酸性硫酸盐土的形成、 特性及其生态环境效应
2014-04-09黄巧义唐拴虎张发宝杨少海
黄巧义, 唐拴虎, 卢 瑛, 张发宝, 杨少海
(1 广东省农业科学院农业资源与环境研究所,农业部南方植物营养与肥料重点实验室,广东省养分资源循环利用与耕地保育重点实验室,广州 510640; 2 华南农业大学资源环境学院,广州 510642)
1 ASS的形成条件和过程
ASS形成过程中的铁(Fe)、 硫(S)生物地球化学循环,在全球物质循环过程中具有重要地位,一直是人们关注的热点[17]。ASS的成土母质为富含还原性硫化物的沉淀物,当硫化物被氧化后形成硫酸及一系列次生铁矿物。ASS成土过程中包含 还原性硫化物(硫化铁为主)沉淀物成土母质的形成,以及成土母质的氧化两个关键阶段[1, 18]。发育完全的ASS土壤剖面上,常呈现两种性质截然不同的土层, 下层富含黄铁矿的中性土壤(还原环境),也称潜在酸性硫酸盐土(Potential Acid Sulfate Soils, PASS),上层富含黄铁矿氧化产物的酸性土壤(氧化环境),也称实际酸性硫酸盐土(Actual Acid Sulfate Soils, AASS)[18-19]。
1.1 ASS成土母质的形成条件与过程


Fe2++H2S→FeS+2H+
[1]
Fe2++2HS→Fe(HS)2→FeS+H2S
[2]
FeS1.1(代表亚稳定态硫化亚铁矿物)+H2S→FeS2+H2(完全厌氧条件)
[3]
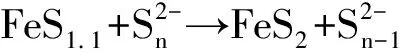
[4]
1.2 ASS成土母质的氧化
形成于还原条件的ASS成土母质,因自然条件变化或者人为干扰等影响,使其环境氧化还原电位提高,ASS成土母质被氧化而形成ASS[1, 33]。例如,北欧地区因均衡性地壳上升及农用埋管排水的综合作用使全新世形成的富含还原态Fe-S矿物的成土母质被氧化形成了ASS[34];泰国曼谷平原则因海岸线的变迁而形成带状ASS[6]。而干旱是导致塞尔维亚、 几内亚(比绍)等非洲国家ASS发育形成的主要因素[6]。
ASS成土母质接触氧气后,还原态硫化铁类矿物[亚稳定态硫化亚铁矿物(FeS1.1)和黄铁矿(FeS2)]发生氧化反应,其反应式为5和6[32, 34]。FeS1.1的反应动力学较快,而黄铁矿慢[34]。溶解态O2和Fe3+是该反应主要的氧化剂,最初氧化产物为元素硫(S0)[32, 35-36]。FeS1.1和FeS2被氧化的过程中,形成多种形态硫、 铁矿物,并产生硫酸、 H+,最终形成酸性极强、 生长障碍因素多及生态危害大的ASS[18, 34, 37]。
10FeS1.1(代表亚稳定态硫化亚铁矿物)+24O2+26H2O→10Fe(OH)3+11H2SO4
[5]
4FeS2+15O2+14H2O→4Fe(OH)3+8H2SO4
[6]
1.3 红树林与ASS
2 ASS中硫的演变
2.1 ASS中硫矿物的动态变化
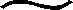
4FeS+3O2+6H2O→4Fe(OH)3+4S0
[7](PAS)
FeS+S0→FeS2
[8](PAS)
[9](PASTZ)
[10](ASTZ)
[11](AS)
[12](AS)
CaCO3+H2SO4+H2O→CaSO4·2H2O+CO2
[13](AS)
