Bone marrow mesenchymal stem cells repair spinal cord ischemia/reperfusion injury by promoting axonal growth and anti-autophagy
2014-04-06FeiYinChunyangMengRifengLuLeiLiYingZhangHaoChenYonggangQinLiGuo
Fei Yin, Chunyang Meng, Rifeng Lu, Lei Li, Ying Zhang, Hao Chen, Yonggang Qin, Li Guo
1 Department of Orthopedics, China-Japan Union Hospital, Jilin University, Changchun, Jilin Province, China
2 Department of Toxicology, School of Public Health, Jilin University, Changchun, Jilin Province, China
Bone marrow mesenchymal stem cells repair spinal cord ischemia/reperfusion injury by promoting axonal growth and anti-autophagy
Fei Yin1, Chunyang Meng1, Rifeng Lu2, Lei Li1, Ying Zhang2, Hao Chen1, Yonggang Qin2, Li Guo2
1 Department of Orthopedics, China-Japan Union Hospital, Jilin University, Changchun, Jilin Province, China
2 Department of Toxicology, School of Public Health, Jilin University, Changchun, Jilin Province, China
Bone marrow mesenchymal stem cells can differentiate into neurons and astrocytes after transplantation in the spinal cord of rats with ischemia/reperfusion injury. Although bone marrow mesenchymal stem cells are known to protect against spinal cord ischemia/reperfusion injury through anti-apoptotic effects, the precise mechanisms remain unclear. In the present study, bone marrow mesenchymal stem cells were cultured and proliferated, then transplanted into rats with ischemia/reperfusion injuryviaretro-orbital injection. Immunohistochemistry and immuno fl uorescence with subsequent quanti fi cation revealed that the expression of the axonal regeneration marker, growth associated protein-43, and the neuronal marker, microtubule-associated protein 2, significantly increased in rats with bone marrow mesenchymal stem cell transplantation compared with those in rats with spinal cord ischemia/reperfusion injury. Furthermore, the expression of the autophagy marker, microtubule-associated protein light chain 3B, and Beclin 1, was signi fi cantly reduced in rats with the bone marrow mesenchymal stem cell transplantation compared with those in rats with spinal cord ischemia/reperfusion injury. Western blot analysis showed that the expression of growth associated protein-43 and neurofi lament-H increased but light chain 3B and Beclin 1 decreased in rats with the bone marrow mesenchymal stem cell transplantation. Our results therefore suggest that bone marrow mesenchymal stem cell transplantation promotes neurite growth and regeneration and prevents autophagy. These responses may likely be mechanisms underlying the protective effect of bone marrow mesenchymal stem cells against spinal cord ischemia/reperfusion injury.
nerve regeneration; bone marrow mesenchymal stem cells; spinal cord ischemia/reperfusion injury; axonal growth; autophagy; repair; NSFC grant; neural regeneration
Funding:This study was supported by the National Natural Science Foundation of China, No. 30972153; the Science and Technology Development Program of Jilin Provincial Science and Technology Department in China, No. 200905183; and the Scientific Research Foundation of Jilin Department of Health of China, No. 2008Z041.
Yin F, Meng CY, Lu RF, Li L, Zhang Y, Chen H, Qin YG, Guo L. Bone marrow mesenchymal stem cells repair spinal cord ischemia/reperfusion injury by promoting axonal growth and anti-autophagy. Neural Regen Res. 2014;9(18):1665-1671.
Introduction
Ischemic preconditioning (Zvara et al., 1999), drug treatment (Tian et al., 2011), and physical therapy (Cakir et al., 2003) have been used to treat and prevent spinal cord ischemia-reperfusion injury. However, these therapeutic strategies do not fundamentally eliminate the occurrence of paralysis; rather, they only relieve the symptoms. A signi fi cant amount of money is spent on these patients who are hospitalized for an extended period of time. Furthermore, their injury places a heavy burden on their family as well as society. Therefore, fi nding a practical and effective strategy to repair spinal cord ischemia/reperfusion injury is necessary.
Bone marrow mesenchymal stem cells are non-hematopoietic stem cells of the bone marrow, and can differentiate into neurons and astrocytes under appropriate conditions (Tsai et al., 2014). Numerous studies suggest that transplanted bone marrow mesenchymal stem cells migrate to damaged tissue, differentiating into tissue-speci fi c cells and repairing the damage to the tissue (Da Silva and Hare, 2013; Calió et al., 2014; Gao et al., 2014). Transplanted bone marrow mesenchymal stem cells have been used to treat stroke (Calió et al., 2014), heart disease (Da Silva and Hare, 2013), and diabetes mellitus (Gao et al., 2014). Our previous study has shown that transplanted bone marrow mesenchymal stem cells differentiate into neurons and astrocytes in the injured spinal cord of rats, and repair spinal cord ischemia/ reperfusion injury through anti-apoptotic effects (Yin et al., 2014).
