The acute effect of the antioxidant drug “U-74389G” on red blood cells levels during hypoxia reoxygenation injury in rats
2014-03-22TsomposPanoulisToutouzasZografosPapalois
C Tsompos, C Panoulis, K Toutouzas, G Zografos, A Papalois
1Department of Obstetrics & Gynecology, Mesologi County Hospital, Etoloakarnania, Greece
2Department of Obstetrics & Gynecology, Aretaieion Hospital, Athens University, Attiki, Greece
3Department of Surgery, Ippokrateion General Hospital, Athens University, Attiki, Greece
4Department of Surgery, Ippokrateion General Hospital, Athens University, Attiki, Greece
The acute effect of the antioxidant drug “U-74389G” on red blood cells levels during hypoxia reoxygenation injury in rats
C Tsompos1*, C Panoulis2, K Toutouzas3, G Zografos4, A Papalois5
1Department of Obstetrics & Gynecology, Mesologi County Hospital, Etoloakarnania, Greece
2Department of Obstetrics & Gynecology, Aretaieion Hospital, Athens University, Attiki, Greece
3Department of Surgery, Ippokrateion General Hospital, Athens University, Attiki, Greece
4Department of Surgery, Ippokrateion General Hospital, Athens University, Attiki, Greece
Objective: To examine the effect of the antioxidant drug “U-74389G”, on rat model and particularly in a hypoxia reoxygenation (HR) protocol. The beneficial effect or non-effectiveness of that molecule was studied hematologically using mean red blood cells levels. Methods: 40 rats of mean weight 231.875 g were used in the study. Red blood cells levels were measured 60 min after reoxygenation (groups A and C) and 120 min after reoxygenation (groups B and D) with administration of the drug U-74389G only in groups C and D. Results: U-74389G administration non-significantly increased the red blood cells level by (0.64±0.32)% (P=0.810 6). Reoxygenation time also non-significantly increased the red blood cells level by (4.63±2.36)% (P=0.068 0). However, U-74389G administration and reoxygenation time together non-significantly increased the red blood cells level by (1.05±0.53)% (P=0.491 1). Conclusions: Results of this study show that U-74389G administration, reoxygenation time and their interaction non significantly increased the red blood cells levels within 2 hours’ time interval.
ARTICLE INFO
Article history:
Received 8 October 2014
Received in revised form 20 October 2014
Accepted 25 October 2014
Available online 20 November 2014
Hypoxia
1. Introduction
Tissue hypoxia and reoxygenation (HR) remain one of the main causes of permanent or transient damage with serious implications on adjacent organs and certainly on patients’ health. The use of antioxidant substances has been a research subject for many years. However, even if important progress has been made, satisfactory answers have not been given yet to fundamental questions, such as, how much powerful should an antioxidant be, when should it be administered, and in which dosage. The particularly satisfactory action of the antioxidant U-74389G in tissue protection has been noted in several performed experiments. Since a careful literature search (PubMed - Medline) was conducted, it was realized that this certain antioxidant has been tried in ischemia reperfusion (IR) experiments. However, just few relative reports were found, not covering completely this particular matter. Also, a lot of publications addressed trials of other similar molecules of aminosteroids (lazaroids) to which the studied molecule also belongs to. U-74389G or better 21-[4-(2,6-di-1-pyrrolidinyl-4-pyrimidinyl)-1-piperazinyl]-pregna-1,4,9(11)-triene-3,20-dione maleate salt1 is an antioxidant which prevents both arachidonic acid-induced and iron-dependent lipid peroxidation. It protects against HR injury in animal heart, liver, and kidney models. These membrane-associating antioxidants2 are particularly effective in preventing permeability changes in brain microvascular endothelial cells monolayers. The same authors3-6 found the influence of U-74389G as depicted at table 1.1 on some biochemic variables serum levels in related IR injury experiments in rats 1 h, 1.5 h, 2 h and interaction of U-74389G and reperfusion time after reperfusion (Table 1).
The aim of this experimental study was to examine the effect of the antioxidant drug “U-74389G” on rat model andparticularly in a HR protocol. The beneficial effect or noneffectiveness of that molecule was studied by measuring the red blood cells (RBC) levels.