PASS的氧化还原电位低,只有热力学稳定性较差的FeS1.1能被氧化,生成氢氧化铁和元素硫S0,尚无酸形成,土壤pH仍较高,反应式为7[46]。在该pH条件下,元素硫S0又能与土壤中残余的FeS1.1反应,形成黄铁矿(反应式8)[29, 47]。接近PASS的TZ区域,氧化还原电位仍较低,只有少量黄铁矿被完全氧化(反应式9),生成硫酸根及氢氧化铁,但该反应速度缓慢,不能使土体酸化[48-50]。而接近上层AASS的TZ区域,土壤pH下降到4.5以下,Fe3+溶解度提高,成为黄铁矿氧化反应的主要氧化剂,进一步加快黄铁矿氧化(反应式10)[32, 50]。同时,在该pH条件下,嗜酸氧化亚铁硫杆菌催化Fe2+氧化形成Fe3+,加快催化该氧化反应;但当pH下降到3.5以下时,大部分释放出的Fe3+被水结合,形成氢氧化铁沉淀[3, 51]。AASS中黄铁矿氧化产生大量硫酸,但大部分被淋溶出土体,当土壤进一步酸化(pH低于4.0),硫酸根与Fe、 K、 Na等元素形成黄钾铁矾[KFe3(SO4)2(OH)6][52]和施氏矿物[Fe8O8(OH)6SO4][53],尤以黄钾铁矾最普遍,其形成过程为反应式11[52]。黄钾铁矾和施氏矿物最终被水化形成针铁矿(FeOOH),同时释放出硫酸和酸(反应式12)[53-55]。开垦耕种的ASS土壤上,常表施石灰,能中和土壤部分酸并生成石膏(反应式13)[32]。
2.2 ASS中各种硫形态含量
3 ASS中铁(Fe)的地球化学动态
3.1 ASS中铁矿物的转化

[14]
FeS1.1(s)+H2S(aq)→FeS2(s)+H2(g)
[15]
FeS1.1(s)+Sn+1(aq)2-→FeS2(s)+Sn(aq)2-
[16]
[17]
Fe8O8(OH)6SO4+2.5H2O→8FeOOH+
[18]
[19]
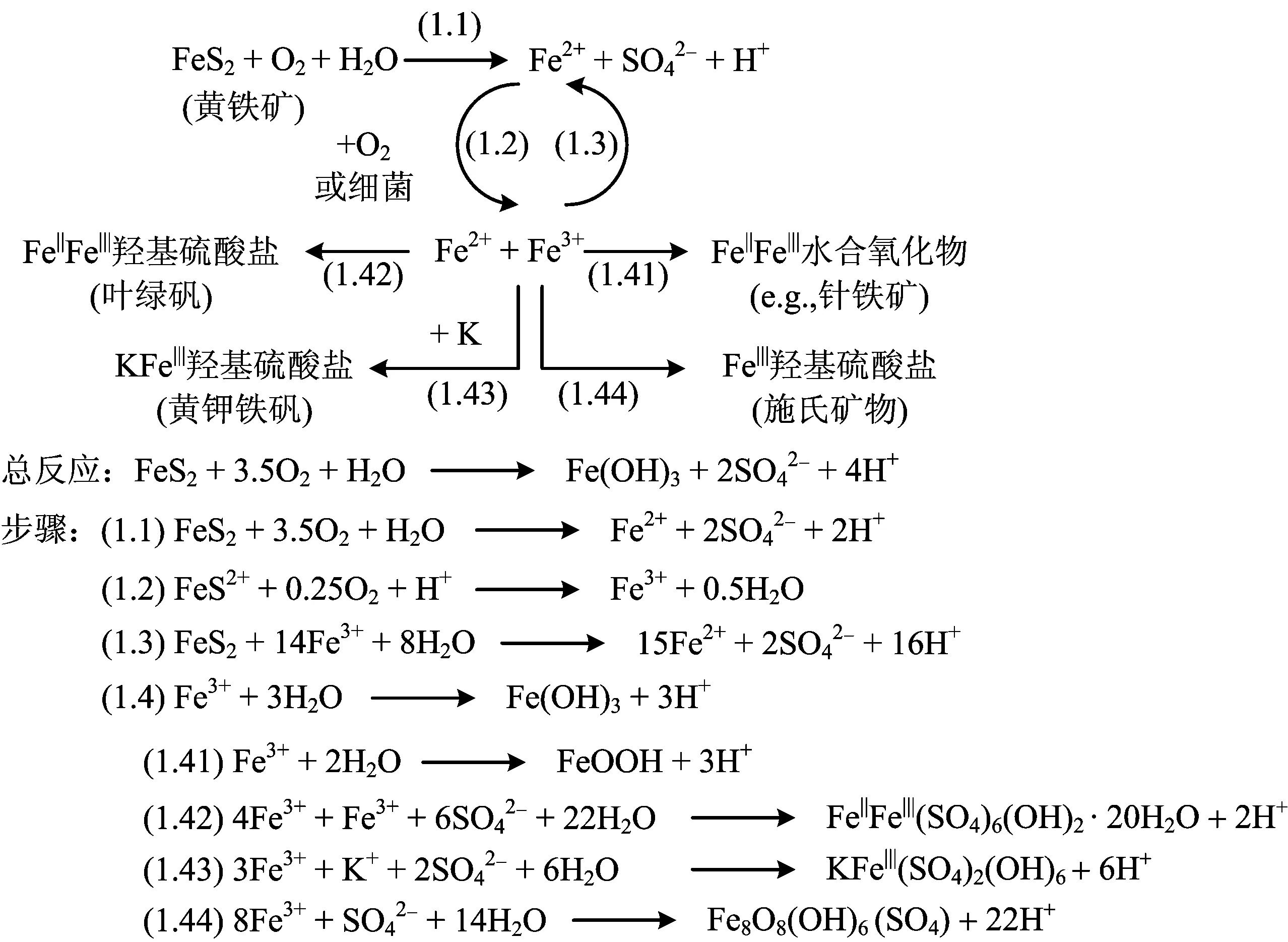
图1 黄铁矿氧化途径及可能产物Fig.1 Steps in pyrite oxidation and possible secondary Fe minerals that may form as weathering products
3.2 ASS中各种铁矿物含量
4 ASS的酸特性
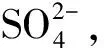
5 ASS的生态环境效应

淹水还原环境下,由于存在硫化物、 有机物质,有利于ASS形成金属硫化物和金属含量较高的有机复合物,将游离的金属固定下来,实现海水净化[86-87]。在氧化环境下,ASS严重酸化,Al、 Cd、 Mn、 Ni、 As等有毒金属及类金属大量活化[88-93],另一方面,P、 Ca、 Mg、 Zn、 Cu、 K、 B等生物生长必需营养元素被固定或流失[58, 94-96],严重毒害实地植物、 动物生长。同时,酸根离子和有毒金属及类金属随水经渗漏、 侧流等途径进入周边水系,酸化污染周边水体环境[15, 97-99],及水系周边生态系统[73, 100]。另外,淹水环境中因潮汐、 作物根系作用以及生物扰动等影响,也会发生氧化反应,使还原性硫化物发生氧化,释放出酸根离子和金属或类金属,进而污染海水环境、 危害滨海生物[101-104]。因此,ASS生态系统在不同环境条件扮演着环境金属和类金属净化者和污染者的双重角色,而从土壤学家的角度出发,ASS主要是环境污染源。
5.1 ASS中有毒金属和类金属的活性及流失风险
ASS产生的酸性离子以及活化的有毒金属和类金属随地表径流、 侧渗、 淋溶等途径进入地下水、 周边河流,污染水体质量[11, 51, 94, 97, 100, 108-109]。ASS影响周边水体生态,一方面因水体本身受污染,导致水生生物生长受害,影响水产养殖[13, 78, 97],且以受污染水灌溉、 饮用等用途的农户,其身体健康也将受到威胁[6, 7, 110];另一方面,污染水的流动、 利用,进一步将污染扩大到周边环境,加上金属的移动性及生物累积效应,致使ASS的污染区域不断扩大。因此,ASS作为一个潜在或现有的环境隐患,若开发不当将引发严重的生态环境问题[6]。
ASS导致的Fe、 Al、 Mn、 Cd等重金属的污染已有目共睹[13, 89, 90, 111-112],而且有些改良措施虽然中和了土壤酸性且固定Al、 Mn、 Ni和Zn等潜在的有毒金属,但却导致As和Fe的活化[13, 113]。同时,Fe-S矿物对痕量元素的生物有效性影响显著,已有研究表明,ASS中Fe矿物的转化影响土壤中稀土元素的含量及分布[114],同时,受ASS影响的江河沉淀物中,痕量元素的含量及活性明显较高[115-116]。
