Schwann cells originating from skin-derived precursors promote peripheral nerve regeneration in rats
2014-04-06PingZhangXiaochengLuJianghaiChenZhenbingChen
Ping Zhang, Xiaocheng Lu, Jianghai Chen, Zhenbing Chen
1 Department of Orthopedics, Wuhan Women and Children Health Care Center, Wuhan, Hubei Province, China
2 Department of Hand Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China
Schwann cells originating from skin-derived precursors promote peripheral nerve regeneration in rats
Ping Zhang1, Xiaocheng Lu2, Jianghai Chen2, Zhenbing Chen2
1 Department of Orthopedics, Wuhan Women and Children Health Care Center, Wuhan, Hubei Province, China
2 Department of Hand Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China
Ping Zhang and Xiaocheng Lu contributed equally to this paper.
Arti fi cial guidance channels containing Schwann cells can promote the regeneration of injured peripheral nerve over long distances. However, primary Schwann cells are not suitable for autotransplantation. Under speci fi c conditions, skin-derived progenitors can be induced to differentiate into Schwann cells. Therefore, adult rat dorsal skin (dermis)-derived progenitors were isolated and induced to differentiate with DMEM/F12 containing B27, neuregulin 1, and forskolin. Immuno fl uorescence staining and reverse transcription polymerase chain reaction (RTPCR) con fi rmed that the resultant cells were indeed Schwann cells. Arti fi cial guidance channels containing skin-derived progenitors, Schwann cells originating from skin-derived progenitors, or primary Schwann cells were used to bridge 5 mm sciatic nerve defects. Schwann cells originating from skin-derived progenitors signi fi cantly promoted sciatic nerve axonal regeneration. The signi fi cant recovery of injured rat sciatic nerve function after the transplantation of Schwann cells originating from skin-derived progenitors was con fi rmed by electromyogram. The therapeutic effect of Schwann cells originating from skin-derived progenitors was better than that of skin-derived progenitors. These findings indicate that Schwann cells originating from skin-derived precursors can promote peripheral nerve regeneration in rats.
nerve regeneration; skin-derived precursors; Schwann cells; peripheral nerve injury; cell transplantation; NSFC grant; neural regeneration
Funding:This work was supported by the National Natural Science Foundation of China, No. 81171194.
Zhang P, Lu XC, Chen JH, Chen ZB. Schwann cells originating from skin-derived precursors promote peripheral nerve regeneration in rats. Neural Regen Res. 2014;9(18):1696-1702.
Introduction
Although the axons of peripheral nerves are capable of regeneration after transection injuries, the functional recovery of target organs is rarely satisfactory (Fawcett and Keynes, 1990; Stoll and Müller, 1999). To achieve better outcomes, the distal and proximal stumps of injured nerves should be directly reconnected without putting them under tension. However, the nerve defects are sometimes too long to be repaired by direct suturing of the nerves without causing tension or leaving a gap. Many techniques have been developed to address this problem. Compared with other approaches, autologous nerve grafting is the gold standard because it provides both a nerve scaffold and activates Schwann cells to facilitate axonal regeneration (Grif fi n et al., 2013; Pabari et al., 2014). However, certain complications of autologous nerve grafting are unavoidable, such as numbness, sensory abnormality, functional deficiency at the donor site, and unsatisfactory outcomes for long defects or large diameter nerves. Therefore, several alternatives have been developed, including acellular nerve allografts and synthetic or biological nerve guidance channels (Lin et al., 2013; Gu et al., 2014). Although the preliminary results when using these materials to repair short gaps after nerve injury are very promising, nerve defects with long gaps failed to regenerate when treated with a conduit alone (di Summa et al., 2011). Because cellular components, such as Schwann cells, appear indispensable during axonal regeneration of peripheral nerves, nerve conduits supplemented with extrinsic Schwann cells were proposed (Erba et al., 2010; Sun et al., 2011). Further research con fi rmed that arti fi cial guidance channels seeded with Schwann cells can remarkably enhance the regeneration of peripheral nerves to repair large gap nerve defects (Evans et al., 1998, 2002; Hadlock et al., 2000; Mosahebi et al., 2001; Levi et al., 2002).
