resin-Pi) under red alder + Douglas fir as partly due to the increased soil phosphatase activity converting Poto Piin the soils.
Under a 63-year old mixed-conifer (Douglas fir, western hemlock, and Sitka spruce) plantation with red alder understory on a mesic andic haplumbrept at the Cascade Head Experimental Forest, Oregon, USA, Zou et al. (1995) reported that labile inorganic P pools (resin- and NaHCO3-extractable Pi) in the soil under red alder + mixed conifers were three to fourfold higher than those under red alder or mixed conifers. They also reported that the mixed conifer stand had the highest soil pH (5.3), but the net P solubilisation rate under this stand (9 mg·kg-1·d-1) was similar to that under the red alder (12 mg·kg-1·d-1), while the red alder + mixed conifers had the lowest pH (4.8) and the highest net P solubilisation rate (51 mg·kg-1·d-1). Soil phosphatase activity under the red alder + mixed conifer stand was also higher than in the pure mixed conifer stand and red alder. These observations led Zou et al. (1995) to suggest that the interaction between red alder and conifers was the cause for the increase in the labile soil P pool.
Further evidence of the role of understory vegetation in enhancing P uptake by trees was provided by Gillespie and Pope (1989). They reported that when walnut (Juglans nigra L.) tree seedlings were interplanted with alfalfa (Medicago sativa L.) grown in pots containing typic argiaquoll soils treated with synthetic hydroxyapatite (a calcium phosphate mineral), the tree seedlings had greater P uptake compared to walnut seedlings grown alone. They considered that the diffusion of solubilised phosphate rock-P to the roots of walnut at the points of root intersection with alfalfa was the mechanism for the greater P uptake by walnut seedlings. This was caused by H+ions diffusing from the roots of alfalfa and decreasing pH, thereby, solubilising the rock phosphate.
Others have demonstrated the facilitation of P uptake in intercropping systems where legumes were cultivated with other crop species. For example, white lupin (Lupinus albus L.) facilitated P uptake by wheat when they were grown together in the field (Gardner and Boundy 1983). In a pot experiment, Li et al. (2003b) showed that chickpea helped wheat to take up P from an organic P source. They suggested that chickpea hydrolysed organic P by the phosphatase released from its roots, thereby, contributing to the P uptake by wheat.
Understory vegetation effect on P availability to P. radiata
Only limited information is available on the effect of understory vegetation on soil P dynamics and P nutrition of P. radiata trees. After 3-4 years of growing P. radiata with various understory species in a moderately fertile pumice soil in the field at Rotorua, New Zealand (Richardson et al. 1993), some species of grass, herbaceous broadleaves and buddleia significantly increased P concentrations in the P. radiata needles (synergism), but broom, gorse, lotus and pampas had no significant effect on needle P concentrations (Richardson et al. 1996). However, the effect of these plant species on P concentrations in other parts of the tree, total P uptake by the tree from the soil, or soil P changes due to these understory species were not reported in their study. The mechanism by which the understory species influenced the P nutrition of the tree was also not reported.
Scott and Condron (2004) conducted a pot experiment in a glasshouse to study the effects of understory species on P. radiata growth, P uptake and soil P changes in four soils, namely a low P, low C soil (320 μg P g-1; 2.5% C), a low P, high C soil (524 μg P g-1; 5.1% C), a high P, low C soil (721 μg P g-1; 2.4% C), and a high P, high C soil (768 μg P g-1; 4.0% C). The understory species tested were lucerne and ryegrass. The experimental treatments included P. radiata trees grown alone, trees and lucerne grown together, trees and ryegrass grown together, lucerne grown alone, and ryegrass grown alone. Lucerne had a positive effect on tree growth and P uptake across all four soils regardless of Piand Poavailability. In addition, lucerne appeared to facilitate redistribution of P from the less labile to the more labile fraction. This study also confirmed that interactions between plant species and soil P and C status strongly affected changes in soil P forms (Scott and Condron 2010). These are interesting findings and help to explain the observation above by Richardson et al. (1996) of the beneficial effects of some understory species on P nutrition of P. radiata.