5.2 ASS对实地及周边生物的影响
ASS对植物生长的毒害不仅因低pH的直接影响,还与Al、 Fe等金属离子活化的毒害作用有关[117],同时,P、 Ca、 Mg、 Zn、 Cu、 K、 B等营养元素有效性低、 土壤结构差等因素均限制了植物的生长[6, 118]。我国受ASS影响的水稻产量远低于全国平均水平,印度尼西亚、 泰国、 几内亚比绍、 斯里兰卡等国家ASS稻区的产量也较低[6]。更有甚者,因长期酸害影响,植被无法生存,土表裸露[119]。据调查,ASS上牧草、 橡树的Co、 Ni、 Mn含量均较常规值高[97, 120]。但Fältmarsch等发现ASS中重金属淋溶流失的比例较大,仅少量被土壤上生长的包菜吸收,因此,重金属对实地植物的影响相对较小[121]。有研究表明,受ASS影响的奶牛的牛奶中Al含量明显偏高,但动物体内金属含量与ASS的关系尚不清晰[97]。
受ASS影响的水域中水生苔藓体内Al、 Cu、 Fe含量显著高于常规值[97]。澳大利亚东部受ASS影响的流域常发生大规模鱼类死亡事件,其原因主要归结于强酸和金属离子含量高[122]。另一方面,鱼类蛋黄、 卵子形成和产卵的过程受到该恶劣环境干扰而导致繁殖失败,使部分鱼类绝种灭亡[122],同时,离子调节系统和器官呼吸作用受到破坏使鱼类行为失常[97]。Faltmarsch 等[97]认为ASS可能还影响区域的老人痴呆症、 帕金森等神经性疾病和心血管疾病的发生率。有学者对澳大利亚ASS土地上的房地产开发后的表土、 粉尘及水样调查表明,其金属含量及活性均在生态临界值以内[7]。Hinwood 等[110]调查ASS区域居民尿液、 脚趾甲及头发中重金属累积情况,结果表明Al、 As、 Cd、 Pb、 Cu及Zn的含量并不高。ASS对人类身体健康的影响已逐渐引起学者的关注,但目前相关证据尚不确凿。
6 展望
1) ASS中Fe-S演变、 循环一直是人们关注的热点。因沉积微环境下各种条件的不同,黄铁矿形成途径、 形态不一致,不同来源、 形态的黄铁矿发育的过程也有所差异。我国前期学者对发育于红树林景观的ASS研究较多,忽视了非红树林海滨沼泽地带Fe-S矿物的形成和累积,亟需加强该方面探讨,以更好规划该问题土壤。该方面应进一步探讨ASS发育过程中不同来源、 形态的黄铁矿对土壤各种理化性状的影响。
2) ASS的形成及发育过程中,Fe-S矿物的转化显著影响着其他金属元素的固定、 活化、 溶解等一系列地球化学过程。其中黄铁矿形成过程中重金属的富集、 黄铁矿氧化过程中重金属的活化是国内外研究热点。近些年,国外有学者开始关注ASS对稀土元素、 痕量元素的影响,但国内尚无相关报道。
3) 尽管我国ASS的面积高达0.11 M hm2,但是从学术界到社会上,对其了解、 认识仍处于表面的危害性。曾有部分学者进行过探讨,但均没有深入到机理层面上,而社会上对该类土壤概念模糊,只注重其生产应用,忽视了其潜在的环境危害、 及产品安全性的考量。在我国大量的ASS被改造成水稻田,采用适当的改良、 排水措施能保证相当的产量收成,被认为是ASS利用的有效途径。然而,ASS开垦种稻后,相应的农艺措施对土壤酸、 重金属的影响如何,其对周边河流、 环境、 居民的影响如何,甚至稻谷的安全性均有待进一步评估。
参考文献:
[1] Dent D L, Pons L J. A world perspective on acid sulphate soils[J]. Geoderma, 1995, 67:263-276.
[2] IUSS Working Group WRB. World reference base for soil resources 2006[M]. Rome, Italy:World Soil Resources Reports No. 103. FAO, 2006.