Because primary Schwann cells are not accessible for harvest and seeding into autografts, the differentiation of embryonic stem cells into Schwann cells and neural stem cells were proposed as candidates for transplantation (Cui et al., 2008; Xu et al., 2012). However, ethical concerns and immunological rejection are associated with these types of cells, which has so far limited their clinical application. Recently, a type of novel multipotent progenitor cell, skin-derived precursors (SKPs), has been reported in the dermis of both neonatal and adult skin (Toma et al., 2001). SKPs are characterized by similar gene expression patterns and functional properties as neural crest-derived cells in the embryonic stage (Toma et al., 2001). A previous study has demonstrated that, under speci fi c differentiating culture conditions, SKPscan be induced to differentiate into neurons and Schwann cells (Fernandes et al., 2004). McKenzie et al. (2006) reported that when SKPs-derived Schwann cells were transplanted into the peripheral nerve of Shiverer mutant mice, which are genetically de fi cient in the basic protein myelin, they were able to myelinate and functionally integrate into the axons of host sciatic nerve. Marchesi et al. (2007) demonstrated that synthetic and collagen-based nerve conduits seeded with SKP-derived Schwann cells could be used to repair a 16 mm gap in rat sciatic nerve. Similarly, Walsh et al. (2009) seeded SKP-SCs into acellular nerve and found that the SKP-SCs remarkably enhanced nerve regeneration in 15 mm nerve defects in rats. Together, these data suggest that SKP-SCs may be an accessible and ef fi cacious cell source for promoting peripheral nerve regeneration. However, to the best of our knowledge, the reported analyses of the effect of SKPs or SKP-SCs on nerve regeneration are often confounded by the different types of synthetic or natural nerve conduits used, and the function of the cells is not separately or independently evaluated. To address this problem, we used silicone tubes seeded with different types of cells to determine the ef fi cacy of SKPs and SKP-SCs for promoting nerve regeneration.
Materials and Methods
Cell culture and differentiation
We prepared cells using previously described protocols (Toma et al., 2001; Fernandes et al., 2004). Brie fl y, 8-weekold male Sprague-Dawley rats of clean grade were provided by the Laboratory Animal Center of Tongji Medical College, Huazhong University of Science and Technology, China (license No. SCXK (E) 2003-0001). The procedures in this study conformed to theGuide for the Care and Use of Laboratory Animalspublished by the US National Institutes of Health (NIH publication No. 85-23, revised 1996), and the protocol was approved by the Institutional Animal Care Committee of Tongji Medical College, Huazhong University of Science and Technology, China. To prepare the SKPs, a group of rats was euthanized by CO2inhalation. The dorsal skins were carefully isolated and minced into small (around 1 mm3) pieces. The minced skin tissue was then digested with collagenase II and then mechanically dispersed into single cells with the tip of a polished Pasteur pipette. Next, the cell suspension was spun down through a 3% bovine serum albumin gradient at 190 ×gto remove any cell debris. Finally, the cells were resuspended in DMEM/F12 (Invitrogen, Grand Island, NY, USA) supplemented with basic fi broblast growth factor (bFGF; 10 ng/mL, R&D Systems, Minneapolis, MN, USA), epidermal growth factor (EGF; 20 ng/mL, R&D Systems), and B27 (1:50; Invitrogen). These primary cells were seeded at 1 × 106cells/mL in a 6-well-plate, which was placed in the incubator at 37°C with 1.5% CO2. Culture medium was replaced every 3 days.
To induce differentiation of the SKPs, the primary cellspheres were collected and mounted on poly-ornithine pre-treated coverslips in the differentiation medium, which was composed of DMEM/F12 supplemented with 1% B27, neuregulin-1 (10 ng/mL, R&D Systems), and 4 μmol/L forskolin (Merck, Whitehouse Station, NJ, USA). Under these conditions, the cells rapidly attached to the culture surface and migrated out of the spheres immediately after adherence. Two weeks after differentiation, the cells were analyzed using immunocytochemistry to estimate the ef fi ciency of SC differentiation.