Field trials were carried out to investigate the growth of second-rotation P. radiata and determine the relationships between needle P concentrations and soil P forms two years after application of two forms of P fertilizer (triple superphosphate and Ben-Guerir phosphate rock in combination with two weed control practices. In the highly P-deficient Kaweka forest soil the presence of understory reduced resin-Piand Olsen P concentrations, but in the moderate P fertility Kinleith forest soil the presence of understory increased Bray-2 P, resin-Pi, and Olsen P concentrations in the soil. The deeper rooted understory species at the Kinleith forest (Himalayan honeysuckle, buddleia and some toetoe) may have enhanced the plant-available P concentrations at the soil surface through litterfall (pumping mechanism) (synergistic effect). Whereas at the Kaweka forest where the soil plant-available P was low, the understory vegetation (bracken fern and some manuka) tended to compete with radiata pine for P (antagonistic effect) (Rivaie and Tillman 2009; Rivaie and Loganathan 2010).
Rivaie (2004) conducted a glasshouse trial to determine changes in soil P fractions in a P-deficient allophanic soil under P. radiata seedlings grown with broom (Cytisus scoparius L.) and ryegrass (Lolium multiflorum) following the application of three rates of triple superphosphate (TSP). When P. radiata was grown with broom, at all P rates the acid phosphatase activity in the rhizosphere of P. radiata was higher than in the bulk soil and the rhizosphere soil of P. radiata grown with ryegrass. In addition, Rivaie and Tillman (2010) reported that without addition of P fertiliser and when broom was grown with P. radiata, the P concentration in needles of radiata was higher than that of radiata pine when grown with ryegrass. However, when P fertiliser was added (50 and 100 μg P g-1 soil) broom had an antagonistic effect on P. radiata. The P. radiata needle P concentration was lower when P. radiata was grown with broom than that when P. radiata was grown with ryegrass. Meanwhile, in the high P fertile soil (application rate of 100 μg P g-1soil), the dry matter yield of radiata was lower when it was grown with broom than when it was grown with ryegrass, suggesting that in moderate to high P fertile soils, P. radiata seedlings grow better with ryegrass than with broom as in the high P fertility soil broom grows robustly. These results lead to clearer understanding of the interaction of P fertiliser, particular understory vegetation and P. radiata, including how understory vegetation alters the availability of P to the trees under different soil P status.
Conclusions
This review of literature has revealed that the above-ground understory biomass is a significant proportion of the total aboveground biomass in P. radiata forests during the first 5-10 years of growth. During this period, competition for nutrients, including P, and for light and water is possible depending on tree age, site P fertility, climate, understory species and their populations. It is also possible that the presence of understory vegetation under P. radiata plantations has synergistic effects on tree growth and nutrition.
As a significant proportion of total above-ground biomass in the forest, therefore, the understory vegetation can have an important effect on P uptake by P. radiata trees, both before and after canopy closure. Before canopy closure, understory vegetation can have either antagonistic or synergistic effects on P uptake by P. radiata, depending on the understory species, soil moisture regime, site P fertility status, and other factors. However, after canopy closure, the understory vegetation dies and the decay of this vegetation may release P for uptake by P. radiata, thus supporting P. radiata growth. If the understory vegetation in the plantations competes with P. radiata for soil P (antagonistic effect), higher rates of P fertiliser need to be applied to the forests or the understory vegetation needs to be controlled by applying costly herbicides. On the other hand, if the understory vegetation increases P availability to P. radiata, then the P re-quirements of P. radiata might be sustained with lower rates of P fertiliser application.
Acknowledgements
The author thanks Prof. R. W. Tillman and Prof P. Loganathan (Massey University) and Dr. T. W. Payn (Centre for Sustainable Forest Management of New Zealand, Forest Research Ltd., Rotorua) for their suggestions on the manuscript.
Adams MA, Attiwill PM, Polglase PJ. 1989. Availability of nitrogen and phosphorus in forest soils in north-eastern Tasmania. Biology and Fertility of Soils, 8: 212-218.