[3] Österholm P, Åström M. Spatial trends and losses of major and trace elements in agricultural acid sulphate soils distributed in the artificially drained Rintala area, W. Finland[J]. Applied Geochemistry, 2002, 17(9):1209-1218.
[4] 章家恩. 酸性硫酸盐土的景观诊断及时空分布与演替模式[J]. 土壤与环境, 1999, 8(2):144-147.
Zhang J E. The landscape diagnosis and their spatial and temporal patterns of distribution and succession of acid sulphate soils[J]. Soil and Environmental Sciences,1999, 8(2):144-147
[5] Andriesse W, van Mensvoort M E F. Acid sulfate soils:distribution and extent[A]. Lal R. Encyclopedia of Soil Science[M]:Boca Raton:CRC Press, 2006. 14-19.
[6] Ljung K, Maley F, Cook Aetal. Acid sulfate soils and human health—a millennium ecosystem assessment[J]. Environment International, 2009, 35:1234-1242.
[7] Ljung K, Maley F, Cooka A. Canal estate development in an acid sulfate soil implications for human metal exposure[J]. Landscape and Urban Planning, 2010, 97:123-131.
[8] 章家恩. 酸性硫酸盐土的酸害暴发机制及其环境影响[J]. 热带地理, 1999, 19(2):137-141.
Zhang J E. The process of acid hazards in acid sulfate soils and its environmental effects[J]. Tropical Geography,1999, 19(2):137-141
[9] Gröger J, Proske U, Hanebuth T J Jetal. Cycling of trace metals and rare earth elements (REE) in acid sulfate soils in the plain of reeds, vietnam[J]. Chemical Geology, 2011, 288:162-177.
[10] Lin C. Acid sulfate soils in Australia:characteristics, problem and management[J]. Pedosphere, 1999, 9:289-298.
[11] Nyberg M E, Österholm P, Nystrand M I. Impact of acid sulfate soils on the geochemistry of rivers in south-western Finland[J]. Environment Earth Science, 2012, 66:157-168.
[12] Smith J, van Oploo P, Marston Hetal. Spatial distribution and management of total actual acidity in an acid sulfate soil environment, McLeods Creek, northeastern NSW, Australia[J]. Catena, 2003, 51:61-79.
[13] Toivonen J, Österholm P. Characterization of acid sulfate soils and assessing their impact on a humic boreal lake[J]. Journal of Geochemical Exploration, 2011, 110:107-117.
[14] Lin C. Could acid sulfate soils be a potential environmental threat to estuarine ecosystems on the south China coast?[J]. Pedosphere, 1999, 9(1):53-59.
[15] Lin C. Analytical methods for environmental risk assessment of acid sulfate soils:A review[J]. Pedosphere, 2001, 11(4):301-310.
[16] 章家恩, 骆世明, 王建武. 酸性硫酸土研究现状与发展趋向[J]. 热带亚热带土壤科学, 1998, 7(4):309-313.
Zhang J E, Luo S M, Wang J W. The present situation and trends in the research of acid sulphate soils[J]. Tropical and Subtropical Soil Sciencs, 1998, 7(4):309-313
[17] Zopfi J, Bottcher M E, Jφrgensen B B. Biogeochemistry of sulfur and iron in Thioploca-colonized surface sediments in the upwelling area of central Chile[J]. Geochimica et Cosmochimica Acta, 2008, 72(3):827-843.
[18] Fanning D S, Rabenhorst M C, Balduff D Metal. An acid sulfate perspective on landscape seascape soil mineralogy in the U.S. Mid-Atlantic region[J]. Geoderma, 2010, 154:457-464.
[19] Wilson B P. Elevations of sulfurous layers in acid sulfate soils:What do they indicate about sea levels during the Holocene in eastern Australia?[J]. Gatena, 2005, 62:45-46.
[20] Keene A F, Johnston S G, Bush R Tetal. Effects of hyper-enriched reactive Fe on sulfidisation in a tidally inundated acid sulfate soil wetland[J]. Biogeochemistry, 2011, 103:263-279.
[21] Berner R A. Sedimentary pyrite formation[J]. American Journal of Science, 1970, 268:1-23.
[22] Wolthers M, Van Der Gaast S J, Rickard D. The structure of disordered mackinawite[J]. American Mineralogist, 2003, 88:2007-2015.
[23] Rickard D, Griffith A, Oldroyd Aetal. The composition of nanoparticulate mackinawite, tetragonal iron(II) monosulfide[J]. Chemical Geology, 2006, 235(3-4):286-298.
[24] Rickard D. Kinetics of Fe-S precipitation:Part 1. Competing reaction mechanisms[J]. Geochimica et Cosmochimica Acta, 1995, 59(21):4367-4379.
[25] Richard D, Luther G W. Chemistry of iron sulfides[J]. Chemical Reviews, 2007, 107(2):514-562.
[26] Rickard D, Morse J W. Acid volatile sulfide (AVS)[J]. Marine Chemistry, 2005, 97(3-4):141-197.
[27] Morse J W, Rickard D. Chemical dynamics of sedimentary acid volatile sulfide[J]. Environmental Science & Technology, 2004, 38:131A-136A.
[28] Morse J W, Cornwell J C. Analysis and distribution of iron sulfide minerals in recent anoxic marine sediments[J]. Marine Chemistry, 1987, 22:55-69.
[29] Butle I B, Böttcher M E, Rickard Detal. Sulfur isotope partitioning during experimental formation of pyrite via the polysulfide and hydrogen sulfide pathways:implications for the interpretation of sedimentary and hydrothermal pyrite isotope records[J]. Earth and Planetary Science Letters, 2004, 228(3-4):495-509.