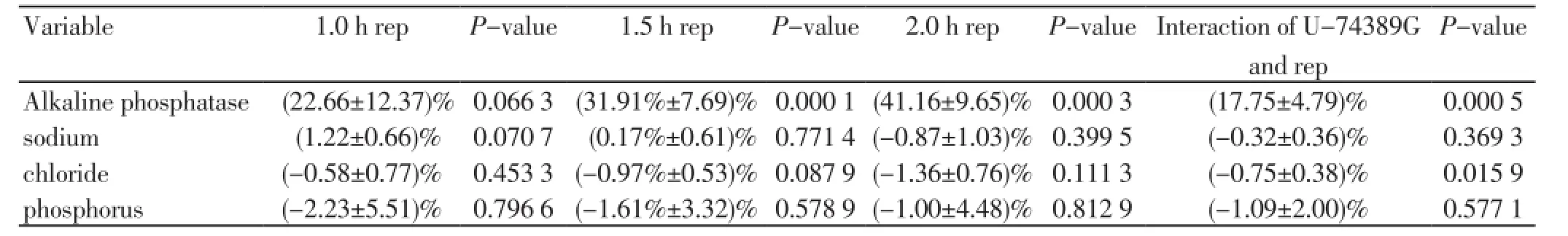
Table 1. The U-74389G influence (+SD) on the levels of some biochemic variables concerning reperfusion (rep) time.
2. Materials and methods
2.1. Animal preparation
This experimental study was laid out at the Exprerimental Research Center of ELPEN Pharmaceuticals Co. Inc. S.A. at Pikermi, Attiki and by Veterinary Address of East Attiki Prefecture under 3693/12-11-2010 & 14/10-1-2012 decisions. All settings needed for the study including of consumables, equipment and substances used, were a courtesy of that S. A. Albino female Wistar rats were used in accordance with accepted standards of humane animal care. They spent in laboratory 7 days before the experiment with easy access to water and food. The experiment was acute, that is, the animal usage was completed by following experimental set of times without awakening and preservation of the rodents. They were randomly assigned to four experimental groups (10 animals in each group).
1) Hypoxia for 45 min followed by reoxygenation for 60 min (group A).
2) Hypoxia for 45 min followed by reoxygenation for 120 min (group B).
3) Hypoxia for 45 min followed by immediate U-74389G intravenous (IV) administration and reoxygenation for 60 min (group C).
4) Hypoxia for 45 min followed by immediate U-74389G IV administration and reoxygenation for 120 min (group D).
The molecule U-74389G dose was 10 mg/kg body weight of animals. The experiment started with prenarcosis. Then, animals were submitted into general anesthesia. Their electrocardiogram and acidometry were continuously monitored. The inferior aorta was prepared so as its flow to be excluded by forceps. After exclusion, the protocol of HR was applied, exactly as described in experimental groups. The molecules were administered at the time of reoxygenation, through inferior vena cava after catheterization achieved after general anesthesia. The RBC measurement was performed at 60 min of reoxygenation (groups A and C) and 120 min of reoxygenation (groups B and D).
Rats were submitted into general anesthesia by initial intramuscular (IM) administration of 0.5 cc compound, which constituted of 0.25 cc xylazine, [25 cc, 20 mg/cc] and 0.25 cc ketamine hydrochloride [1 000, 100 mg/cc, 10 cc]. Before initiation of laparotomy, 0.03 cc butorphanol [10 mg/ cc, 10 cc] anesthetic agent was administered subcutaneous (SC). Continuous oxygen supply was administered during whole experiment performance. Ischemia was caused by clamping inferior aorta, over renal arteries, for 45 min after laparotomic access was achieved. Reoxygenation was induced by removing the clamp and reestablishment of inferior aorta patency. Forty (40) female Wistar albino rats were used of mean weight 231.875 g [Std. Dev: 36.597 03 g], with min weight ≥ 165 g and max weight < 320 g. Rats weight could be potentially a confusing factor, e.g. fatter rats to have greater RBC levels. This suspicion will be investigated.
2.2. Model of hypoxia reoxygenation injury
Control groups: 20 control rats of mean weight 252.5 g [Std. Dev: 39.319 88 g] were subjected to hypoxia for 45 min followed by reoxygenation.
Group A: Reoxygenation which lasted 60 min concerned 10 controls rats of mean weight 243 g [Std. Dev: 45.777 24 g], mean RBC levels 6.983 106/mm3 [Std. Dev: 0.583 629 4 106/ mm3] (Table 2).

Table 2. Weight and RBC mean values and Std. Dev. of groups.
Group B: Reoxygenation which lasted 120 min concerned 10 controls rats of mean weight 262 g [Std. Dev: 31.109 13 g], mean RBC levels 7.362 106/mm3 [Std. Dev: 0.648 344 9 106/ mm3] (Table 2).