Adams JA, Walker TW. 1975. Some properties of a chronosequence of soils from granite in New Zealand. 2. Forms and amounts of phosphorus. Geoderma, 13: 41-51.
Afif-Khouri E, Cámara Obregón, MA, Oliveira-Prendes JA, Gorgoso-Varela JJ. Canga-Líbano E. 2010. Relationship among soil parameters, tree nutrition and site index of Pinus radiata D. Don in Asturias, NW Spain. Forest Systems, 19: 77–88.
Anderson G. 1980. Assessing organic phosphorus in soils. In: F.E. Khasawneh, E.C. Sample and E.J. Kamprath (eds), The role of Phosphorus in Agriculture. Wisconsin, USA: American Society of Agronomy, pp.411-428.
Ballard R. 1980. Phosphorus nutrition and fertilisation of forest trees. In: F.E. Khasawneh, E.C. Sample and E.J. Kamprath (eds), The role of Phosphorus in Agriculture. Wisconsin, USA: American Society of Agronomy, pp. 763-804.
Bao CS, Sang YR, Wang CK, Jin XY. 1996. The characteristics of the nutrient cycling in birch stands with different age stages. Journal of Northeast Forestry University (English Edition), 7(1): 7-10.
Beets PN, Pollock DS. 1987. Accumulation and partitioning of dry matter in Pinus radiata as related to stand age and thinning. New Zealand Journal of Forestry Science, 17: 246-271.
Bi H, Long Y, Turner J, Lei Y, Snowdon P, Li Y, Harper R, Zerihun A, Ximenes F. 2010. Additive prediction of aboveground biomass for Pinus radiata (D. Don) plantations. Forest Ecology and Management, 259: 2301–2314.
Binkley D, Lousier JD, Cromack KJr. 1984. Ecosystem effects of Sitka alder in a Douglas-fir plantation. Forest Science, 30: 26-35.
Binkley D, Giardina C, Bashkin MA. 2000. Soil phosphorus pools and supply under the influence of Eucalyptus saligna and nitrogen-fixing Albizia falcataria. Forest Ecology and Management, 128: 241-247.
Bowen GD, Rovira AD. 1991. The rhizosphere: the hidden half of the hidden half. In: Y. Waisel, A. Eshel and U. Kafkafi (eds), Plant Roots: the Hidden Half. Marcel Dekker, New York: pp.641-669.
Brockerhoff EG, Jactel H, Parrotta JA, Quine CP, Sayer J. 2008. Plantation forests and biodiversity: oxymoron or opportunity? Biodiversity Conservation, 17: 925–951.
Burkes CE, Will RE, Barron-Gafford GA, Teskey RO, Shiver B. 2003. Biomass Partitioning and Growth Efficiency of Intensively Managed Pinus taeda and Pinus elliotii Stands of Different Planting Densities. Forest Science, 49: 224-234.
Chauhan BS, Stewart JWB, Paul EA. 1981. Effect of labile inorganic phosphate status and organic carbon additions on the microbial uptake of phosphorus in soils. Canadian Journal of Soil Science, 61: 373-385.
Chen CR, Condron LM, Davis MR, Sherlock RR. 2000. Effects of afforestation on phosphorus dynamics and biological properties in a New Zealand grassland soil. Plant and Soil, 220: 151-163.
Chen CR, Condron LM, Davis MR, Sherlock RR. 2002. Phosphorus dynamics in the rhizosphere of perennial ryegrass (Lolium perenne L.) and radiata pine (Pinus radiata D. Don.). Soil Biology and Biochemistry, 34: 487-499.
Chen CR, Condron LM, Turner BL, Mahieu N, Davis MR, Xu ZH, Sherlock RR. 2004. Mineralisation of soil orthophosphate monoesters under pine seedlings and ryegrass. Australian Journal of Soil Research, 42: 189-196.
Clinton PW, Frampton CM, Mead DJ. 1994. Modelling competitive pasture effects on nutrient uptake by Pinus radiata. New Zealand Journal of Forestry Science, 24: 268-278.
Condron LM, Davis MR, Newman RH, Cornforth S. 1996. Influence of conifers on the forms of phosphorus in selected New Zealand grassland soils. Biology and Fertility of Soils, 21: 37-42.