[30] Rickard D. Kinetics of pyrite formation by the H2S oxidation of iron (II) monosulfide in aqueous solutions between 25 and 125℃:the rate equation[J]. Geochimica et Cosmochimica Acta, 1997, 61(1):115-134.
[31] Rickard D, Luther G W. Kinetics of pyrite formation by the H2S oxidation of iron (II) monosulfide in aqueous solutions between 25 and 125℃:the mechanism[J]. Geochimica et Cosmochimica Acta, 1997, 61(1):135-147.
[32] Boman A, Åström M, Frojdo S. Sulfur dynamics in boreal acid sulfate soils rich in metastable iron sulfide-The role of artificial drainage[J]. Chemical Geology, 2008, 255:68-77.
[33] Nelson V G. Formation of acid sulfate soil and its implications to brackishwater ponds[J]. Aquacultural Engineering, 1995, 44(4):297-316.
[34] Boman A, Frojdo S, Backlund Ketal. Impact of isostatic land uplift and artificial drainage on oxidation of brackish-water sediments rich in metastable iron sulfidemain[J]. Geochimica et Cosmochimica Acta, 2010, 74:1268-1281.
[35] Silverman M P. Mechanism of bacterial pyrite oxidation[J]. Journal of Bacteriology, 1967, 94(4):1046-1051.
[36] Ward N J, Sullivan L A, Fyfe D Metal. The process of sulfide oxidation in some acid sulfate soil materials[J]. Australian Journal of Soil Research, 2004, 42(4):449-458.
[37] Le T M H, Collins R N, Waite T D. Influence of metal ions and pH on the hydraulic properties of potential acid sulfate soils[J]. Journal of Hydrology, 2008, 356:261-270.
[38] 杨萍如, 何金海, 刘腾辉. 红树林及其土壤[J]. 自然资源学报, 1987, 2(1):32-37.
Yang P R, He J H, Liu T H. Mangrove and its soil[J]. Journal of Natural Resources, 1987, 2(1):32-37.
[39] Lin C, Melville M D. Mangrove soil:a potential contamination source to estuarine ecosystems of Australia[J]. Wetlands, 1992, 11:68-75.
[40] 林慧娜, 傅娇艳, 吴浩, 等. 中国主要红树林湿地沉积物中硫的分布特征及影响因素[J]. 海洋科学, 2009, 33(12):79-82.
Lin H N, Fu J Y, Wu Hetal. Distribution and influential factors of sulfur in mangrove wet-lands in China[J]. Marine Sciences, 2009, 33(12):79-82.
[41] 龚子同, 张效朴. 中国的红树林与酸性硫酸盐土[J]. 土壤学报, 1994, 31(1):86-93.
Gong Z T, Zhang X P. Mangroves and acid sulfate soils in China[J]. Acta Pedologica Sinica, 1994, 31(1):86-93.
[42] 黄宇年, 陆发憙. 广东咸酸田土壤硫化学研究[J]. 土壤学报, 1988, 25(2):101-109.
Huang Y N, Lu F X. A study on the sulfur chemistry of acid sulfate paddy soils of Guangdong Province[J]. Acta Pedologica Sinica, 1988, 25(2):101-109.
[43] 廖家隆, 姚素平, 丁海. 滨海红树林泥炭沉积物中硫的赋存特点及其控制因素[J]. 高校地质学报, 2008, 14(4):620-630.
Liao J L, Yao S P, Ding H. The characteristics and controlling factors of sulfur in the sediments of coastal Mangrove peat[J]. Geological Journal of China Universities, 2008, 14(4):620-630.
[44] 张汝国. 珠江口红树林硫的累积和循环研究[J]. 热带亚热带土壤科学, 1996, 5(2):67-73.
Zhang R G. Study on sulphur accumulation and cycling in mangrove forest in Pear River mouth[J]. Tropical and Subtropical Soil Science, 1996, 5(2):67-73
[45] 桑树勋, 刘焕杰, 施健. 海南岛红树林泥炭中硫及其成因研究[J]. 煤田地质与勘探, 1993, 21(1):12-17.
Sang S X, Liu H J, Shi J. Study of sulphur and its genesis in mangrove peats of Hainan Island[J].Coal Geology & Exploration, 1993, 21(1):12-17.
[46] Burton E D, Bush R T, Sullivan L A. Reduced inorganic sulfur speciation in drain sediments from acid sulfate soil landscapes[J]. Environmental Science & Technology, 2006, 40(3):888-893.
[47] Bush R T. Micromorphology and mineralogy of iron sulfides in acid sulphate soils:Their formation and behaviour[D]. New South Wales:PhD dissertation University of New South Wales, 2001.
[48] Bloomfield C. Acidification and Ochre formation in Pyritic soil [A]. Dost H. Proceedings of the international symposium on acid sulphate soil[C]:Wageningen, The Netherlands:ILRI Publication, 1973, 2:40-51.
[49] Evangelou V P, Zhang Y L. A review:Pyrite oxidation mechanisms and acid mine drainage prevention[J]. Critical Reviews in Environmental Science and Technology, 1995, 25(2):141-199.
[50] Nordstrom D L. Aqueous pyrite oxidation and the consequent formation of secondary iron minerals[A]. Kittrick J A, Fanning D S, Hosner L R. Acid sulfate weathering[M]. Madison:Soil Science Society of America Special Publication, 1982. 37-56.
[51] Sohlenius G, Öborn I. Geochemistry and partitioning of trace metals in acid sulphate soils in Sweden and Finland before and after sulphide oxidation[J]. Geoderma, 2004, 122(2-4):167-175.
