Acute and sub-acute toxicity study of Clerodendrum inerme, Jasminum mesnyi Hance and Callistemon citrinus
2014-03-22
1Department of Pharmacognosy, Hindu College of Pharmacy, Sonepat, Haryana, India
2Department of Pharmaceutical Sciences and Drug Research, Panjabi University, Patiala, India
Acute and sub-acute toxicity study of Clerodendrum inerme, Jasminum mesnyi Hance and Callistemon citrinus
Bharat Bhushan1, Satish Sardana1, Gulshan Bansal2*
1Department of Pharmacognosy, Hindu College of Pharmacy, Sonepat, Haryana, India
2Department of Pharmaceutical Sciences and Drug Research, Panjabi University, Patiala, India
Objective: To study acute and sub-acute toxicity study ofClerodendrum inerme(C. inerme),Jasminum mesnyi(J. mesnyi) Hance andCallistemon citrinus(C. citrinus). Methods: The acute toxicity test was conducted in Swiss albino mice. The extracts ofC. inerme,J. mesnyiHance and C. citrinus was administered in single dose of 0.5, 1.0, 2.0, 3.0, 4.0 and 5.0 g/kg and observed for behavioral changes and mortality, if any. In sub-acute toxicity study, Wistar rats of either sex were administered 1/5th of the maximum tolerated dose,p.o. for 4 weeks. Rats were observed weekly for any change in their body weight, food and water intake during 28 d of the treatment. At the end of 28 d, blood samples of the rats were collected for hematological and biochemical study.
Results: In acute toxicity study, all four extracts of three plants were found to be well tolerated up to the dose of 2 000 mg/kg. These produced neither mortality nor any change in the behavior in mice. In sub-acute toxicity study, all four extracts of three plants at the LD50dose level did not produce any significant alteration in hematological and biochemical parameters in rats.
Conclusions: The results demonstrated that there is a wide margin of safety for the therapeutic use of each of the four extracts of three plants. The findings also corroborated the traditional use of these extracts.
ARTICLE INFO
Article history:
Received 19 April 2014
Received in revised form 15 September 2014
Accepted 25 September 2014
Available online 20 December 2014
Acute toxicity
Subacute toxicity
Albino rats
Clerodendrum inerme
Jasminum mesnyi Hance
Callistemon citrinus
1. Introduction
During last decade there has been an exponential growth in use of herbal products for treatment of various types of diseases[1]. In general, treatment involving herbal drugs spans a long duration of time. In contrast to general old age myth that herbal drugs are safe and do not have toxic effects, These drugs may cause some moderate to severe side effects due to complex nature of their chemical compositions. Hence, there is a need to establish safety to herbal drugs through validated scientific toxicity studies or protocols.
Clerodendrum inerme(C. inerme),Jasminum mesnyi(J.mesnyi) Hance andCallistemon citrinus(C. citrinus) (Figure 1) are used for treatment of diabetes mellitus in traditional system of medicine in India.C. inermebelonging to family Verbenacae has been used as antidiabetic agent in folklore medicinal system of India. It is reported to have antibacterial, hepatoprotective, anticarcinogenic, uterine and intestine stimulating properties. The various constituents characterised in its leaves include phenylethanoid glycoside, neo-clerodane diterpinoids antiviral proteins (CIP-29 and CIP-34) and three iridoid glucoside (Inerminoside A1, C and D[2-4].J. mesnyiHance belonging to family Oleaceae is an evergreen shrub having bright yellow flowers. It is native of China and grown in Indian gardens[5]. The major constituents present in this plant include β-sitosterol, α-amyrin, β-glucoside flavonoids, constituents include rutin and secoiridoid glucosides (9-hydroxyjasminoside, 9-hydroxy jasminosidic acid, Jasmoside and jasminoside)[6,7].C.citrinusbelonging to family Myrtaceae is woody aromatic tree widely distributed in the wet tropics, especially Australia, South America and tropical Asia. It is mainly used as an ornamental plant. The major constituents present inC. citrinusinclude oxygenated monoterpenes, monoterpene hydrocarbons, 1,8 cineole, α-pinene, β -pinene, α-terpinene and α-terpineole. Its leaves are employed as substitute of tea to have a delightful and refreshing flavor[8,9,10]. However, its medicinal uses are not reported widely and its constituents are being investigated for herbicidal properties and for potential in human medicine. Despite their traditional use in treatment of diabetes mellitus, there is no systematic study on exploration of antidiabetic potential of these plants. Further, diabetes mellitus, being a chronic disease, needs a long term treatment and chronic consumption of these herbs may cause mild to severe side or toxic effects. However, there is no report on toxicity evaluation of these

Figure 1.C. inerme (A), J. mesnyi Hance (B) and C. citrinus (C).