Condron L M, Turner BL, Cade-Menun BJ. 2005. Chemistry and dynamics of soil organic phosphorus. In: J.T. Sims and A.N. Sharpley (eds), Phosphorus: Agriculture and the Environment. Madison, Wisconsin: ASA/CSSA/SSSA, pp. 87-121.
Condron L, Newman S. 2011. Revisiting the fundamentals of phosphorus fractionation of sediments and soil. Journal Soil Sediments, 11: 830-840.
Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, Mueller-Dombois. 1995. Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology, 76: 1407-1424.
Davis MR, Lang MH. 1991. Increased nutrient availability in topsoils under conifers in the South Island high country. New Zealand Journal of Forestry Science, 21: 165–179.
Evans J. 2009. Planted forests: uses, impacts and sustainability. Wallingford, United Kingdom, CABI and Rome, FAO, 213 pp.
Forrest WG, Ovington JD. 1970. Organic matter changes in an age series of Pinus radiata plantations. Journal of Applied Ecology, 7: 177-186.
Frederick DJ, Madgwick HAI, Jurgensen MF, Oliver GR. 1985. Dry matter, energy, and nutrient contents of 8-year-old stands of Eucalyptus regnans, Acacia dealbata, and Pinus radiata in New Zealand. New Zealand Journal of Forestry Science, 15: 142-157.
Frossard E, Condron LM, Oberson A, Sinaj S, Fardeau JC. 2000. Processes governing phosphorus availability in temperate soils. Journal of Environmental Quality, 29: 15-23.
Gadgil RL, Charlton JFL, Sandberg AM, Allen PJ. 1988. Establishment of selected legumes in a mid-rotation Pinus radiata plantation. New Zealand Journal of Forestry Science, 18: 210-220.
Gadgil RL, Charlton JFL, Sandberg AM, Allen PJ. 1992. Nutritional relationships between pampas grass (Cortaderia spp.) and Pinus radiata. New Zealand Journal of Forestry Science, 22: 3-11.
Gardner WK, Boundy KA. 1983. The acquisition of phosphorus by Lupinus albus L. IV. The effect of intercropping wheat and white lupin on the growth and mineral composition of the two species. Plant and Soil, 70: 391-402.
Giardina CP, Huffman S, Binkley D, Caldwell BA. 1995. Alders increase soil phosphorus availability in a Douglas-fir plantation. Canadian Journal of Forest Research, 25: 1652-1657.
Gillespie AR, Pope PE. 1989. Alfalfa N2-fixation enhances the phosphorus uptake of walnut in interplantings. Plant and Soil, 113: 291-293.
Grove TS, Malajczuk N. 1984. Biomass production by trees and understory shrubs in an age-series of Eucalyptus diversicolor F. Muell. stands. Forest Ecology and Management, 11: 59-74.
Haynes RJ. 1984. Lime and phosphate in the soil-plant system. Advances in Agronomy, 37: 249-315.
Hedley MJ, White RE, Nye PH. 1982. Plant-induced changes in the rhizosphere of rape (Brassica napus Var. Emerald) seedlings. III. Changes in L-value, soil phosphate fractions and phosphatase activity. New Phytologist, 95: 69-82.
Hinsinger P. 2001. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant and Soil, 237: 173-195.
Horst WJ, Kamh M, Jibrin JM, Chude VO. 2001. Agronomic measures for increasing P availability to crops. Plant and Soil, 237: 211-223.
Hunter IR, Graham JD. 1983. Three-year response of phosphorus-deficient Pinus radiata to various rates of superphosphate fertiliser. New Zealand Journal of Forestry Science, 13: 229-238.
Hunter IR, Hunter JAC. 1991. Apparent phosphorus uptake and change in nitrogen content of Pinus radiata growing on soils of different phosphorus retention, treated with superphosphate and A-grade rock phosphate. New Zealand Journal of Forest Science, 21: 50-61.