Spinal cord injury induces apoptosis (type I programmedcell death) and autophagic death (type II programmed cell death) (Shimizu et al., 2014). Autophagy is the basic catabolic mechanism involving cell degradation of unnecessary or dysfunctional cellular components through lysosomes (Guan et al., 2013). Autophagy has a double action because it can either promote neuronal injury (Baba et al., 2009) or repair nerve tissue (Wang et al., 2014). Kanno et al. (2009) have shown that the time course of Beclin 1 expression is similar to that of apoptosis in the injured region of spinal cord hemi-transection injury in the mouse (Yong et al., 1998; Citron et al., 2000). Varying degrees of neurological dysfunction in the hindlimb occur after spinal cord ischemia/reperfusion injury (Zivin and DeGirolami, 1980; Zvara et al., 1999; Calió et al., 2014; Yin et al., 2014), in which one possible mechanism is autophagy-induced neuronal injury after spinal cord injury (Baba et al., 2009) Axonal damage-induced conduction block has also been postulated to be a mechanism underlying spinal cord injury-induced neurological disorders (Schwab and Bartholdi, 1996; Bock et al., 2013; Park et al., 2013). Few reports have investigated if the protective effect of transplanted bone marrow mesenchymal stem cells in spinal cord ischemia/ reperfusion injury is associated with axonal regeneration or autophagy. Therefore, the aim of the present study was to explore this association.
Materials and Methods
Animals
A total of 40 healthy and clean, adult (weighing 220 ± 20 g) and neonatal (5-7 days old) male/female Sprague-Dawley rats were purchased from the Center of Laboratory Animals, Jilin University, China (license No. SCXK(Ji)2008-0005). All rats were fed the standard diet and given water, housed at 20-22°C under a 12-hour light/dark cycle. The experimental protocols were conducted in accordance with the Animal Care and Use Committee of Jilin Province, China. The 40 rats were randomly and equally assigned to control, sham surgery, model, and stem cell therapy groups.
Isolation and culture of bone marrow mesenchymal stem cells
Neonatal rats were sacri fi ced and immersed in 75% ethanol for 15 minutes, as previously described (Guo et al., 2005). The femur and tibia were then aseptically collected. The metaphysis was exposed and washed with aseptic Dulbecco’s modifi ed Eagle’s medium/Ham’s nutrient mixture F-12 (DMEM/ F12; Gibco, Grand Island, NY, USA). The bone marrow was obtained and made into a single cell suspension, which was then centrifuged. The remaining cells were resuspended in DMEM/F12 containing fetal bovine serum (Hyclone, Logan, UT, USA), counted, and placed in a 75 mL-culture flask at 1 × 106/mL, and then incubated in DMEM/F12, supplemented with 10% fetal bovine serum, and 100 U/mL penicillin/100 μg/mL streptomycin (Sigma-Aldrich, St. Louis, MO, USA), in a 5% CO2incubator with saturated humidity at 37°C. The medium was replaced 72 hours after planting. All cells were digested with trypsin and passaged 7 days later.
The spinal cord ischemia/reperfusion injury model
A model of spinal cord ischemia/reperfusion injury was prepared, as previously described (Zivin and DeGirolami, 1980). In the model and stem cell therapy groups, rats were intraperitoneally injected with 10% chloral hydrate (3 mL/kg) and were fixed by lying the rats on their side. A 5-cm incision was made down from the lower edge midline of the left ribs. The left kidney was then located, followed by the abdominal aorta along the renal artery, which was ligated with a 10-g bulldog clamp below the renal artery for 1 hour. The bulldog clamp was then removed and the abdominal cavity closed after it was washed with penicillin. Rats in the sham surgery group only received laparotomy without ligation of the abdominal aorta. The model was deemed as being successfully established if neurological de fi cits appeared in the hindlimb. Controls were not given any treatment.
Bone marrow mesenchymal stem cell transplantation
Passage 4 bone marrow mesenchymal stem cells were collected and made into single cells. In the stem cell therapy group, bone marrow mesenchymal stem cells (5 × 106, about 0.1 mL) were intravenously injected by retro-orbital injection 1 and 24 hours after reperfusion, repectively, as previously described (Yin et al., 2014). The rats in the model and sham surgery groups were administered an equal volume of PBS. The rats were sacri fi ced by cervical dislocation, 7 days after reperfusion. L3-4spinal cord segments were made into 4-5-μm-thick paraf fi n sections.