Estimation of the ef fi ciency of SC differentiation by immuno fl uorescence staining
For immuno fl uorescence staining, SKP spheres were collected and fi xed in 4% paraformaldehyde at room temperature for 15 minutes. The spheres were permeabilized with 0.5% saponin and incubated with primary antibodies overnight. The differentiated SKPs were fi xed in 4% paraformaldehyde and permeabilized with 0.4% Triton X-100. Next, the samples were incubated with rabbit anti-rat S100 monoclonal antibody (1:200; Abcam, Cambridge, MA, USA), mouse anti-rat βIII-tubulin monoclonal antibody (1:200; Cell Signaling, Danvers, MA, USA), or mouse anti-Nestin (1:200; Abcam) overnight at room temperature. All samples were then incubated with Alexa 488-goat anti-mouse IgG (1:1,000; Molecular Probes, Eugene, OR, USA) or Alexa 555-goat anti-rabbit IgG (1:1,000; Molecular Probes) for 1 hour at room temperature. DAPI was used for nuclear counterstaining. Staining was visualized using a fluorescence microscope (Olympus, Tokyo, Japan).
Validation of the ef fi ciency of SC differentiation by reverse transcription PCR (RT-PCR)
RT-PCR was used to validate the immunostaining results and was performed as previously described (Chen et al., 2005). Brie fl y, the RNA samples were diluted to 10 ng/μL, 1 ng/μL, 100 pg/μL, and 10 pg/μL before reverse transcription, and subjected to the following PCR protocol. One microliter of RT reaction solution was added to 9 μL of a PCR mixture composed of 0.1 μL of deoxy-NTPs (100 mm), 1.1 μL of PCR Gold buffer, 0.9 μL of MgCl2(25 mm), 0.15 μL of AmpliTaq Gold DNA polymerase (5 U/μL; Applied Biosystems, Life Technologies, Grand Island, NY, USA), and 0.1 μL of each oligonucleotide primer (100 μm; Invitrogen). For S100β cDNA ampli fi cation, the following primers were used: 5′-ATG TCC GAG CTG GAG AAG GC-3′ (sense) and 5′-TCG TCC AGC GTC TCC ATC AC-3′ (antisense), producing a fragment of 188 bp. The samples were subjected to 7 minutes at 95°C; 50 cycles of 10 seconds at 95°C, 15 seconds at 63°C, and 25 seconds at 72°C; and fi nally 7 minutes at 72°C in a GeneAmp PCR System 2400 (Applied Biosystems). An internal control was created using ribosomal protein L19 for normalization standard with the following primers: 5′-AAG AAG GTC TGG TTG GAT CCC AAT G-3′ (sense) and 5′-AGG CTG TGA TAC ATA TGG CGG TCA A-3′ (antisense). Those samples were subjected to 7 minutes at 95°C; 50 cycles of 15 seconds at 95°C, 20 seconds at 59°C, and 45 seconds at 72°C; and fi nally 7 minutes at 72°C.
Transplantation of different cell types to repair rat sciatic nerve defects
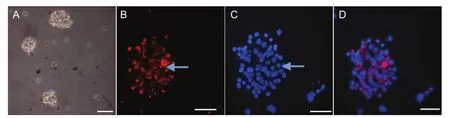
Figure 1 Isolation and culture of skin-derived progenitors (SKPs) by immuno fl uorescence staining.
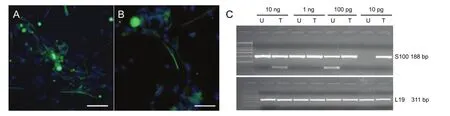
Figure 2 Schwann cell puri fi cation after differential adhesion treatment.
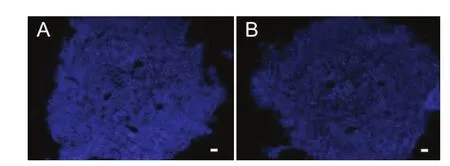
Figure 3 Transplanted green fl uorescent protein (GFP)-positive cells are visible in the newly regenerated nerve (immuno fl uorescence staining).