Hunter IR, Rodgers BE, Dunningham A, Prince JM, Thorn AJ. 1991. An atlas of radiata pine nutrition in New Zealand. New Zealand Ministry of Forestry. FRI Bulletin No. 165, 24 pp.
Jiang SN, Zhai MP. 2000. Nitrogen transfer between N2-fixing plant and non-N2-fixing plant. Journal of Forestry Research, 11(2): 75–80.
Junk A, Seeling B, Gerke J. 1993. Mobilization of different phosphate fractions in the rhizosphere. Plant and Soil, 155/156: 91-94.
Khanna PH, Ulrich B. 1984. Soil characteristics influencing nutrient supply in forest soils. In: G.D. Bowen and E.K.S. Nambiar (eds). Nutrition of Plantation Forests. Academic Press Inc. Ltd., New York, USA, pp.79-117.
Klemmedson JO. 1994. New Mexican locust and parent material: influence of forest floor and soil macronutrients. Soil Science Society of America Journal, 58: 974-980.
Knight PJ. 1978. Fertiliser practice in New Zealand forest nurseries. New Zealand Journal of Forestry Science, 8: 27-53.
Li L, Zhang F, Li X, Christie P, Sun J, Yang S, Tang C. 2003a. Interspecific facilitation of nutrient uptake by intercropped maize and faba bean. Nutrient Cycling in Agroecosystems, 65: 61-71.
Li L, Tang C, Rengel Z, Zhang F. 2003b. Chickpea facilitates phosphorus uptake by intercropped wheat from an organic phosphorus source. Plant and Soil, 248: 297-303.
Long JN, Turner J. 1975. Above-ground biomass of understory and overstorey in an age sequence of four Douglas-fir stands. Journal of Applied Ecology, 12: 179-188.
MacLean DA, Wein RW. 1977. Nutrient accumulation for postfire jack pine and hardwood succession patterns in New Brunswick. Canadian Journal of Forest Research, 7: 562-578.
Maclaren JP. 1993. Radiata pine growers’ manual. FRI Bulletin No. 184. Rotorua, New Zealand, New Zealand Forest Research Institute, 140 pp.
Madgwick HAI, Jackson DS, Knight PJ. 1977. Above-ground dry matter, energy, and nutrient contents of trees in an age series of Pinus radiata plantations. New Zealand Journal of Forestry Science, 7: 445-468.
Madgwick HAI. 1985. Dry matter and nutrient relationships in stands of Pinus radiata. New Zealand Journal of Forestry Science, 15: 324-336.
Madgwick HAI, Oliver GR. 1985. Dry matter content and production of close-spaced Pinus radiata. New Zealand Journal of Forestry Science, 15: 135-141.
Madgwick HAI, Sims A, Oliver GR. 1988. Nutrient content and uptake of close-spaced Pinus radiata. New Zealand Journal of Forestry Science, 18: 65-76.
Magid J, Tiessen H, Condron LM. 1996. Dynamics of organic phosphorus in soils under natural and agricultural ecosystems. In: A. Piccolo (ed), Humic Substances in Terrestrial Ecosystems., Amsterdam: Elsevier, pp. 429–466.
Manley B, Maclaren P. 2009. Modelling the impact of carbon trading legislation on New Zealand’s plantation estate. New Zealand Journal of Forestry, 54: 39–44.
Mason EG, Milne PG. 1999. Effects of weed control, fertilization, and soil cultivation on the growth of Pinus radiata at midrotation in Canterbury, New Zealand. Canadian Journal of Forest Research, 29: 985-992.
May B, Carlyle C, Lowther R, Keeley P, Bernie C. Michael M. 2009. A decision support system for maximising profit from nutrient management of mid-rotation radiata pine. Project PNO: 03.3907. Melbourne, Australia: Forest and Wood Products Australia Limited, p.165.
McGill WB, Cole CV. 1981. Comparative aspects of cycling of organic C, N, S, and P through soil organic matter. Geoderma, 26: 267-286.
Mead DJ, Gadgil RL. 1978. Fertiliser use in established radiata pine stands in New Zealand. New Zealand Journal of Forestry Science, 8: 105-134.