Immunohistochemistry for microtubule-associated protein 2, axonal regeneration marker growth associated protein-43, and microtubule-associated protein light chain 3B
After 7 days of reperfusion, the spinal cord was fi xed in 10% formalin buffer, embedded in paraf fi n, sliced into sections, and dehydrated in graded ethanol. After antigen retrieval at 98°C, the spinal cord was incubated with endogenous peroxidase blockers for 10 minutes, washed with PBS, and blocked with goat serum for 30 minutes. The spinal cord section was then incubated with rabbit anti-microtubule-associated protein 2 polyclonal antibody (1:400; Proteintech Group, Chicago, IL, USA), rabbit anti-growth associated protein-43 polyclonal antibody (1:500; Proteintech Group), or rabbit anti-rat light chain 3B monoclonal antibody (1:200; Abcam, Burlingame, CA, USA) overnight at 4°C. The spinal cord was incubated with biotinylated goat anti-rabbit IgG (ready-to-use; Fuzhou Maixin Biotechnology Development, Fuzhou, China), followed by streptavidin-peroxidase (readyto-use; Fuzhou Maixin Biotechnology Development) for 40 minutes at room temperature. Staining was visualized with 3,3′-diaminobenzidine (Fuzhou Maixin Biotechnology Development), and spinal cords were then counterstained with hematoxylin, dehydrated with graded ethanol, permeabilized with xylene, and then mounted with neutral resin. The data were analyzed using Image Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA). Five fi elds (at 400 × magni fi cation) of the spinal cord of each rat were selected and the mean optical density was calculated.
Immuno fl uorescence for Beclin 1 in the spinal cord
Paraffin sections were dewaxed, dehydrated, subjected to antigen retrieval at 98°C, incubated with PBS containing 1% Triton X-100, and then blocked with goat serum for 30 minutes. These sections were treated with rabbit anti-Beclin 1 polyclonal antibody (1:300; Proteintech Group), overnight at 4°C, then Alexa Fluor® 488 Goat anti-rabbit IgG (1:400; Molecular Probes, Eugene, OR, USA) for 40 minutes at room temperature. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich), and sections were then mounted with glycerol. The data were analyzed with Image Pro Plus 6.0 (Media Cybernetics). Five fi elds (400 × magni fi cation) of the spinal cord of each rat were selected, and the mean optical density was calculated.
Western blot analysis of growth associated protein-43, neuro fi lament-H, light chain 3B, and Beclin 1
Total protein was extracted from the rat spinal cord, separated by Tris-sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred to polyvinylidene fl uoride membranes (Millipore, Schwalbach, Germany). Membranes were blocked and supplemented with 5% skimmed milk powder in Tris-buffered saline with Tween, 20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl and 0.05% Tween 20, slowly shaking for 2 hours at room temperature. Rabbit anti-growth associated protein-43 polyclonal antibody (1:1,000), rabbit anti-neuro fi lament-H (marker for mature neuronal axons) (Yabe et al., 2001) polyclonal antibody (1:1,000; Proteintech Group), rabbit anti-rat light chain 3B monoclonal antibody (1:1,000), or rabbit-Beclin 1 polyclonal antibody (1:1,000) were added overnight at 4°C. Mouse anti-rat β-actin monoclonal antibody (1:2,000; Proteintech Group) served as the reference marker/positive control. After three washes with Tris-buffered saline with Tween, membranes were incubated with horseradish peroxidase -conjugated goat anti-rabbit/ mouse IgG (1:2,000; Proteintech Group) for 1 hour at 37°C. Following three washes with Tris-buffered saline with Tween, Immobilon™ Western Chemiluminescent horseradish peroxidase substrate kit (Millipore, Billerica, MA, USA) was used, followed by band visualization and fixing. The data were analyzed using Quantity one image analytical system (BioRad, Hercules, CA, USA). Relative expression was calculated and expressed as a ratio of integrated optical density of the target band to β-actin.
Statistical analysis
All data were expressed as the mean ± SD, and were analyzed by one-way analysis of variance followed by the Fisher’s least signi fi cant difference test. A value ofP< 0.05 was considered statistically significant. SPSS 17.0 software (SPSS, Chicago, IL, USA) was used.
Results
Bone marrow mesenchymal stem cells elevated the expression of microtubule-associated protein 2 and growth associated protein-43 in the spinal cord of rats with spinal cord ischemia/reperfusion injury
Quanti fi cation of immunohistochemistry revealed that the expression of microtubule-associated protein 2 and growth associated protein-43 was signi fi cantly reduced in the spinal cord of rats from the model group at 7 days after reperfusion (P< 0.05; Figure 1). Furthermore, neuronal processes were absent. Compared with the model group, the expression of microtubule-associated protein 2 and growth associated protein-43 was significantly higher in the spinal cord of rats from the stem cell therapy group (P< 0.05; Figure 1). Moreover, long processes were present in the transplanted neurons.
Bone marrow mesenchymal stem cells decreased the expression of Beclin 1 and light chain 3B in the spinal cord of rats with spinal cord ischemia/reperfusion injury
Immunofluorescence and immunohistochemistry results demonstrated low-level expression of light chain 3B and Beclin 1 in the spinal cord of rats from the control and sham surgery groups at 7 days after reperfusion (Figure 2). Light chain 3B and Beclin 1 expression signi fi cantly increased in the model group (P< 0.05; Figure 2). The expression of light chain 3B and Beclin 1 was significantly lower in the stem cell therapy group than that in the model group (P< 0.05; Figure 2).