Lazaroid (L) group: 20 rats of mean weight 211.25 g [Std. Dev: 17.537 55 g] suffered by hypoxia for 45 min followed by reoxygenation in the beginning of which 10 mg U-74389G/kgbody weight were IV administered.
Group C: Reoxygenation which lasted 60 min concerned 10 L rats of mean weight 212.5 g [Std. Dev: 17.834 11 g], mean RBC levels 7.081 106/mm3 [Std. Dev: 0.508 056 2 106/mm3] (Table 2).
Group D: Reoxygenation which lasted 120 min concerned 10 L rats of mean weight 210 gr [Std. Dev: 18.104 63 g], mean RBC levels 7.354 106/mm3 [Std. Dev: 0.442 422 6 106/mm3] (Table 2).
3. Results
Every weight rats group initially was compared with each one from 3 remained groups applying statistical paired t-test (Table 3). Any emerging significant difference among RBC levels will be investigated whether owed in the above probable mentioned significant weight correlations. Every RBC levels rats group initially was compared with each one from 3 remained groups applying statistical paired t-test (Table 3).
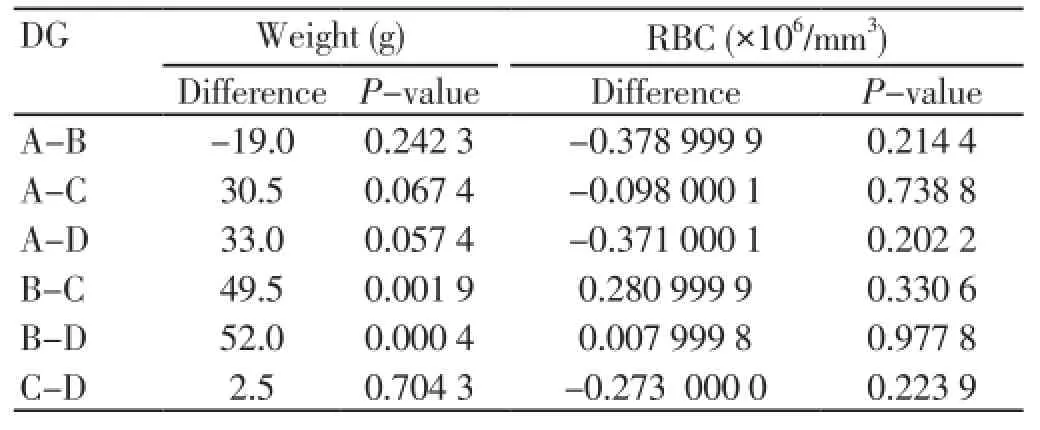
Table 3. Statistical significance of mean values difference for groups (DG) after statistical paired t test application.
Applying generalized linear models (glm) with dependent variable the red blood cells levels and independent variables the U-74389G administration or no, the reoxygenation time and their interaction, resulted in: U-74389G administration non significantly increased the red blood cells level by 0.045 000 1 106/mm3 [-0.315 108 9 106/mm3 - 0.405 109 2 106/mm3] (P=0.801 7). This finding was in accordance with the results of paired t-test (P=0.819 5). Reoxygenation time also non-significantly increased the red blood cells level by 0.326 106/mm3 [-0.018 144 5 106/mm3 - 0.670 144 5 106/mm3] (P=0.062 7), in accordance also with pairedt-test (P=0.073 3). However, U-74389G administration andreoxygenation time together non-significantly increased the red blood cells level by 0.074 181 9 106/mm3 [-0.141 785 2 106/mm3 - 0.290 149 106/mm3] (P=0.491 1). Reviewing the above and Table 3, Tables 4 and 5 sum up concerning the increasing influence of U-74389G in connection with reoxygenation time. Inserting the rats weight also as an independent variable at generalized linear models analysis, a non-significant relation results in red blood cells levels (P=0.093 7), so as to further investigation is not needed.
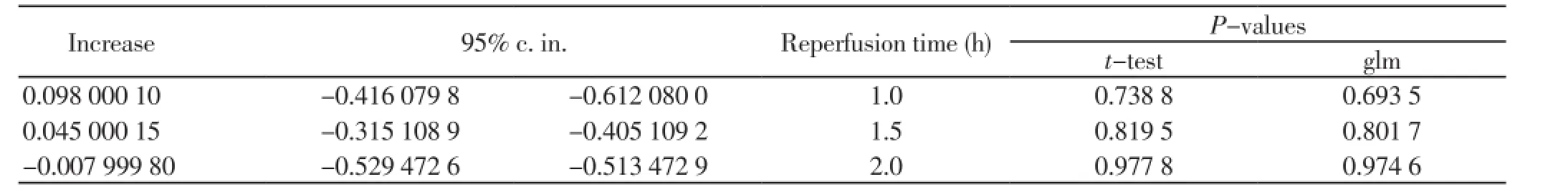
Table 4. The increasing influence of U-74389G in connection with reperfusion time.