Mead DJ. 2005a. Opportunities for increasing plantation productivity. How much? How quickly? How realistic? Biomass and Bioenergy, 28: 249–266.
Mead DJ. 2005b. Fertilising. In: M. Colley (ed), NZIF forestry handbook. Christchurch, New Zealand: New Zealand Institute of Forestry, pp.110–111.
Mead DJ. 2010. Results of 16 years of study in a temperate silvopastoral experiment with Pinus radiata in New Zealand. In: L.R. Kellimore (ed), Handbook on agroforestry: management practices and environmental impact. New York, USA: Nova Science Publishers, pp. 225–249.
Mitchell AD. 2000. Magnesium fertiliser effects on forest soils under Pinus radiata. PhD Thesis, Massey University. New Zealand.
Morgan JAW, Bending GD, White PJ. 2005. Biological costs and benefits to plant-microbe interactions in the rhizosphere. Journal of Experimental Botany, 56: 1729-1739.
Nambiar EKS, Zed PG. 1980. Influence of weeds on the water potential, nutrient content and growth of young radiata pine. Australian Forestry Research, 10: 279-288.
Norrish K, Rosser H. 1983. Mineral Phosphate. In: J.J. Lenaghan and G. Katsantoni (eds), Soils: an Australian Viewpoint. Melbourne: Division of Soils, CSIRO / London: Academic Press, pp. 335-361.
O’Connell AM, Grove TS. 1996. Biomass production, nutrient uptake and nutrient cycling in the Jarrah (Eucalyptus marginate) and Karri (Eucalyptus diversicolor) forests of South-western Australia. In: P. M. Attiwill and M. A. Adams (eds), Nutrition of Eucalyptus. Australia: CSIRO, pp. 155-190.
Ouro G, Pérez-Batallón P, Merino A. 2001. Effects of sylvicultural practices on nutrient status in a Pinus radiata plantation: Nutrient export by tree removal and nutrient dynamics in decomposing logging residues. Annals of Forest Science, 58: 411–422.
Parfitt RL, Tate KR, Yeates GW, Beets PN. 1994. Phosphorus cycling in a sandy podsol under Pinus radiata. New Zealand Journal of Forestry Sci-ence, 24: 253–267.
Payn TW, Skinner MF, Clinton PW. 1998. Future nutrient requirements of New Zealand plantation forests. In: L.D. Currie and P. Loganathan (eds), Long-term Nutrient Needs for New Zealand’s Primary Industries. Occasional report No. 11. New Zealand: Fertiliser and Lime Research Centre, Massey University, pp. 97-110.
Payn TW, Skinner MF, Hill RB, Thorn AJ, Scott J, Downs S, Chapman H. 2000. Scaling up or scaling down: the use of foliage and soil information for optimising the phosphate nutrition of radiata pine. Forest Ecology and Management, 138: 79-89.
Radersma S, Grierson PF. 2004. Phosphorus mobilization in agroforestry: Organic anions, phosphatase activity and phosphorus fractions in the rhizosphere. Plant and Soil, 259: 209-219.
Richardson B, Vanner A, Davenhill N, Balneaves J, Miller K, Ray J. 1993. Interspecific competition between Pinus radiata and some common weed species - first-year results. New Zealand Journal of Forestry Science, 23(2): 179-193.
Richardson B, Vanner A, Ray J, Davenhill N, Coker G. 1996. Mechanisms of Pinus radiata growth suppression by some common forest weed species. New Zealand Journal of Forestry Science, 26(3): 421-437.
Richardson B, Watt MS, Mason EG, Kriticos DJ. 2006. Advances in modelling and decision support systems for vegetation management in young forest plantations. Forestry, 79: 29–42.
Rivaie AA. 2004. Understory Effects on Phosphorus Fertiliser Response of Second-Rotation Pinus radiata. PhD Thesis. Massey University, New Zealand.
Rivaie AA, Tillman RW. 2009. Growth Response of Second-rotation Pinus radiata on an Orthic Allophanic soil to P Fertiliser and Weed Control. Taiwan Journal of Forest Science, 24(4): 243-256.