Bone marrow mesenchymal stem cells affected the expression of growth associated protein-43, neuro fi lament-H, light chain 3B and Beclin 1 in the spinal cord of rats with spinal cord ischemia/reperfusion injury by western blot analysis
Western blot analysis of spinal cord samples showed that the expression of growth associated protein-43 and neurofi lament-H was markedly lower (P< 0.05), but light chain 3B and Beclin 1 was signi fi cantly higher in the model group compared with the control and sham surgery groups at 7 days after reperfusion (P< 0.05; Figure 3). In the stem cell therapy group, the expression of growth associated protein-43 and neuro fi lament-H expression was markedly higher, but light chain 3B and Beclin 1 expression was significantly lower than that of the model group (P< 0.05; Figure 3).
Discussion
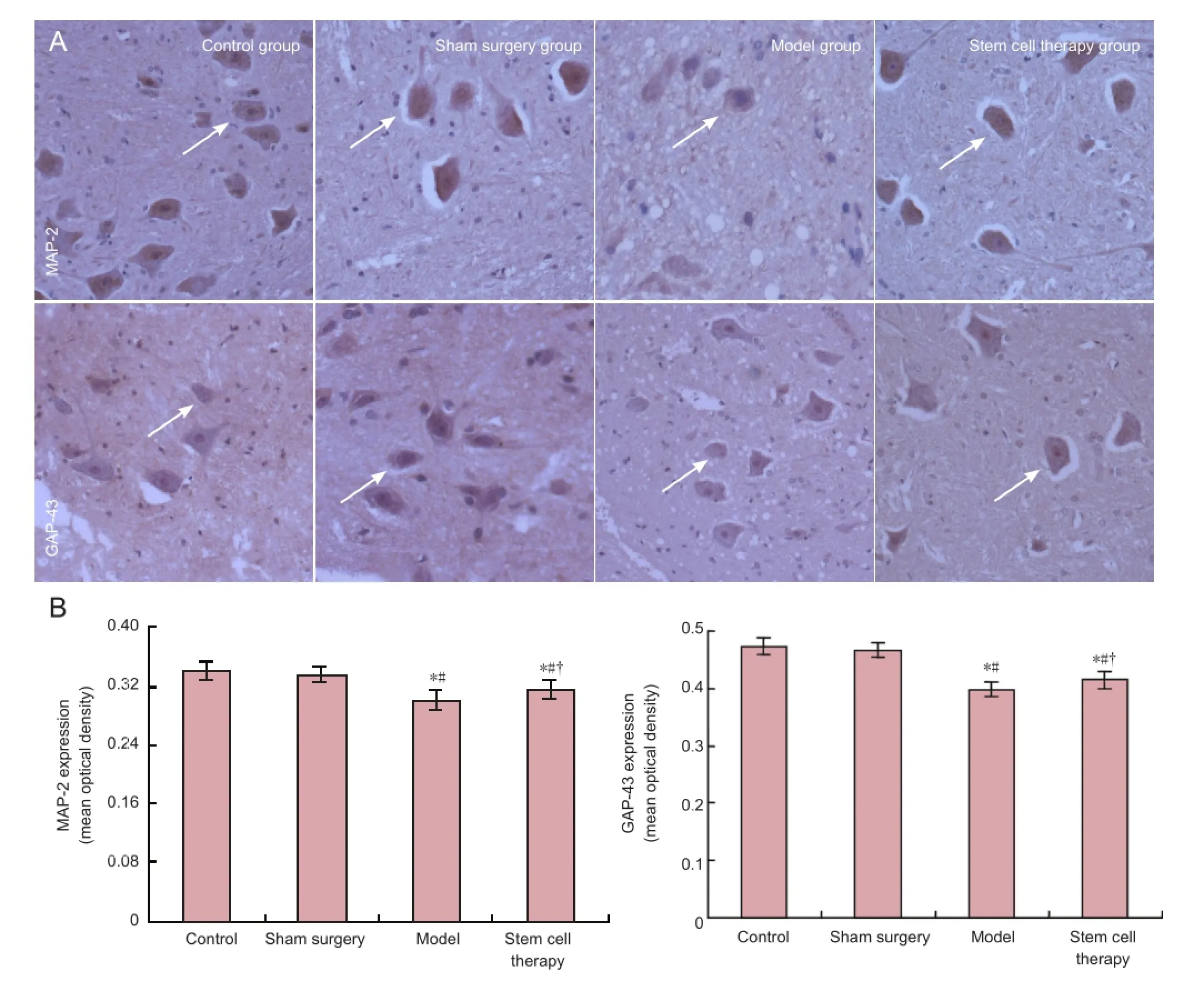
Figure 1 Effects of bone marrow mesenchymal stem cells on the expression of microtubule-associated protein 2 (MAP-2) and growthassociated protein 43 (GAP-43) in the spinal cord of rats with spinal cord ischemia/reperfusion injury.