2. Methods and materials
Swiss albino mice (25-30 g) and Wistar albino rats of either sex (150-250g) were procured from Lala Lajpat Rai University of Veterinary & animal Sciences, Hisar and housed in Animal House of Hindu College of Pharmacy (Sonepat, India) (Reg No. 585/02/CPCSEA) under controlled environmental conditions (25±2) oC with natural light/ dark cycle. The animals were allowed free access to food (standard pellet diet, Golden feed, Delhi, India) and water and acclimatized for at least a week before the commencement of the experiment. All experiments were duly approved by the Institutional Animal Ethics committee.
The leaves ofC. inerme,J. mesnyiHance andC. citrinuswere collected from healthy plants in Sonipat(India) in July 2009. The leaves were authenticated by Dr. H.B. Singh (Scientist F and Head Raw Materials Herbarium and Museum, NISCAIR, Delhi) with a Voucher number -Niscair/RHMD/Consult/-2009-10/1241/45.
2.1. Chemicals and reagents
The chemicals and solvents required for chemical evaluation and extracts were procured commercially from E. Merck (Mumbai, India), s.d. fine chemicals (Mumbai, India) and C.D.H. Private Limited, (New Delhi, India). Soxhlet extraction assembly of 2 L capacity (Borosil, New Delhi, India) was used for extraction of plant material. The extracts were concentrated on rotary vacuum evaporator (Hi - Con, New Delhi, India)
2.2. Preparation of extracts
The leaves were dried at room temperature under wellventilated shade by spreading them uniformly. The dried leaves were sorted, powdered, weighed (about 270 g) and extracted with petroleum ether to remove fatty constituents and chlorophyll. The marc was then subjected to successive solvent extraction with different solvents viz. Ethyl acetate, Chloroform, Ethanol and Water in soxhlet apparatus using about 800 mL of each solvent. Each extract was dried under vacuum and percent yield was calculated as % w/w with respect to total weight of dried leaves taken for extraction[13,14].
2.3. Acute toxicity study
The acute toxicity studies were carried out on Swiss albino mice of either sex weighing 25-30 g by the method described by Miller and Tainter[15,16]. The toxicity study was conducted at six doses (0.5, 1.0, 2.0, 3.0, 4.0 and 5.0 g/kg) of each of ethyl acetate, chloroform, ethanolic and aqueous extracts ofC. inerme,J. mesnyiHance andC. citrinus. The animals were divided into different groups with 6 animals in each group and fasted overnight. Each extract was administered orally at each of the six dose levels. The animals were observed for first 12 h for any toxic symptoms and for 24 h for any mortality. The number of animals dying during the period was noted. The LD50 was calculated by the method of Miller and Tainter. The percent mortality was calculated followed by calculation of the corrected percent mortality using the formula:
For 0% death = 100 × (0.25/n)
For 100% death = 100 × [(n-0.25)/n]
Where, n is the number of animals in each group. Corrected percent mortality was then transformed into probit values[17,18]. A graph of percent mortality (in probits) was plotted against log dose. The dose corresponding to probit 5 was read to be LD50.
2.4. Sub-acute toxicity study
Healthy Wistar albino rats of either sex weighing (150-250 g) were divided into different groups with 6 rats in each group. The control group received vehicle alone and the other groups received maximum therapeutic dose (MTD) of ethanolic, aqueous, ethyl acetate and chloroform extracts ofC. inerme,J. MesnyiHance andC. citrinusfor 28 d. The MTD was calculated from LD50 determined through acute toxicity study. The animals were monitored for body weight, mortality, food and water intake daily. After 28 days, all animals were fasted overnight and anaesthetized with ether[19,20]. The blood samples were collected in heparinised tubes for determining haematological parameters such as haemoglobin, RBC count, WBC count, blood urea, creatinine, SGOT, SGPT and blood sugar level[21,22,23].
2.5. Statistical analysis
All results were expressed as Mean ± Standard error. Data were analyzed using two-way analysis of variance (ANOVA) followed by Tukey’s test. The results were regarded as significant atP<0.05.