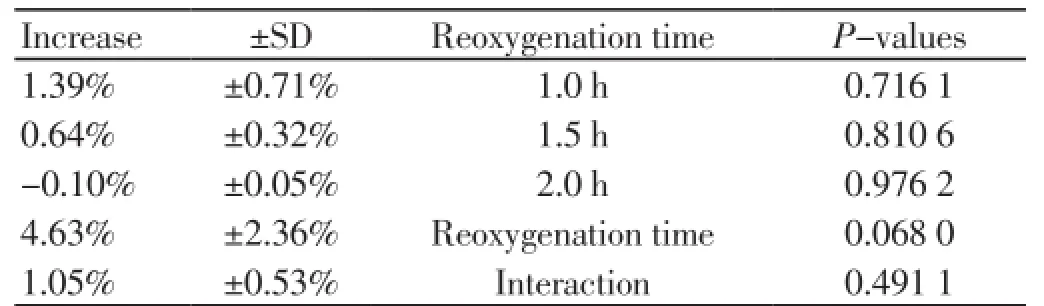
Table 5. The (%) increasing influence of U-74389G in connection with reoxygenation time.
4. Discussion
Lee CH et al studied[7] Wistar rats after three hours ileal IR injury and noticed significant decreases in circulating red blood cells levels than control ones. Wang MX et al investigated[8] the effect of IR-induced Wistar rat mesenteric microcirculatory dysfunctions randomly distributed into 5 groups. I/R led to circulating red blood cells among all IR-induced manifestations, significantly reduced in mesenteric post-capillary venules. Ghibu S et al studied[9] the effects of post-ischemic recovery and oxidative stress in isolated perfused rat hearts challenged with an IR sequence. Red cells are a good model to investigate oxidative damage in biological membranes; lipoic acid induced a slight increase in coronary flow in isolated perfused hearts, after global total ischemia. Gumuştaş K et al investigated[10] the effects of superoxide dismutase (SOD) expression in hyperglycemic rats after cerebral ischemia reperfusion injury. Superoxide dismutases SOD in red blood cells were detected in hyperglycemic rats with cerebral IR injury induced by two common carotid artery occlusions. Treatment prophylaxis increased SOD in IR. Arumugam TV et al subjected[11] female Wistar rats to renal IR. Pre-ischemic treatment with the C5a receptor (C5aR) antagonist substantially inhibited or prevented IR-induced tissue hemorrhage, hematuria, vascular leakage and microvascular permeability. Thisantagonist, however, did not inhibit complement-mediated lysis of red blood cells, suggesting unimpaired formation of the membrane attack complex. Bekyarova G et al supposed[12] that burns are followed by oxidative changes in red blood cells, probably as a result of IR which takes place in the microvasculature of rats injured tissues. This leads to a marked decrease in erythrocyte deformability, one of the most prominent factors for hemorheological disorders in the early post-burn period. Decreased erythrocyte deformability is partly related with oxidative membrane damage of red blood cells in the early post burn period. Lennon GM et al applied[13] acute unilateral renal IR to four groups of ten adult male Wistar rats. The vascular perfusion indexes (VPI) for each vascular region of the kidney were similar in control and ischemic kidneys that were not subjected to reflow. In contrast, VPI was markedly decreased in the inner stripe and inner medulla in animals in which revascularisation had occurred, and the vasculature in these regions was histologically shown to be packed with red blood cells. Post-ischemic renal failure is associated with hyperperfusion (vascular hyperemia) of the medulla (outer medulla) resulting from blockage of the vasculature that occurs during revascularisation.
U-74389G also influences the RBC level. Pratt MF examined[14] the effects of 21-aminosteroid U-74389G on pig model random skin flap survival/necrosis pathophysiology. Demonstrated mechanisms of skin flap failure included the alteration of erythrocyte flexibility with resultant accumulation of damaging oxygen-free radicals. Random skin flap survival was improved significantly with U-74389G administration.
U-74389G administration, reoxygenation time and their interaction non significantly increase the red blood cells level within 2 hours’ time interval. This drug may need more time in order to reveal its significant acute increasing capacity. Clinical trials with larger samples and longer study times may prove it.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgment
This study was funded by Scholarship by the Experimental Research Center ELPEN Pharmaceuticals (E.R.C.E), Athens, Greece. The research facilities for this project were provided by the aforementioned institution.