The main marker of spinal cord injury is axonal injury because of conduction block-induced neurological deficit (Schwab and Bartholdi, 1996; Bock et al., 2013; Park et al., 2013). A central hallmark of spinal cord injury is axonal damage (Schwab and Bartholdi, 1996). Axonopathy is associated with the destruction of the fast axonal transport mechanism and phosphorylated neuro fi lament protein alteration (Coleman and Perry, 2002; Tobias et al., 2003; Petzold, 2005). In the present study, we investigated the expression of growth associated protein-43, microtubule-associated protein 2, and neuro fi lament-H. Growth associated protein-43 is expressed in developing and regenerating neurons, during axonal regeneration, the regeneration of growth cone navigation, and synaptic remodeling, which is also the most common marker of axonal regeneration (Deumens et al., 2005; Petzold, 2005). Cytoskeletal protein microtubule-associated protein 2 maintains structural integrity of neurons and is very sensitive to ischemia. The immune response of microtubule-associated protein 2 has been shown to be sensitive, and is a selective and early marker of ischemic injury in the central nervous system (Dawson and Hallenbeck, 1996). Our previous results have shown that neurological impairment in the rat hindlimb after spinal cord ischemia/reperfusion injury is improved after bone marrow mesenchymal stem cell transplantation (Yin et al., 2014), a fi nding that was also observed in the present study. Our current results showed that the expression of growth associated protein-43 and microtubule-associated protein 2 was reduced in the injured spinal cord of rats, suggesting that spinal cord injury damages neuronal structure and weakens the capability of nerve regeneration and synapse reconstruction. We also found that the expression of growth associated protein-43 and microtubule-associated protein 2 was markedly higher in the stem cell therapy group compared with the model group. These results indicate that stem cell therapy repairs neuronal structure as well as enhancing nerve regeneration and synapse reconstruction. Neurofilament-H is important for maintaining the stability of mature neuronal axons (Yabe et al., 2001). Immunohistochemistry results from our previous study demonstrated that the markedly decreased expression of neurofilament-H in the spinal cord ischemia/ reperfusion injury group occurs simultaneously with the lowering of axon number in the injured region. This correlated with the neurological dysfunction in the hindlimb (Yin et al., 2014). After bone marrow mesenchymal stem cell transplantation, neuro fi lament-H (western blot) expression noticeably increased and neurological function in the hindlimb improved (Yin et al., 2014). Our present results showed that the expression of growth associated protein-43, microtubule-associatedprotein 2, and neuro fi lament-H increased in the injured spinal cord after bone marrow mesenchymal stem cell transplantation, indicating that cell transplantation promotes axonal regeneration, a hypothesis that is consistent with a study by Park et al. (2013).
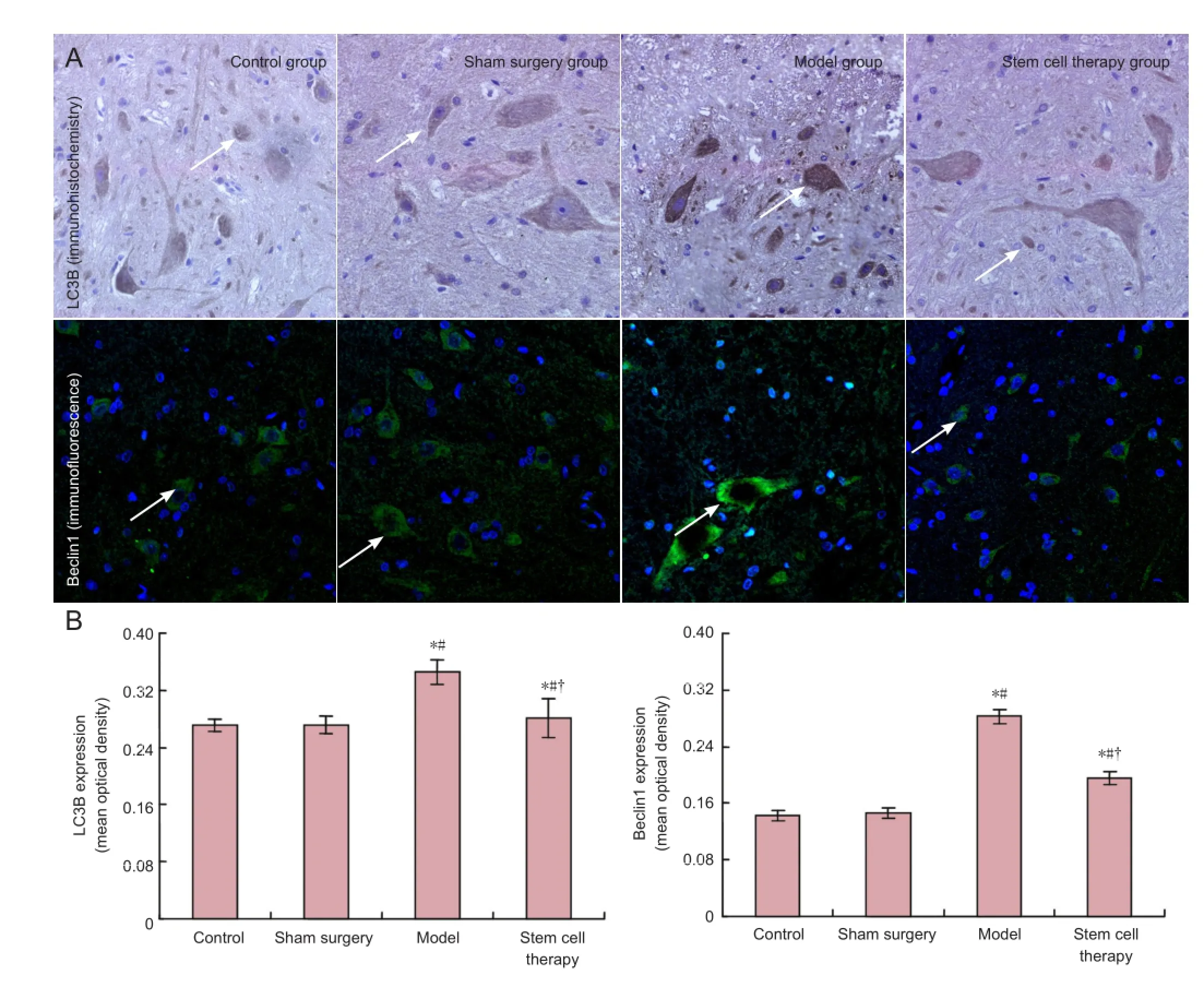
Figure 2 Effects of bone marrow mesenchymal stem cells on the expression of microtubule-associated light chain 3B (LC3B) and Beclin 1 in the spinal cord of rats with spinal cord ischemia/reperfusion injury.