3. Results
The % yield of each extract of each of the selected plants is given in Table 1. Ethanol extract ofC. inermewas obtained with maximum yield (22.8%) whereas chloroform extract ofC. citrinuswas obtained in minimum amount (0.1%).
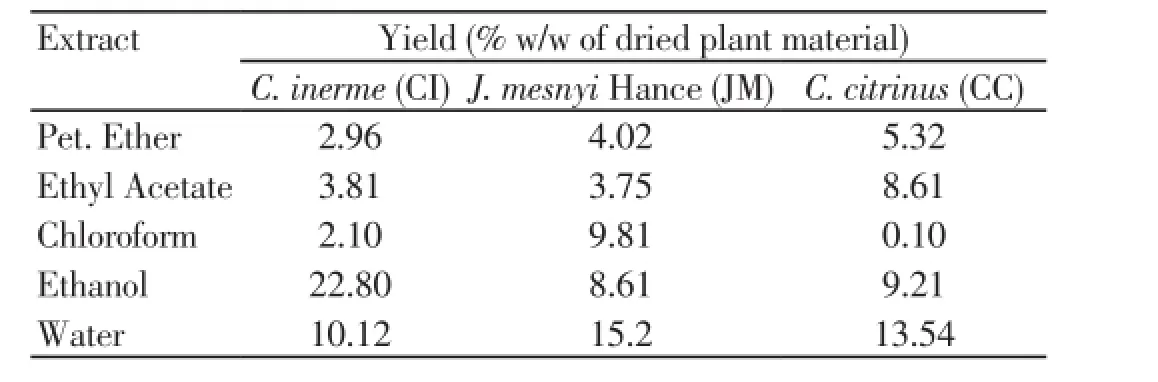
Table 1. Successive extract of leaves of C. inerme (CI), J. mesnyi Hance (JM), and C. citrinus (CC) Extract
3.1. Acute toxicity
It was carried out to determine LD50of each extract ofC. inerme,J. mesnyiHance andC. citrinus. Each extract was found to be non-toxic upto a dose of 2 g/kg in mice. The LD50of each extract is given in Table 2. The 1/5th of LD50of each extract was taken as its MTD for subsequentsub-acute toxicity studies.
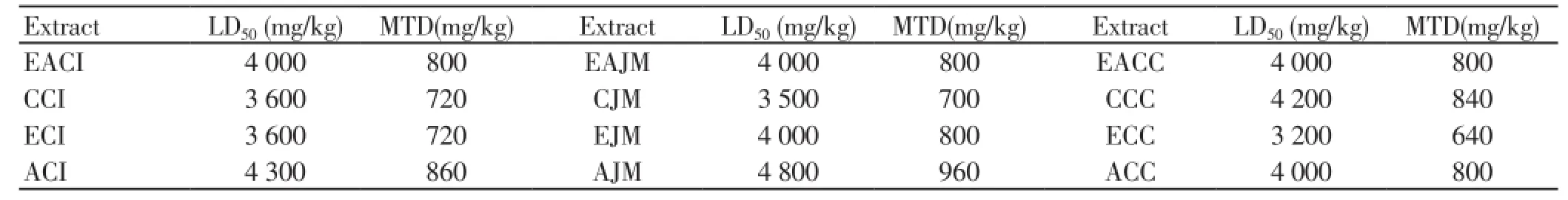
Table 2. LD50 and MTD of the ethyl acetate (EA), chloroform(C), ethanolic (E) and aqueous (A) extracts of C. inerme (CI), J. Mesnyi Hance (JM) and C. citrinus (CC).