[52] Breemen N V. Genesis, morphology, and classification of acid sulfate soils in coastal plains[A]. Kittrick J A, Fanning D S, Hosner L R. Acid sulfate weathering[M]. Madison:Soil Science Society of America Special Publication, 1982, 95-108.
[53] Sullivan L A, Bush R T. Iron precipitate accumulations associated with waterways in drained coastal acid sulfate landscapes of eastern Australia[J]. Marine and Freshwater Research, 2004, 55(7):727-736.
[54] Burton E D, Bush R T, Sullivan L A. Sedimentary iron geochemistry in acidic waterways associated with coastal lowland acid sulfate soils[J]. Geochimica et Cosmochimica Acta, 2006, 70:5455-5468.
[55] Burton E D, Bush R T, Sullivan L Aetal. Reductive transformation of iron and sulfur in schwertmannite-rich accumulations associated with acidified coastal lowlands[J]. Geochimica et Cosmochimica Acta, 2007, 71:4456-4473.
[56] Backlund K, Boman A, Fröjdö S. An analytical procedure determination of sulphur species and isotopes in boreal acid sulphate soils sediments[J]. Agricultural and Food Science, 2005, 14:70-82.
[57] Breemen N V. Soil forming processes in acid sulphate soils [A]. Drost H. Acid sulphate soil[M]. Wageningen, The Netherlands:International Institute for land Reclamation and Improvement, 1973, 18:66-130.
[58] Hartikainen H, Yli-Halla M. Oxidation-induced leaching of sulphate and cation from acid sulphate soils[J]. Water, Air, and Soil Pollution, 1986, 27:1-3.
[59] 刘振乾, 王建武, 骆世明, 等. 酸性硫酸盐土中硫形态转化过程的水分制约作用[J]. 生态科学, 2002, 21(1):68-71.
Liu Z Q, Wang J W, Luo S Metal. Effects of moisture on the transformation of sulfur forms in acid sulfate sois[J]. Ecology Science, 2002, 21(1):68-71.
[60] 王建武, 骆世明. 酸性硫酸盐土中硫的形态含量分布和酸化特征[J]. 应用生态学报, 1999, 10(5):583-588.
Wang J W, Luo S M. Characteristics of sulfur forms content distribution and its acidification of acid sulfate soils[J]. Chinese Journal of Applied Ecology, 1999, 10(5):583-588.
[61] 钟继洪. 酸性硫酸盐土中的硫及其形态演变探讨[J]. 生态学杂志, 2003, 22(5):98-101.
Zhong J H. Succession of sulfur forms in acid sulfate soils[J]. Chinese Journal of Ecology, 2003, 22(5):98-101.
[62] Claff S R, Sullivan L A, Burton E Detal. Partitioning of metals in a degraded acid sulfate soil landscape, influence of tidal[J]. Chemosphere, 2011, 85:1220-1226.
[63] Claff S R, Burton E D, Sullivan L Aetal. Metal partitioning dynamics during the oxidation and acidification of sulfidic soil[J]. Chemical Geology, 2011, 286:146-157.
[64] Bush R T, Mcgrath R, Sullivan L A. Occurrence of marcasite in an organic-rich Holocene estuarine mud[J]. Soil Research, 2003, 42(6):617-621.
[65] Smith J, Melville M D. Iron monosulfide formation and oxidation in drain-bottom sediments of an acid sulfate soil environment[J]. Applied Geochemistry, 2004, 19:1837-1853.
[66] Luther G W. Pyrite synthesis via polysulfide compounds[J]. Geochimica et Cosmochimica Acta, 1991, 55(10):2839-2849.
[67] Bigham J M, Nordstrom D K. Iron and aluminum hydroxysulfates from acid sulfate waters[J]. Reviews in Mineralogy and Geochemistry, 2000, 40:351-403.
[68] Johnston S G, Keene A F, Bush R Tetal. Iron geochemical zonation in a tidally inundated acid sulfate soil wetland[J]. Chemical Geology, 2011, 280:257-270.
[69] Acero P, Ayora C, Torrento Cetal. The behavior of trace elements during schwertmannite precipitation and subsequent transformation into goethite and jarosite[J]. Geochimica et Cosmochimica Acta, 2006, 70:4130-4139.
[70] Madden M E E, Madden A S, Rimstidt J Detal. Jarosite dissolution rates and nanoscale mineralogy[J]. Geochimica et Cosmochimica Acta, 2012, 91:306-321.
[71] Claff S R, Sullivan L A, Burton E Detal. A sequential extraction procedure for acid sulfate soils, partitioning of iron[J]. Geoderma, 2010, 155:224-230.
[72] Smith A M L, Hudson-Edwards K A, Dubbin W Eetal. Dissolution of jarosite [KFe3(SO4)2(OH)6] at pH 2 and 8:Insights from batch experiments and computational modelling[J]. Geochimica et Cosmochimica Acta, 2006, 3(70):608-621.
[73] Lin C, Islam M M, Bush R Tetal. Acid release from an acid sulfate soil sample under successive extractions with different extractants[J]. Pedosphere, 2000, 10(3):221-228.
[74] Joukainen S, Yli-Halla M. Environmental impacts and acid loads from deep sulfidic layer of two well-drained acid sulfate soils in western Finland[J]. Agriculture, Ecosystems & Environment, 2003, 95:297-309.
[75] 刘兆辉, 王遵亲. 我国滨海酸性硫酸盐土壤中几种不同形态的酸[J]. 土壤学报, 1992, 29(4):401-407.