Apoptosis and autophagy are closely related biological processes. Recent studies have veri fi ed that neuronal apoptosis and autophagic cell death occur during spinal cord injury. Light chain 3B and Beclin 1 are two markers of autophagy. Light chain 3 is related to the formation of autophagy (Kabeya et al., 2000). Light chain 3B, an isoform of light chain 3, is associated with autophagy, and is often used to be a marker for monitoring autophagy. Beclin 1, an autophagic regulator, takes part in the initiation of autophagosome formation (Miracco et al., 2010). Our results showed high expression of light chain 3B and Beclin 1 in the spinal cord of rats with spinal cord ischemia/reperfusion injury, which was signi fi cantly diminished after bone marrow mesenchymal stem cell transplantation, suggesting that autophagy contributes to neuronal cell death in the injured spinal cord. Transplanted bone marrow mesenchymal stem cells signi ficantly lowered the occurrence of autophagy. Our previous study has con fi rmed that hindlimb function improves after cell transplantation (Yin et al., 2014), suggesting that the improvement of neurological function in the hindlimb may be associated with the reduction in autophagy. Baba et al. (2009) suggest that autophagy promotes neuronal death. The expression of Beclin 1 expression has been shown to increase 4 hours after spinal cord hemisection injury in rats, and peaking at 3 days and lasting until 21 days (Kanno et al., 2009). Furthermore, autophagy has been shown to be expressed after spinal cord injury, with active effects on nerve tissue repair (Wang et al., 2014). In the present study, autophagy promoted neuronal cell death after spinal cordischemia/reperfusion injury. Moreover, transplanted bone marrow mesenchymal stem cells decreased the occurrence of autophagy, and this effect may possibly be a mechanism that underlies the bone marrow mesenchymal stem cell transplantation-mediated repair of spinal cord ischemia/reperfusion injury.
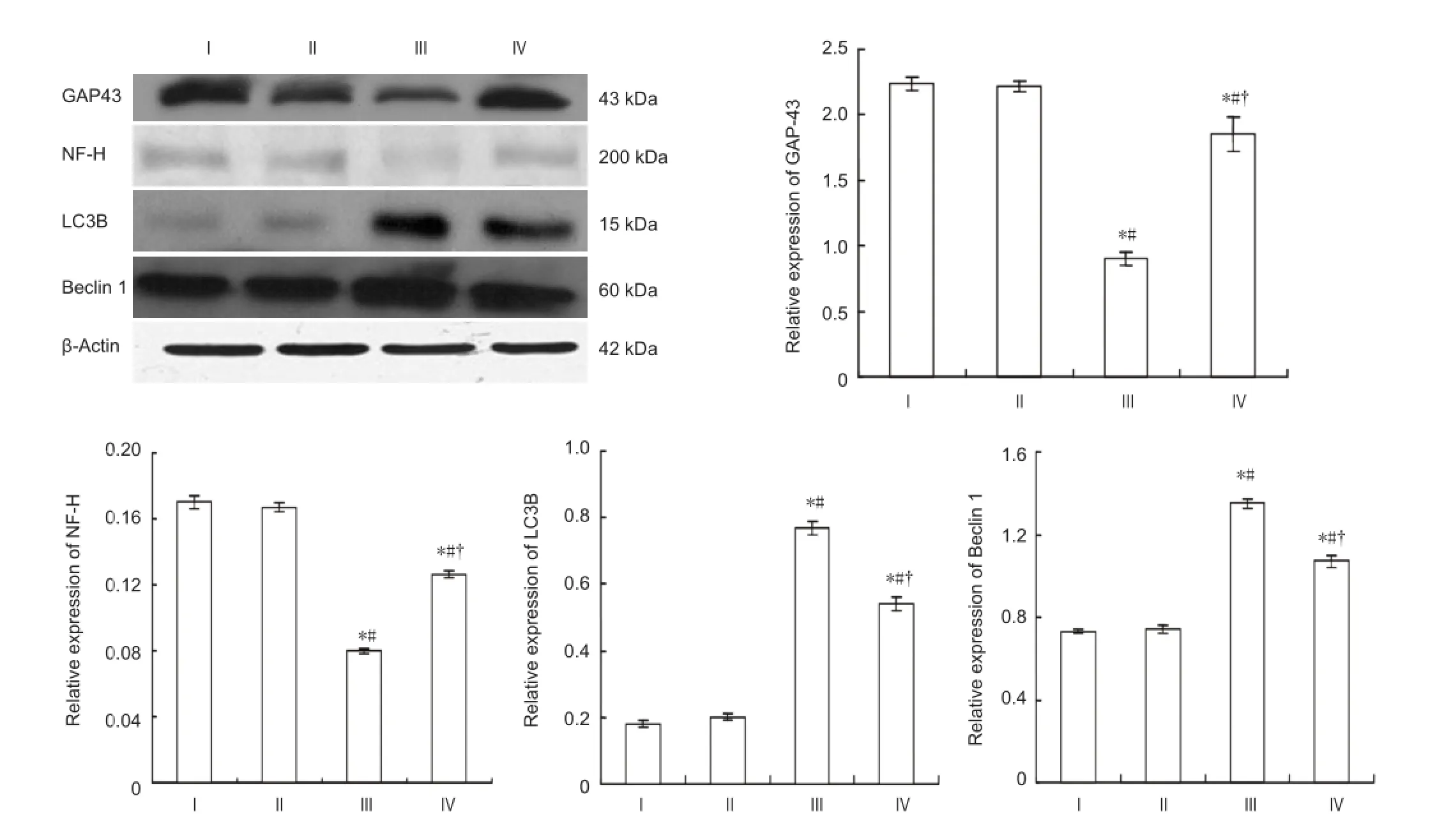
Figure 3 Effect of bone marrow mesenchymal stem cells on the expression of growth associated protein-43 (GAP-43), neuro fi lament-H (NF-H), light chain 3B (LC3B), and Beclin 1 in the spinal cord of rats with spinal cord ischemia/reperfusion injury (western blot assay).