[1] Cayman Chemical Company. U-74389G.[Online]. Avaliable from: https://www.caymanchem.com/app/template/Product.vm/ catalog/75860.
[2] Fenglin Shi, Jennifer Cavitt, Kenneth L Audus. 21-aminosteroid and 2-(aminomethyl)chromans inhibition of arachidonic acidinduced lipid peroxidation and permeability enhancement in bovine brain microvessel endothelial cell monolayers. Free Radical Biol Med 1995; 19(3): 349-357.
[3] C Tsompos, C Panoulis, K Toutouzas, G Zografos, A Papalois. The effect of the antioxidant drug “U-74389G” on alkaline phosphatase levels during ischemia reperfusion injury in rats. J Exp Clin Med 2014; 31: 99-102.
[4] C Tsompos, C Panoulis, K Toutouzas, G Zografos, A Papalois. The effect of the antioxidant drug “U-74389G” on sodium levels during ischemia reperfusion injury in rats. Crit Care Shock 2014; 2: 31-36.
[5] C Tsompos, C Panoulis, K Toutouzas, G Zografos, A Papalois. The effect of the antioxidant drug “U-74389G” on chloride during ischemia reperfusion injury in rats. Med Rev 2014; 50(2): 40-44.
[6] C Tsompos, C Panoulis, K Toutouzas, G Zografos, A Papalois. The effect of the antioxidant drug “U-74389G” on phosphorus levels during ischemia reperfusion injury in rats. Psychogeriatria Polska 2013; 10(3): 103-108.
[7] Lee CH, Hsiao CC, Hung CY, Chang YJ, Lo HC. Long-term enteral arginine supplementation in rats with intestinal ischemia and reperfusion. J Surg Res 2012; 175(1): 67-75.
[8] Wang MX, Liu YY, Hu BH, Wei XH, Chang X, Sun K, et al. Total salvianolic acid improves ischemia-reperfusioninduced microcirculatory disturbance in rat mesentery. World J Gastroenterol 2010; 16(42): 5306-5316.
[9] Ghibu S, Lauzier B, Delemasure S, Amoureux S, Sicard P, Vergely C, et al. Antioxidant properties of alpha-lipoic acid: effects on red blood membrane permeability and adaptation of isolated rat heart to reversible ischemia. Mol Cell Biochem 2009; 320(1-2): 141-148.
[10] Gumuştaş K, Meta Guzeyli FM, Atukeren P, Sanus GZ, Kemerdere R, Tanriverdi T, et al. The effects of vitamin E on lipid peroxidation, nitric oxide production and superoxide dismutase expression in hyperglycemic rats with cerebral ischemia-reperfusion injury. Turk Neurosurg 2007; 17(2): 78-82.
[11] Arumugam TV, Shiels IA, Strachan AJ, Abbenante G, Fairlie DP, Taylor SM, et al. A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int 2003; 63(1): 134-142.
[12] Bekyarova G, Yankova T. Alpha-Tocopherol and reduced glutathione deficiency and decreased deformability of erythrocytes after thermal skin injury. Acta Physiol Pharmacol Bulg 1998; 23(2): 55-59.
[13] Lennon GM, Ryan PC, Gaffney EF, Fitzpatrick JM. Changes in regional renal perfusion following ischemia/reperfusion injury to the rat kidney. Urol Res 1991; 19(4): 259-264.
[14] Pratt MF. Edmund Prince Fowler Award Thesis. Evaluation of random skin flap survival in a porcine model. Laryngoscope 1996; 106(6): 700-712.
ment heading
10.1016/S2221-6189(14)60068-8
*Corresponding author: Tsompos Constantinos, 87 Neratziotissis street, Marousi 15124Attiki, Greece.
Tel: 00306946674264, 00302631360237
Fax: 00302106811215
E-mail: Constantinostsompos@yahoo.com
Reoxygenation
U-74389G
Red blood cells
杂志排行
Journal of Acute Disease的其它文章
- Acute and sub-acute toxicity study of Clerodendrum inerme, Jasminum mesnyi Hance and Callistemon citrinus
- Different profiles of mental and physical health and stress hormone response in women victims of intimate partner violence
- Time-critical AMI Detection: A novel and fast technique using the 12-lead ECG
- Epidemiological survey on scorpionism in Gotvand County, Southwestern Iran: an analysis of 1 067 patients
- Successful treatment of lower urinary tract obstruction with peritonealamniotic and vesicoamniotic shunting
- Simvastatin-induced Toxic Epidermal Necrolysis