Ninety 8-week-old Sprague-Dawley male rats were randomly and equally divided into three groups: SKPs, SCs, and SKPSCs. The animals were anesthetized, and the right sciatic nerve was exposed and transected. A 5-mm-segment of the nerve in the proximal stump was excised. A custom-made silicone tube with 1 mm diameter was used to bridge the gap between the proximal and distal nerve stumps. The nerve stumps were sutured to the tube with 8-0 Prolene suture. The SKPs, SKP-SCs, and SCs were resuspended in DMEM/F12 at a density of 1 × 106cells/mL and 15 μL of the cell suspension was injected into each tube. Eight weeks after the operation, the bridged sciatic nerve was exposed. After removing the silicone tube, we found that the nerve gaps had been connected by regenerated nerve tissue in all groups. To confi rm these results, green fl uorescent protein (GFP)-transgenic rats were provided by Prof. Hugo Vankelecom (University of Leuven, Belgium). GFP-transgenic rat derived SKPs, SKPSCs, and SCs were also harvested and transplanted into host animals as described above.
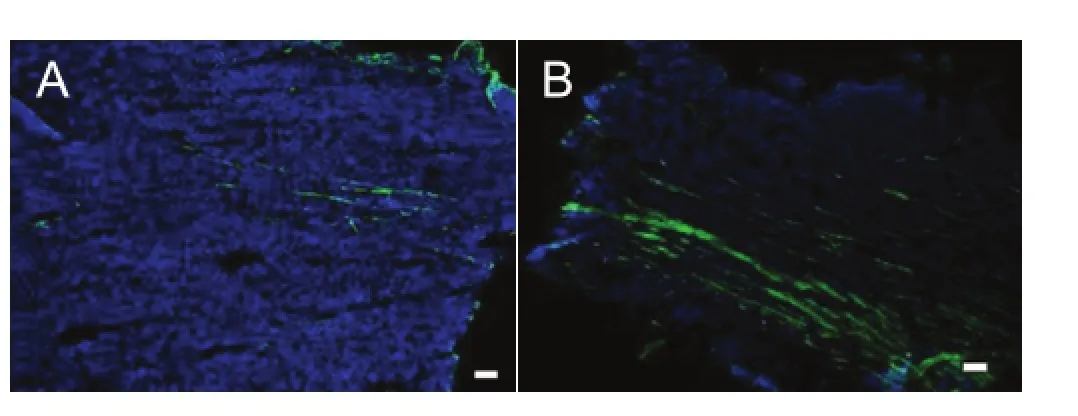
Figure 5 Schwann cells sorted in newly regenerated nerve (immuno fl uorescence staining).
Rat neurological function detected by electromyography (EMG)
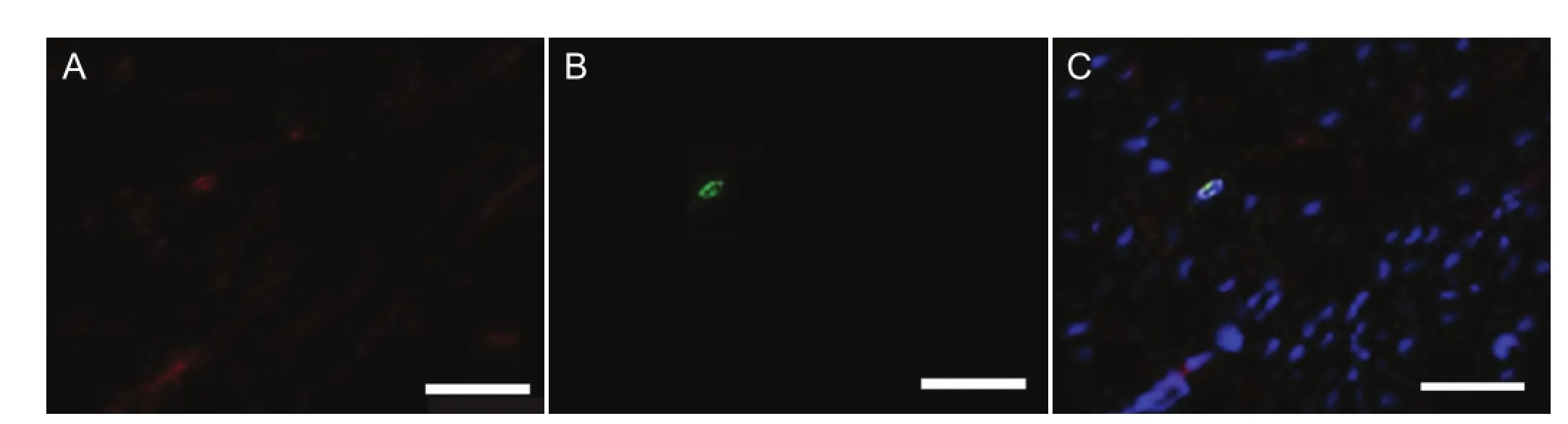
Figure 4 Transplanted green fl uorescent protein (GFP)-positive cells integrated into regenerated nerve (immuno fl uorescence staining).