In summary, transplanted bone marrow mesenchymal stem cells contribute to the growth and regeneration of axons. Anti-autophagy resulting from bone marrow mesenchymal stem cell transplantation may be a mechanism by which spinal cord ischemia/reperfusion injury is repaired, thereby providing a new therapeutic target for the treatment of spinal cord ischemia/reperfusion injury.
Author contributions:Yin F conducted the majority of the experiment and wrote the manuscript. Meng CY, Lu RF, Li L, Zhang Y, Chen H, Qin YG, and Guo L completed statistical analyses, conceived and designed the study, and revised the manuscript. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Baba H, Sakurai M, Abe K, Tominaga R (2009) Autophagy-mediated stress response in motor neuron after transient ischemia in rabbits. J Vasc Surg 50:381-387.
Bock P, Spitzbarth I, Haist V, Stein VM, Tipold A, Puff C, Beineke A, Baumgärtner W (2013) Spatio-temporal development of axonopathy in canine intervertebral disc disease as a translational large animal model for nonexperimental spinal cord injury. Brain Pathol 23:82-99.
Cakir O, Erdem K, Oruc A, Kılınc N, Eren N (2003) Neuroprotective effect of N-acetylcysteine and hypothermia on the spinal cord ischemia-reperfusion injury. Cardiovasc Surg 11:375-379.
Calió ML, Marinho DS, Ko GM, Ribeiro RR, Carbonel AF, Oyama LM, Ormanji M, Guirao TP, Calió PL, Reis LA, Simões MdJ, Lisbôa-Nascimento T, Ferreira AT, Bertoncini CRA (2014) Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radic Biol Med 70:141-154.
Citron BA, Arnold PM, Sebastian C, Qin F, Malladi S, Ameenuddin S, Landis ME, Festoff BW (2000) Rapid upregulation of caspase-3 in rat spinal cord after injury: mRNA, protein, and cellular localization correlates with apoptotic cell death. Exp Neurol 166:213-226.
Coleman MP, Perry VH (2002) Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci 25:532-537.
Da Silva J, Hare J (2013) Cell-based therapies for myocardial repair: emerging role for bone marrow-derived mesenchymal stem cells (MSCs) in the treatment of the chronically injured heart. Methods Mol Biol 1037:145-163.
Dawson DA, Hallenbeck JM (1996) Acute focal ischemia-induced alterations in MAP2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab 16:170-174.