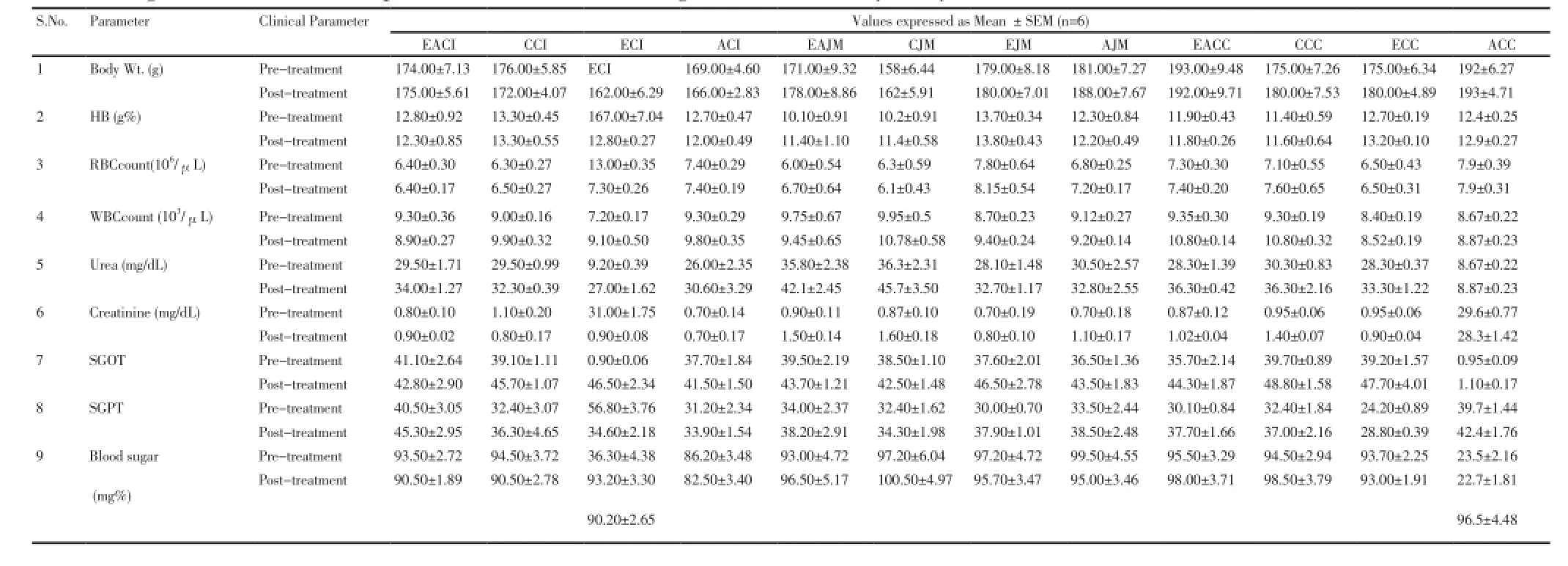
Table 3. Haematological and biochemical parameters of animals during sub-acute toxicity study.
3.2 Sub-acute toxicity study
None of the extract of any of the three plants produced any mortality in animals at the MTD administered (Table 2) over 28 d. No sign of observable toxicity was detected during the experimental period. All the haematological parameters such as haemoglobin, RBC count, WBC count, urea, creatinine level, blood sugar level and biochemical parameters such as SGOT, SGPT were determined before the start of dosing (pre-treatment) as well as at the end of the study (post-treatment) (Table 3). All parameters were found within the normal range. These results suggested that the selected herbs can be used for treatment of chronic diseases without exhibiting any side/toxic effect.
4. Discussion
In conclusion, Acute and sub-acute studies onC. inerme,J. mesnyiHance andC. citrinuswere carried out as a prerequisite to exploration of antidiabetic potential of these plants. The LD50 of each of the ethyl acetate, chloroform, ethanolic and aqueous extracts of each plant was determined and MTD was calculated. Each extract was evaluated for its sub-acute toxicity at its MTD and was found to be non toxic. The selected plants can be further explored for their therapeutic potential in different chronic diseases.
Conflict of interest statement
We declare that we have no conflict of interest
[1] Nagarajan S. Cultivation and Utilization of Medicinal Plants. Jammu-Tawi: CSIR; 1982, p. 584-604.
[2] Calis I, Hasny M, Yuruker A. Inerminosides A1, C and D, three iridoid glycosides fromClerodendrum inerme.J Phytochem1994; 37(4): 1083-1085.
[3] Pandey R, Verma RK, Gupta MM. Neo-clerodane diterpenoids fromClerodendrum inerme.Phytochem2005; 66(6): 643-648.
[4] Kanchanapoom T, Kasai R, Chumsri P, Hiraga Y, Yamasaki K. Mega stigmane and irioid glucosides fromClerodendrum inerme.J Phytochem2001; 58: 333-336.
[5] Rastogi RP. Compendium of Indian Medicinal Plants. New Delhi: NISCAIR; 2007, p. 368.