Figure 6 The electrophysiological analysis of rat sciatic nerves treated with different cells 12 weeks after transplantation.
Twelve weeks after the operation, EMG analysis (Shanghai Haishen Medical Electronic Instrumentation (NID-092), Shanghai, China) was performed. One electrode was inserted into the gastrocnemius muscle and the others were placed on the proximal and distal sciatic nerves at the grafting site. The distal motor latency and amplitude of the EMG signal were measured for rats from each group.
Statistical analysis
The data were expressed as mean ± SD and were analyzed by one-way analysis of variance followed by Tukey-Kramer multiple-comparison tests to determine if there were any signi fi cant differences among the groups using NCSS 2004 statistical software (NCSS, LLC, Kaysville, UT, USA).
Results
Isolation and culture of SKPs
Primary skin dermal cells from adult rats were cultured for 3-4 days, at which point a few small non-adherent spheres were observed, but the majority of cells in culture were adhered to the surface of the plastic culture dishes. Within the following 10 days, the number of spheres and the sphere size increased. Secondary cells were also able to generate spheres in the sphere-forming medium. Further generation of spheres was obtained for more than six passages, indicating that the cells from the SKP spheres were able to self-renew. Fourteen days later, immunofluorescence staining revealed that nearly all cells in the spheres were nestin-positive, but none of them expressed NeuN or NFM-L. These results agree with observations from previous studies (Hou et al., 2006) (Figure 1).
Differentiation and puri fi cation of SKP-SCs
To induce differentiation of the SKPs towards Schwann cells, SKPs were cultured with Schwann cell induction medium. Similar to SKP spheres in differentiation culture medium, small SKP-SC aggregates attached to the culture surface after 24 hours, and then cells around the aggregates formed processes and migrated out of the aggregates over the following 24 to 48 hours. Two weeks after induction of differentiation, the cells were stained for Schwann cell marker S100β. At that time point, the immunocytochemistry results showed that the majority of cells in these culture conditions had begun to differentiate (70% in the Schwann cell conditions). However, the percentage of S100β-positive cells did not increase after three passages. Therefore, we used differential adhesion to enrich the SKP-SCs in culture. We observed that thepurity of the SKP-SCs increased after differential adhesion. Semi-quantitative PCR showed that the expression of S100β by cells after purification using differential adhesion was higher than that in untreated cells (Figure 2).
Bridging nerve defects using nerve conduits seeded with cells
Gross appearance
Eight weeks after the operation, the new segment of nerve was slightly whiter and smaller in diameter than the proximal and distal nerve stumps in all three groups. There were no significant macroscopic differences in the regenerated nerve sections among the three groups.
Schwann cell sorting and axonal regeneration
Eight weeks after the operation, the GFP-positive cells were visible in the longitudinal section of new growth nerve by fluorescence microscopy (Figure 3). Although the percentage of the total cell number that was GFP-positive was similar among the three groups, the distributions of the GFP cells were different. For example, the GFP-positive cells appeared in both ends and the center of the nerve in the longitudinal section of the SKP group, whereas in the SKPSC and SC groups, more GFP-positive cells were found in the central area. Immuno fl uorescence staining showed that most of the GFP-positive cells in the SKP-SC and SC groups also expressed S100 (Figure 4). These data confirmed the survival of SKPs and SKP-SCs in the host nerve, and their integration into the axonal regeneration after grafting. Interestingly, immuno fl uorescence staining of the longitudinal sections of the regenerated nerve segments revealed that the S100-positive cells, including extrinsic and intrinsic cells, in the SKP-SC and SC groups were more spatially localized than in the SKP group (Figure 5). Together, these results suggest that SKP-SCs may more actively promote axonal regeneration after cell transplantation compared with naive SKPs.