Liu Z H, Wang Z Q. Some acid forms in acid sulfate soils in coastal region of China[J]. Acta Pedologica Sinica, 1992, 29(4):401-407.
[76] 刘振乾, 王建武, 骆世明, 等. 酸性硫酸盐土酸消长的水动力机制研究[J]. 土壤学报, 2002, 39(5):726-734.
Liu Z Q, Wang J W, Luo S Metal. Hydrodynamic mechanism on the change of different forms of acid in acid sulfate soil[J]. Acta Pedologica Sinica, 2002, 39(5):726-734.
[77] Hicks W, Bowman G R F. Environmental impacts of acid sulfate soils near Cairns, QLD[J]. CSIRO Land Water, 1999, 8.
[78] Powell B, Martens M. A review of acid sulfate soil impacts, actions and policies that impact on water quality in the Great Barrier Reef catchments, including a case study on remediation at East Trinity[J]. Marine Pollution Bulletin, 2005, 51:149-164.
[79] Lin C, Lancaster G, Sullivan L Aetal. Actual acidity and its assessment in acid sulfate soils [A]. Slavich P. Proceedings of workshop on assessment and remediation of acid sulfate soils[C]. Lismore, Australia:Acid Sulfate Soil Management Advisory Committee, 2000.
[80] Lin C, Melville M D, Valetine N. Characteristics of soluble and exchangeable acidity in an extremely acidified acid sulfate soil[J]. Pedosphere, 1999, 9(4):323-330.
[81] Lin C, O'Brien K, Lancaster Getal. An improved analytical procedure for determination of total actual acidity in acid sulfate soils[J]. Science of The Total Environment, 2000, 252:57-61.
[82] 林初夏, 吴志峰. 酸性硫酸盐土的酸度类型及其测定方法[J]. 生态环境, 2003, 12(4):505-507.
Lin C X, Wu Z F. Forms of acidity in acid sulfate soils and their determinations[J]. Ecology and Environment, 2003, 12(4):505-507.
[83] Sherman R E, Fahey T J, Battles J J. Small-scale disturbance and regeneration dynamics in a neotropical mangrove forest[J]. Journal of Ecology, 2000, 88(1):165-178.
[84] Dittmar T, Hertkorn N, Kattner Getal. Mangroves, a major source of dissolved organic carbon to the oceans[J]. Global Biogeochemical Cycles, 2006, 20(1):B1012.
[85] 林鹏. 中国红树林研究进展[J]. 厦门大学学报, 2001, 40(2):592-603.
Lin P. A review on the mangrove research in China[J]. Journal of Xiamen University, 2001, 40(2):592-603.
[86] Zhou Y W, Zhao B, Peng Y Setal. Influence of mangrove reforestation on heavy metal accumulation and speciation in intertidal sediments[J]. Marine Pollution Bulletin, 2010, 60:1319-1324.
[87] Nath B, Birch G, Chaudhuri P. Trace metal biogeochemistry in mangrove ecosystems:A comparative assessment of acidified (by acid sulfate soils) and non-acidified sites[J]. Science of The Total Environment, 2013:463-464, 667-674.
[88] Minh L Q, Tuong T P, van Mensvoort M E Fetal. Contamination of surface water as affected by land use in acid sulfate soils in the Mekong River Delta, Vietnam[J]. Agriculture, Ecosystems & Environment, 1997, 61:19-27.
[89] Minh L Q, Tuong T P, van Mensvoort M E Fetal. Aluminum contaminant transport by surface runoff and bypass flow from an acid sulphate soil[J]. Agricultural Water Management, 2002, 56:179-191.
[90] Johnston S G, Keene A F, Burton E Detal. Arsenic mobilization in a seawater inundated acid sulfate soil[J]. Environmental Science and Technology, 2010, 44(6):1968-1976.
[91] 李金培. 有机无机改良剂对酸性硫酸盐土化学动力学与水稻生长的影响[J]. 华南农业大学学报, 1993, 14(1):16-23.
Li J P. The effect of organic matter and inorganic amendnents on the chemical kinetics and the growth of rice in an acid sulphate soil[J]. Journal of South China Agricultural University, 1993, 14(1):16-23.
[92] 刘兆辉, 王遵亲. 我国酸性硫酸盐土壤中铁锰形态转化及迁移[J]. 土壤学报, 1994, 31(4):376-384.
Liu Z H, Wang Z Q. Transformation and movement of iron and manganese in acid sulfate soils of China[J]. Acta Pedologica Sinica, 1994, 31(4):376-384.
[93] 王建武, 骆世明, 冯远娇. 酸性硫酸盐土中铝的形态[J]. 应用生态学报, 2000, 11(5):735-740.
Wang J W, Luo S M, Feng Y J. Aluminum forms in acid sulfate soils[J]. Chinese Journal of Applied Ecology, 2000, 11(5):735-740.
[94] Åström M. Mobility of Al P and alkali and alkaline earth metals in acid sulphate soils in Finland[J]. Science of the Total Environment, 1998, 215:19-30.
[95] Golez N V, Kyuma K. Influence of pyrite oxidation and soil acidification on some essential nutrient elements[J]. Aquacultural Engineering, 1997, 16:107-124.
[96] Moore P A, Patrick W H. Aluminium, boron and molybdenum availability and uptake by rice in acid sulfate soils[J]. Plant and Soil, 1991, 136:171-181.
[97] Faltmarsch R M, Åström M E, Vuori K M. Environmental risks of metals mobilised from acid sulphate soils in Finland:a literature review[J]. Boreal Environment Research, 2008, 13:444-456.