Deumens R, Koopmans GC, Joosten EAJ (2005) Regeneration of descending axon tracts after spinal cord injury. Prog Neurobiol 77:57-89.
Gao X, Song L, Shen K, Wang H, Qian M, Niu W, Qin X (2014) Bone marrow mesenchymal stem cells promote the repair of islets from diabetic mice through paracrine actions. Mol Cell Endocrinol 388:41-50.
Guan JL, Simon AK, Prescott M, Menendez JA, Liu F, Wang F, Wang C, Wolvetang E, Vazquez-Martin A, Zhang J (2013) Autophagy in stem cells. Autophagy 9:830-849.
Guo L, Yin F, Meng HQ, Ling L, Hu-He TN, Li P, Zhang CX, Yu S, Duan DS, Fan HX (2005) Differentiation of mesenchymal stem cells into dopaminergic neuron-like cells in vitro. Biomed Environ Sci 18:36-42.
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720-5728.
Kanno H, Ozawa H, Sekiguchi A, Itoi E (2009) Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis 33:143-148.
Miracco C, Cevenini G, Franchi A, Luzi P, Cosci E, Mourmouras V, Monciatti I, Mannucci S, Biagioli M, Toscano M, Moretti D, Lio R, Massi D (2010) Beclin 1 and LC3 autophagic gene expression in cutaneous melanocytic lesions. Hum Pathol 41:503-512.
Park JH, Min J, Baek SR, Kim SW, Kwon IK, Jeon SR (2013) Enhanced neuroregenerative effects by scaffold for the treatment of a rat spinal cord injury with Wnt3a-secreting fi broblasts. Acta Neurochir (Wien) 155:809-816.
Petzold A (2005) Neuro fi lament phosphoforms: Surrogate markers for axonal injury, degeneration and loss. J Neurol Sci 233:183-198.
Schwab ME, Bartholdi D (1996) Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 76:319-370.
Shimizu S, Yoshida T, Tsujioka M, Arakawa S (2014) Autophagic cell death and cancer. Int J Mol Sci 15:3145-3153.
Tian F, Xu LH, Zhao W, Tian LJ, Ji XL (2011) The optimal therapeutic timing and mechanism of puerarin treatment of spinal cord ischemia-reperfusion injury in rats. J Ethnopharmacol 134:892-896.
Tobias CA, Shumsky JS, Shibata M, Tuszynski MH, Fischer I, Tessler A, Murray M (2003) Delayed grafting of BDNF and NT-3 producing fi broblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp Neurol 184:97-113.
Tsai HL, Chiu WT, Fang CL, Hwang SM, Renshaw PF, Lai WF (2014) Different forms of tenascin-C with tenascin-R regulate neural differentiation in bone marrow-derived human mesenchymal stem cells. Tissue Eng Part A 20:1908-1921.
Wang ZY, Lin JH, Muharram A, Liu WG (2014) Beclin-1-mediated autophagy protects spinal cord neurons against mechanical injury-induced apoptosis. Apoptosis 19:933-945.
Yabe JT, Wang FS, Chylinski T, Katchmar T, Shea TB (2001) Selective accumulation of the high molecular weight neuro fi lament subunit within the distal region of growing axonal neurites. Cell Motil Cytoskeleton 50:1-12.
Yin F, Guo L, Meng CY, Liu YJ, Lu RF, Li P, Zhou YB (2014) Transplantation of mesenchymal stem cells exerts anti-apoptotic effects in adult rats after spinal cord ischemia-reperfusion injury. Brain Res 1561:1-10.
Yong C, Arnold PM, Zoubine MN, Citron BA, Watanabe I, Berman NE, Festoff BW (1998) Apoptosis in cellular compartments of rat spinal cord after severe contusion injury. J Neurotrauma 15:459-472.
Zivin JA, DeGirolami U (1980) Spinal cord infarction: a highly reproducible stroke model. Stroke 11:200-202.
Zvara DA, Colonna DM, Deal DD, Vernon JC, Gowda M, Lundell JC (1999) Ischemic preconditioning reduces neurologic injury in a rat model of spinal cord ischemia. Ann Thorac Surg 68:874-880.
Copyedited by Mark F, Frenchman B, Yu J, Qiu Y, Li CH, Song LP, Zhao M
Li Guo, Ph.D., Department of Toxicology, School of Public Health, Jilin University, Changchun 130021, Jilin Province, China, gli@jlu.edu.cn.
10.4103/1673-5374.141801
http://www.nrronline.org/
Accepted: 2014-07-14
杂志排行
中国神经再生研究(英文版)的其它文章
- Histological assessment in peripheral nerve tissue engineering
- Genetic factors for nerve susceptibility to injuries -lessons from PMP22 de fi ciency
- Oxidative phosphorylated neuro fi lament protein M protects spinal cord against ischemia/reperfusion injury
- Ginsenoside Rd inhibits apoptosis following spinal cord ischemia/reperfusion injury
- Factors affecting directional migration of bone marrow mesenchymal stem cells to the injured spinal cord
- Craniocerebral injury promotes the repair of peripheral nerve injury