Neurological function
Twelve weeks after the operation, EMG tests were performed on samples from all groups. The distal motor latency in the rat sciatic nerves of the SKP, SKP-SC, and SC groups were similar (P> 0.05). In contrast, the amplitude in the SKP-SC and SC groups was larger than that in the SKP group (P<0.05; Figure 6).
Discussion
Peripheral nerve defects can be repaired using nerve guidance channels seeded with stem or progenitor cells. In the present study, the functions of SKPs and SKP-SCs during nerve regeneration were analyzed. Three major conclusions can be drawn from these results. First, SKP-SCs can be purifi ed and enriched under speci fi c culture conditions. Second, although the SKPs differentiated into Schwann cells in the microenvironment of the peripheral nerve, only portions of the transplanted cells were induced towards a neural fate, which implies that the pre-differentiation procedure is necessary for the Schwann cell graft application. Third, SKP-SCs can survive and proliferate after being transplanted into the rat sciatic nerve. They were able to robustly integrate into the axonal regeneration and myelination, and induced similar effects on nerve regeneration as primary Schwann cells. Thus, these data suggest that SKP-SCs could be a valuable source for Schwann cell transplantation to resolve the lack of such cells for cell therapy treatments of peripheral nerve injuries.
对于单个机床监控功能通过双击机床列表中对应的机床进入,单个机床监控界面如图6和图7所示,包括加工状态、主轴控制、NC程序、参数设置、报警信息和系统信息6个类型,能够实现对机床各类型信息的全面详细监控。
After peripheral nerve injury, regeneration of the axon can occur spontaneously, but the nerve defect will not likely be recovered without a bridge material. While autologous nerve grafts are considered to be the gold standard for the repair of peripheral nerve defects, the complications associated with this technique limit its clinical application, at least in some patients. A number of nerve conduits have been assessed in the pursuit of a suitable approach for bridging nerve defects. Although reports in the literature have demonstrated that large nerve gaps of various lengths have been regenerated using synthetic or biological conduits. In addition, numerous studies have revealed the potential effects of different cell types for improving the ef fi cacy of nerve conduits transplanted for nerve regeneration. Schwann cells have been shown to enhance axonal regeneration in a rat sciatic nerve injury model. Bone marrow stem cells also likely play an active role in conduit-guided nerve regeneration.
Fernandes et al. (2008) demonstrated that SKPs are progenitor cells with similar molecular and functional properties as embryonic neural crest stem cells. In medium supplemented with serum and growth factors, human SKPs differentiate into both neuronal cells and astrocytes (Toma et al., 2001; Fernandes et al., 2004). Moreover, differentiation culture medium supplemented with growth factors promotes SKPs differentiation towards Schwann cells in signi fi cantly higher percentages than other culture systems. Based on these studies, SKP-SCs and SKPs were considered to be an accessible cell source for cell therapy of peripheral nerve injuries. In several studies, SKPs and/or SKP-SCs were seeded into different scaffolds to try to repair nerve gaps after nerve injuries, leading to satisfactory outcomes. However, there are few reports that evaluated the independent function of SKPs and SKP-SCs during nerve regeneration. Herein, we used silicone tubes seeded with different types of seed cells to determine the effects of SKPs, SKP-SCs, and primary Schwann cells.
In this study, we used Schwann cell differentiation medium, DMEM/F12 supplemented with neuregulin-1 and forskolin, to induce the differentiation of SKPs. After 10 days in culture, around 80% of the cells in the Schwann cell differentiation medium expressed S100 , which is consistent with the results from prior reports. However, the purity of Schwann cells did not significantly change even after the cultures were passaged several times. When we compared the effects of the different cell types on nerve regeneration, the homogeneity of the cell population is one of the most important factors to arrive at an accurate conclusion. Therefore, differential adhesion was used to increase thepurity of the Schwann cells in culture before cell transplantation. Numerous experiments have shown that differential adhesion can be used to purify and enrich primary Schwann cells in culture without changing their molecular and functional properties. Here, we obtained SKP-SCs at 95% purity in culture using this technique. This result is comparable to the cell purities reported by other studies in which SKP-SCs were assessed as a source for cell transplantation (Belicchi et al., 2004; McKenzie et al., 2006; Marchesi et al., 2007; Walsh et al., 2009).