[98] Russell D J, Helmke S A. Impacts of acid leachate on water quality and fisheries resources of a coastal creek in northern Australia[J]. Marine and Freshwater Research, 2002, 53:19-33.
[99] Stephens F J, Ingram M. Two cases of fish mortality in low pH, aluminium rich water[J]. Journal of Fish Diseases, 2006, 29:765-770.
[100] Hinwood A L, Horwitz P, Appleyard Setal. Acid sulphate soil disturbance and metals in groundwater:Implications for human exposure through home grown produce[J]. Environmental Pollution, 2006, 143:100-105.
[101] Ferreira T, Otero X, Vidal-Torrado Petal. Redox processes in mangrove soils under in relation to different environmental conditions[J]. Soil Science Society of America, 2007, 71(2):484-491.
[102] Marchand C, Allenbach M, Lallier-Vergès E. Relationships between heavy metals distribution and organic matter cycling in mangrove sediments (Conception Bay, New Caledonia)[J]. Geoderma, 2011, 160(3-4):444-456.
[103] Amaral V, Cabral H N, Bishop M J. Resistance among wild invertebrate populations to recurrent estuarine acidification[J]. Estuarine, Coastal and Shelf Science, 2011, 93(4):460-467.
[104] Amaral V, Cabral H N, Bishop M J. Prior exposure influences the behavioural avoidance by an intertidal gastropod,Bembiciumauratum, of acidified waters[J]. Estuarine, Coastal and Shelf Science, 2013, 136(1):82-90.
[105] Åström M. Partitioning of transition metals in oxidized and reduced zones of sulphide-bearing fine-grained sediments[J]. Applied Geochemistry, 1998, 13(5):607-617.
[106] Åström M. Effect of widespread severely acidic soils on spatial features and abundance of trace elements in streams[J]. Journal of Geochemical Exploration, 2001, 73:181-191.
[107] Roos M, Åström M. Hydrogeochemistry of rivers in an acid sulphate soil hotspot area in western Finland[J]. Agricultural and Food Science, 2005, 14:24-33.
[108] Åström M, Nystrand M, Gustafsson J Petal. Lanthanoid behaviour in an acidic landscape[J]. Geochimica et Cosmochimica Acta, 2010, 74:829-845.
[109] Macdonald B C T, White I, Åström M Eetal. Discharge of weathering products from acid sulfate soils after a rainfall event, Tweed River, eastern Australia[J]. Applied Geochemistry, 2007, 22:2695-2705.
[110] Hinwood A, Horwitz P, Rogan R. Human exposure to metals in groundwater affected by acid sulfate soil disturbance[J]. Arch Environ Contam Toxicol, 2008, 55:538-545.
[111] Lin C, Mcconchie D, Bush R Tetal. Characteristics of some heavy metals in acid sulfate topsoils, Eastern Australia[J]. Pedosphere, 2001, 11(1):31-37.
[112] Lin C, Melville M D. Controls of soluble Al in experimental acid sulfate conditions and acid sulfate soils[J]. Pedosphere,1997, 7(2):97-102.
[113] Burton E D, Bush R T, Sullivan L Aetal. Mobility of arsenic and selected metals during re-flooding of iron- and organic-rich acid-sulfate soil[J]. Chemical Geology, 2008, 253:64-73.
[114] Welch S A, Christy A G, Isaacson Letal. Mineralogical control of rare earth elements in acid sulfate soils[J]. Geochimica et Cosmochimica Acta, 2009, 73:44-64.
[115] Morgan B, Rate A W, Burton E D. Trace element reactivity in FeS-rich estuarine sediments:Influence of formation environment and acid sulfate soil drainage[J]. Science of the Total Environment, 2012, 438:463-476.
[116] Morgan B, Rate A W, Burton E Detal. Enrichment and fractionation of rare earth elements in FeS- and organic-rich estuarine sediments receiving acid sulfate soil drainage[J]. Chemical Geology, 2012, 308-309:60-73.
[117] Kang D, Seo Y, Lee Betal. Identification and crop performance of acid sulfate soil-tolerant rice varieties[J]. The Korean Society of Crop Science, 2010, 13(2):75-81.
[118] 臧小平, 张承林, 孙光明,等. 酸性硫酸盐土壤上施用磷矿粉对水稻养分有效性的影响[J]. 植物营养与肥料学报, 2003, 9(2):203-207.
Zang X P, Zhang C L, Sun G Metal. Effect of phosphate rocks on nutrient availability of rice in acid sulphate soils[J]. Plant Nutrition and Fertilizer Science, 2003, 9(2):203-207.
[119] Rosicky M, Sullivan L, Slavich Petal. Factors contributing to the acid sulfate soil scalding process in the coastal flood plains of New South Wales, Australia[J]. Soil Research, 2004, 42:587-594.
[120] Faltmarsch R, Österholm P, Greger Metal. Metal concentrations in oats (AvenasativaL.) grown on acid sulphate soils[J]. Agricultural and Food Science, 2009, 18:45-56.
[121] Fältmarsch R, Österholm P, Gunnar J. Chemical composition of cabbage (BrassicaOleraceaL. var. capitata) grown on acid sulfate soils[J]. Journal of Plant Nutrition and Soil Science, 2010, 173:423-433.
[122] Russell D J, Preston K M, Mayer R J. Recovery of fish and crustacean communities during remediation of tidal wetlands affected by leachate from acid sulfate soils in north-eastern Australia[J]. Wetlands Ecology and Management, 2011, 19:89-108.