Rivaie AA, Loganathan P. 2010. Phosphorus fertiliser source and weed control effects on growth of 3-year-old second-rotation Pinus radiata on Orthic Pumice Soil in New Zealand. Southern Forest: a Journal of Forest Science, 72(2): 75-81.
Rivaie AA, Tillman RW. 2010. Phosphorus fertiliser induced changes in the soil plant-available P, the P nutrition and the growth of Pinus radiata seedlings grown in association with understory. Journal of Forestry Research, 21(2): 129-139.
Rivaie AA. 2011. Growth response of broom (Cytisus scoparius) growing with and without radiate pinne (Pinus radiate) seedlings to different P levels in soil. Journal of Forestry Research, 22(4): 597-602.
Romanyà J, Vallejo VR. 2004. Productivity of Pinus radiata plantations in Spain in response to climate and soil. Forest Ecology and Management, 195: 177–189.
Rubilar R, Blevins L, Toro J, Vita A, Muñoz F. 2008. Early response of Pinus radiata plantations to weed control and fertilization on metamorphic soils of the Coastal Range, Maule Region, Chile. Bosque, 29: 74–78.
Salam AK, Afandi, Sriyani N, Kimura M. 2001. Soil enzymatic activities in a hilly coffee plantation in Lampung Province, South Sumatra, Indonesia, under plant cover management. Soil Scince and Plant Nutrition, 45: 695–702.
Sarno, Lumbanraja J, Afandi, Adachi T, Oki Y, Senge M, Watanabe A. 2004. Effect of weed management in coffee plantation on soil chemical properties. Nutrient Cycling in Agroecosystems, 69: 1–4.
Scott JT, Condron LM. 2004. Short term effects of radiata pine and selected pasture species on soil organic phosphorus mineralisation. Plant and Soil, 266: 153–163.
Scott JT, Condron LM. 2010. Isotopic exchange kinetics of soil P under Pinus radiata and Lucerne Understory. In: Proceeding of 19thWorld Congress of Soil Science, Soil Solutions for a Changing World, Brisbane1 - 6 August 2010, Australia, pp. 129–132.
Skinner MF, Lowe AT, Nicholson GM, Prince J. 1991. Availability of phosphorus in New Zealand forest soils. A new approach with the Bray reagent. In: R.E. White and L.D. Currie (eds), Soil and Plant Testing for Nutrient Deficiencies and Toxicities. Occasional report No. 5. Palmerston North, New Zealand: Fertiliser and Lime Research Centre, Massey University, pp. 143–147.
Skinner MF, Payn TW. 1993. Nutritional requirements of plantation forests in New Zealand. In: Issues challenging the Fertiliser Industry. Proceedings of the New Zealand Fertiliser Manufacturers’ Research Association 22ndTechnical Conference, Dunedin 9-11 November 1993, pp. 130–142.
Smith CT, Lowe AT, Beets PN. 1994. Nutrient accumulation in second-rotation Pinus radiata after harvest residue management and fertiliser treatment of coastal sand dunes. New Zealand Journal of Forestry Science, 24: 362–389.
Snowdon P. 2002. Growth of Pinus elliottii, P. pinaster and P. radiata on coastal dune soils near Jervis Bay, Australian Capital Territory. Australian Forestry, 66: 161–169.
Syers JK, Evans TD, Williams JDH, Murdoch JT. 1971. Phosphate sorption parameters of representative soils from Rio Grande do Sul, Brazil. Soil Science, 112: 267–275.
Tappeiner JC, Alm AA. 1975. Undergrowth vegetation effects on the nutrient content of litterfall and soils in red pine and birch stands in Northern Minnesota. Ecology, 56: 1193–1200.
Tarafdar JC, Claassen N. 1988. Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatase produced by plant roots and microorganisms. Biology and Fertility of Soils, 5: 308–312.
Tiessen H, Stewart JWB, Moir JO. 1983. Changes in organic and inorganic phosphorus composition of two grassland soils and their particle size fractions during 60-70 years of cultivation. Journal of Soil Science, 34: 815–823.
Toal ME, Yeomans C, Killham K, Meharg AA. 2000. A review of rhizosphere carbon flow modelling. Plant and Soil, 222: 263-281.