Previously, SKP-SCs have been shown to proliferate and produce myelin duringin vitroculture. SKPs or SKP-SCs were transplanted into rats with peripheral nerve injuries or Shiverer mutant mice, and they were integrated into axonal myelination in the peripheral nervous system. Similar results were obtained after cells were transplanted into the central nervous system (McKenzie et al., 2006). In addition, undifferentiated SKPs were seeded into a collagen conduit to bridge nerve defect gaps. Eight weeks after cell transplantation, the immunohistochemistry results showed expression of S100 and myelin basic protein in donor cells, indicating the differentiation of SKPs in peripheral nerve injury conditions, as well as the cell therapy potential of SKPs for nerve regeneration (Marchesi et al., 2007). Recently, nerve-derived Schwann cells or SKP-SCs were seeded into acellular nerve grafts to evaluate the regeneration potential of SKP-SCs after peripheral nerve damage. In their hands, SKP-SCs remarkably enhanced nerve regeneration 4-8 weeks after transplantation when compared with the control group, in which only basal media were used (Walsh et al., 2009). In the present study, 5-mm-long nerve defects were bridged by a silicone tube seeded with nerve-derived SCs, SKPs, or SKP-SCs. Eight weeks after transplantation, the gross fi ndings showed that the nerve gaps were fi lled in all three groups. However, the diameters of the regenerated nerve within the silicone tube were signi fi cantly smaller in the SKP group than in the SKPSC and SC groups, which had similarly sized regenerated nerves. The number of axons in the regenerated region of the SKP-SC group was similar to that in the SCs group as assessed by immunohistochemistry, whereas the number was greater than in the SKP group. It has been suggested that transplanted SCs enhance nerve regeneration by producing and secreting neurotrophin and cytokines in the host nerve (McKenzie et al., 2006). Here, we found fewer S100-positive cells in the SKP group than in the SC and SKP-SC groups. One major reason for the greater axonal regeneration in the SC and SKP-SC groups may be fewer donor cells with the Schwann cell phenotype were found in the SKP group compared with the SKP-SC and SC groups. Moreover, immunostaining showed that there were more GFP-positive cells surrounding the regenerated axons in the SC and SKPSC groups than in the SKP group. Together with the EMG results, these fi ndings suggest that the percentage of SC-like cells in the donor cell population may be a key factor for successful nerve regeneration in such nerve gap models. In conclusion, SKP-SCs are an accessible alternative for Schwann cells that may be able to improve axonal regeneration after peripheral nerve injury.
Author contributions:Zhang P obtained the data, conducted the experiments, and performed the data analysis. Lu XC conceived and designed the study and provided technical and material support. Chen ZB wrote the manuscript. Chen JH provided technical support and revised the manuscript. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Belicchi M, Pisati F, Lopa R, Porretti L, Fortunato F, Sironi M, Scalamogna M, Parati EA, Bresolin N, Torrente Y (2004) Human skinderived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. J Neurosci Res 77:475-486.
Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H (2005) The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 146:3985-3998.
Cui L, Jiang J, Wei L, Zhou X, Fraser JL, Snider BJ, Yu SP (2008) Transplantation of embryonic stem cells improves nerve repair and functional recovery after severe sciatic nerve axotomy in rats. Stem Cells 26:1356-1365.
di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G (2011) Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 181:278-291.
Erba P, Mantovani C, Kalbermatten DF, Pierer G, Terenghi G, Kingham PJ (2010) Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet Surg 63:e811-817.
Evans GR, Brandt K, Katz S, Chauvin P, Otto L, Bogle M, Wang B, Meszlenyi RK, Lu L, Mikos AG, Patrick CW (2002) Bioactive poly(L-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials 23:841-848.
Evans PJ, Mackinnon SE, Levi AD, Wade JA, Hunter DA, Nakao Y, Midha R (1998) Cold preserved nerve allografts: changes in basement membrane, viability, immunogenicity, and regeneration. Muscle Nerve 21:1507-1522.
Fawcett JW, Keynes RJ (1990) Peripheral nerve regeneration. Annu Rev Neurosci 13:43-60.
Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, Kaplan DR, Labosky PA, Rafuse V, Hui CC, Miller FD (2004) A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol 6:1082-1093.