[6] Kuwajima H, Matsuuchi K, Inoue K, Fujita T, Inouye H. Secoiridoid glucosides from Jasminum mesnyi Hance. Phytochemistry 1985; 24(6): 1299-1303.
[7] Matsuda S, Inouye H, Zasshi Y. Two secoiridoid glycosides from Jasminum mesnyi Hance. Phytochem 1984; 21(4): 1232.
[8] Oyedeji OO, Lawal OA, Shode FO, Oyedeji AO. Chemical composition and antibacterial activity of the essential oils of Callistemon citrinus and Cllistemon viminalis from South Africa. Molecules 2009; 14: 1990-1998.
[9] Harborne JB. Phytochemical Method: A guide to modern techniques of plant analysis. 2nd ed. New York: Chapman and Hall; 2005, p. 40-56.
[10] Mukherjee K. Quality control of herbal drugs. 1st ed. New Delhi: Business horizons; 2002, p. 9, 15, 559.
[11] Chatterjee A. The treatise on Indian Medicinal Plants. New Delhi: Publications and information directorate; 1997, p. 17-18.
[12] Parsad SK, Kulshreshtha A, Qureshi TN. Antidiabetic activity of some herbal plants in streptozotocin induced diabetic albino rats. Pakistan J Nutr 2009; 5: 551-557.
[13] Pullaiah T. Antidiabetic plants in India and herbal based antidiabetic research. India: Regeny publications; 2003, p. 144, 209.
[14] Deshpade J. A handbook of medicinal herbs. India: Agrobios; 2006, p. 117-118.
[15] Ghosh MN. Fundamentals of experimental pharmacology. 4th ed. Kolkata: Hilton and Company; 2008, p. 176-183.
[16] Devbhuti D, Gupta JK, Devbhuti P, Bose H. Phytochemical and acute toxicity study on Bryophyllum calycinum. Acta Pol Pharm 2008; 65(4): 501-504.
[17] Bhardwaj S, Gupta D. Study of acute, subacute and chronic toxicity test. Int J Adv Pharm Biol Sci 2012; 1(2): 103-129.
[18] Biswas NR, Sen S, Singh S, Gopal N, Pandey RM, Giri D. Sub-acute toxicity study of a polyherbal drug in rats. Indian J Pharmacol 1998; 30(1): 239-244.
[19] Bonge KP, Penlap BV, Mbofung, CM. Acute and sub-acute toxicity of the methanol extract from Holarrhena floribunda. Eur J Exp Biol 2012; 2(4): 1284-1288.
[20] Burger C, Fischer DR, Filo VC. Acute and subacute toxicity of the hydroalcoholic extract from Wedelia paludosa in mice. J Pharm Sci 2005; 8(2): 370-373.
[21] Hilaly J, Hilaly JE, Isrili ZH, Badiaa L. Acute and chronic toxicological studies of Ajugaiva in experimental animals. J Ethnopharmacol 2004; 91: 43-50.
[22] Hussain T, Farred S, Siddiqui H, Vijaykumar M, Venkateswara R. Acute and sub-acute oral toxicity evaluation of Tephrosia purpurea extract in rodents. Asian Pac J Trop Dis 2012; 8: 129-132.
[23] Joshi CS, Priya ES, Venkatraman S. Acute and subacute toxicity studies on the polyherbal antidiabetic formulation Diakyur in experimental animal models. J Health Sci 2007; 53(2): 245-249.
ment heading
10.1016/S2221-6189(14)60069-X
*Corresponding author: Gulshan Bansal, Ph.D, Associate Professor, Department of Pharmaceutical Sciences and Drug Research, Panjabi University, Patiala, India.
Tel: +91-175-3046255
Fax: +91-175-2283073
Email: gulshanbansal@rediffmail.com; gulshanbansal@gmail.com
杂志排行
Journal of Acute Disease的其它文章
- The acute effect of the antioxidant drug “U-74389G” on red blood cells levels during hypoxia reoxygenation injury in rats
- Different profiles of mental and physical health and stress hormone response in women victims of intimate partner violence
- Time-critical AMI Detection: A novel and fast technique using the 12-lead ECG
- Epidemiological survey on scorpionism in Gotvand County, Southwestern Iran: an analysis of 1 067 patients
- Successful treatment of lower urinary tract obstruction with peritonealamniotic and vesicoamniotic shunting
- Simvastatin-induced Toxic Epidermal Necrolysis