Trolove SN, Hedley M J, Kirk GJD, Bolan NS, Loganathan P. 2003. Progress in selected areas of rhizosphere research on P acquisition. Australian Journal of Soil Research, 41: 471–499.
Turner J, Lambert MJ. 1985. Soil phosphorus forms and related tree growth in a long term Pinus radiata phosphate fertiliser trial. Communication in Soil Science and Plant Analysis, 16: 275–288.
Turner J, Lambert MJ. 1986. Effects of forest harvesting nutrient removals on soil nutrient reserves. Oecologia, 70: 140–148.
Vance CP, Uhde-stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations for securing a non renewable resource. New Phytologist, 157: 423–447.
Wagner RG, Little KM, Richardson B, McNabb K. 2006. The role of vegetation management for enhancing productivity of the world’s forest. Forestry, 79: 57–79.
Walker TW, Syers JK. 1976. The fate of phosphorus during pedogenesis. Geoderma, 15: 1–19.
Watt MS, Whitehead D, Mason EG, Richardson B, Kimberley MO. 2003a. The influence of weed competition for light and water on growth and dry matter partitioning of young Pinus radiata, at a dryland site. Forest Ecology and Management, 183: 363–376.
Watt MS, Clinton PW, Whitehead D, Richardson B, Mason EG, Leckie AC. 2003b. Above-ground biomass accumulation and nitrogen fixation of broom (Cytisus scoparius L.) growing with juvenile Pinus radiata on a dryland site. Forest Ecology and Management, 184: 93–104.
Watt MS, Clinton PC, Parfitt RL, Ross C, Coker G. 2009. Modelling the influence of site and weed competition on juvenile modulus of elasticity in Pinus radiata across broad environmental gradients. Forest Ecology and Management, 258: 1479–1488.
Watt M, Palmer DJ, Kimberley MO, Hock BK, Payn TW, Lowe DJ. 2010. Development of models to predict Pinus radiata productivity throughout New Zealand. Canadian Journal of Forest Research, 40: 488–499.
Webber B, Madgwick HAI. 1983. Biomass and nutrient content of a 29-year-old Pinus radiata stand. New Zealand Journal of Forestry Science, 13: 222–228.
Will GM. 1959. Nutrient return in litter and rainfall under some exotic conifer stands in New Zealand. New Zealand Journal of Agricultural Research, 2: 719–734.
Will GM. 1968. The uptake, cycling and removal of mineral nutrients by crops of Pinus radiata. Proceedings of the New Zealand Ecological Society, 15: 20–24.
Zabowski D, Skinner MF, Payn TW. 2007. Nutrient release by weathering: implications for sustainable harvesting of Pinus radiata in New Zealand soils. New Zealand Journal of Forestry Science, 37(3): 336–354.
Zas R, Serrada R. 2003. Foliar nutrient status and nutritional relationships of young Pinus radiata D. Don plantations in northwest Spain. Forest Ecology and Management, 174: 167–176.
Zhang F, Shen J, Li L, Liu X. 2004. An overview of rhizosphere processes related with plant nutrition in major cropping systems in China. Plant and Soil, 260: 89–99.
Zou X, Binkley D, Caldwell BA. 1995. Effects of dinitrogen-fixing trees on phosphorus biogeochemical cycling in contrasting forests. Soil Science of American Journal, 59: 1452–1458.
杂志排行
Journal of Forestry Research的其它文章
- Effects of gaps on regeneration of woody plants: a meta-analysis
- Changes induced by osmotic stress in the morphology, biochemistry, physiology, anatomy and stomatal parameters of almond species (Prunus L. spp.) grown in vitro
- Characterization of expressed genes in the establishment of arbuscular mycorrhiza between Amorpha fruticosa and Glomus mosseae
- Dependences of longleaf pine (Pinus palustris) natural reproduction on environments above ground
- Phytosociology, structure and dynamics of Pinus roxburghii associations from Northern Pakistan
- A modified Murashige and Skoog media for efficient multipleshoot induction in G. arborea Roxb.