Fernandes KJ, Toma JG, Miller FD (2008) Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential. Philos Trans R Soc Lond B Biol Sci 363:185-198.
Grif fi n JW, Hogan MV, Chhabra AB, Deal DN (2013) Peripheral nerve repair and reconstruction. J Bone Joint Surg Am 95:2144-2151.
Gu X, Ding F, Williams DF (2014) Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 35:6143-6156.
Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP (2000) A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng 6:119-127.
Hou SY, Zhang HY, Quan DP, Liu XL, Zhu JK (2006) Tissue-engineered peripheral nerve grafting by differentiated bone marrow stromal cells. Neuroscience 140:101-110.
Levi AD, Dancausse H, Li X, Duncan S, Horkey L, Oliviera M (2002) Peripheral nerve grafts promoting central nervous system regeneration after spinal cord injury in the primate. J Neurosurg 96:197-205.
Lin MY, Manzano G, Gupta R (2013) Nerve allografts and conduits in peripheral nerve repair. Hand Clin 29:331-348.
Marchesi C, Pluderi M, Colleoni F, Belicchi M, Meregalli M, Farini A, Parolini D, Draghi L, Fruguglietti ME, Gavina M, Porretti L, Cattaneo A, Battistelli M, Prelle A, Moggio M, Borsa S, Bello L, Spagnoli D, Gaini SM, Tanzi MC, et al. (2007) Skin-derived stem cells transplanted into resorbable guides provide functional nerve regeneration after sciatic nerve resection. Glia 55:425-438.
McKenzie IA, Biernaskie J, Toma JG, Midha R, Miller FD (2006) Skinderived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci 26:6651-6660.
Mosahebi A, Simon M, Wiberg M, Terenghi G (2001) A novel use of alginate hydrogel as Schwann cell matrix. Tissue Eng 7:525-534.
Pabari A, Lloyd-Hughes H, Seifalian AM, Mosahebi A (2014) Nerve Conduits for Peripheral Nerve Surgery. Plast Reconstr Surg 133: 1420-1430.
Stoll G, Müller HW (1999) Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol 9:313-325.
Sun F, Zhou K, Mi WJ, Qiu JH (2011) Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats. Biomaterials 32:8118-8128.
Toma JG, Akhavan M, Fernandes KJL, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD (2001) Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 3:778-784.
Walsh S, Biernaskie J, Kemp SW, Midha R (2009) Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience 164:1097-1107.
Xu L, Zhou S, Feng GY, Zhang LP, Zhao DM, Sun Y, Liu Q, Huang F (2012) Neural stem cells enhance nerve regeneration after sciatic nerve injury in rats. Mol Neurobiol 46:265-274.
Copyedited by McCarty W, Robert J, Yu J, Li CH, Song LP, Zhao M
Correction Announcement
The Figure 2A in the article entitled “Overexpression of cytoglobin gene inhibits hypoxic injury to SH-SY5Y neuroblastoma cells” published inNeural Regeneration Research[2013;8(23):2198-2203] was mistaken. Corrected Figure 2A is shown as follows:
We are particularly grateful to Professor Roland H. Wenger from Institute of Physiology, University of Zürich-Irchel, Switzerland for pointing out the error.
Hereby Certi fi ed!
Editorial Board ofNeural Regeneration Research
Zhenbing Chen, M.D., Ph.D., Department of Hand Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, China, 2990430205@qq.com.
10.4103/1673-5374.141805
Jianghai Chen, M.D., Ph.D., Department of Hand Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, China, Chenjianghai2002@hotmail.com.
http://www.nrronline.org/
Accepted: 2014-07-21
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Histological assessment in peripheral nerve tissue engineering
- Genetic factors for nerve susceptibility to injuries -lessons from PMP22 de fi ciency
- Bone marrow mesenchymal stem cells repair spinal cord ischemia/reperfusion injury by promoting axonal growth and anti-autophagy
- Oxidative phosphorylated neuro fi lament protein M protects spinal cord against ischemia/reperfusion injury
- Ginsenoside Rd inhibits apoptosis following spinal cord ischemia/reperfusion injury
- Factors affecting directional migration of bone marrow mesenchymal stem cells to the injured spinal cord
