Acute type B aortic dissection: update on proper management
2014-03-22GeorgiosGeropapasGeorgeGalyfosIoannisStefanidisIoannisStamatatosStavrosKerasidisSotiriosGiannakakisGeorgiosKastrisiosGerasimosPapacharalampousChrisostomosMaltezos
Georgios Geropapas, George Galyfos, Ioannis Stefanidis, Ioannis Stamatatos, Stavros Kerasidis, Sotirios Giannakakis, Georgios Kastrisios, Gerasimos Papacharalampous, Chrisostomos Maltezos
Department of Vascular Surgery, KAT General Hospital, Athens, Greece
Acute type B aortic dissection: update on proper management
Georgios Geropapas*#, George Galyfos#, Ioannis Stefanidis, Ioannis Stamatatos, Stavros Kerasidis, Sotirios Giannakakis, Georgios Kastrisios, Gerasimos Papacharalampous, Chrisostomos Maltezos
Department of Vascular Surgery, KAT General Hospital, Athens, Greece
This study aims to collect and present all current literature data on the diagnostic and therapeutic management of acute type B aortic dissection. It includes a comprehensive literature search utilizing the following keywords: ‘acute aortic dissection’, ‘type B aortic dissection’, ‘conservative management’, ‘endovascular repair’, ‘open surgery’ and ‘diagnosis’. Uncomplicated acute type B aortic dissection can be effectively managed using conservative management, although open repair is indicated only for complicated cases. Endovascular repair shows promising results in selected patients with increased perioperative risk and without contraindications. Recent evidence supports endovascular repair even in uncomplicated cases, although more data on long-term outcomes are needed. Early risk stratification and evaluation of the patient is crucial for selection of optimal management.
ARTICLE INFO
Article history:
Received 8 January 2015
Received in revised form 11 January 2015
Accepted 14 January 2015
Available online
Acute aortic dissection
1. Introduction
Acute aortic dissection (AAD) remains a potentially life-threatening condition that is followed by a 2-3 times higher risk for rupture compared to that of aneurysms[1]. Although aortic dissection is the most common etiology for acute aortic syndrome, other processes such as intramural hematoma and penetrating atherosclerotic ulcers are being increasingly recognized[2]. The incidence is approximately 3-5/100 000 in western countries with an observed increase during the past decades[3]. Moreover, men suffer more frequently than women do from this acute condition, showing almost a two times higher incidence[4]. Additionally, AAD seems to present in a particular circadian or seasonal pattern. A timeframe between 6.00 am and 12.00 pm as well as winter months seem to show an increased prevalence[5].
Regarding pathophysiology, an endothelial damage of the aortic wall allows the blood to flow through the different aortic wall and to form a false lumen that could apply pressure to the true lumen of the aorta[5]. This leads to a dissection of the aortic wall that could expand either proximally or distally. Acute type B aortic dissection (ATBAD) (identified within 2 weeks of symptom onset), as described using the Stanford classification, accounts for 25%-40% of all aortic dissections[6]. Stanford type B or DeBakey III aortic dissection originates in the descending thoracic aorta without retrograde extension into the ascending aorta, and involves the aorta distal to the left subclavian artery[7]. Acute type B dissections may be classified as uncomplicated or complicated. Approximately 25% of patients presenting with ATBAD are complicated at admission by malperfusion syndrome or hemodynamic instability, resulting in a high risk of early death when untreated. Complicated type B aortic dissection refers to malperfusion syndrome involving visceral, renal, or extremity ischemia, rupture or impending rupture, uncontrolled hypertension, persistent abdominal or chest pain, or findings of rapid expansion on computed tomography (CT) imaging[7].
The selection of optimal therapeutic strategy for ATBAD, in contrast to type A dissection, seems to still be under debate. Therefore, this review aims to collect and present current literature data on proper management of ATBAD as well as to make useful conclusions for all physicians.
2. Risk factors and clinical presentation
Many risk factors have been identified to be associated with the development of acute aortic syndromes and AAD (Table 1). Almost 75% of these patients suffer from arterial hypertension[8]. Male sex, age and smoking have also been identified as major risk factors[9,10]. Although trauma and endovascular interventions are a leading cause for aortic dissection in patients of all ages[11], hereditary syndromes remain the commonest predisposing factor in children and younger individuals[12]. Marfan syndrome among other connective tissue disorders (e.g. Ehlers-Danlos) is an important risk factor for aortic dissection, especially in young patients and adolescents, and thresholds for prophylactic aortic replacement are typically lower for this specific patient group[13]. However, type B dissection is less frequent than type A in this particular group of patients[13]. Additionally, cocaine use is also implicated in 1.8% of patients with AAD, as underlined by Dean et al[14].

Table 1 Major risk factors for acute aortic syndromes and aortic dissection.
Recently, the International Registry of Acute Aortic Dissection (IRAAD) declared that about one fifth of these patients do not present with an aortic dilatation[15]. The risk of ATBAD was thought to increase with descending thoracic aortic diameter. However, the majority of patients with ATBAD present with a descending aortic diameter less than 5.5 cm before dissection. Therefore, aortic diameter measurements do not seem to be a useful parameter to prevent aortic dissection, and other methods are needed to identify patients at risk for ATBAD[16]. Furthermore, inflammatory or infectious diseases could also predispose to type B aortic dissection[17,18]. Finally, aortic atherosclerosis seems to play an important role in aortic dissection development, and is more associated with distal than with proximal aortic dissection, according to recent data[19].
Regarding clinical presentation, pain is the most commonly reported presenting symptom of AAD regardless of patient age, sex, or other associated clinical complaint[20]. It is usually described as tearing, stabbing, or sharp in character. Almost 17% of individuals will feel the pain migrate as the dissection extends down the aorta, while the location of pain is associated with the location of the dissection[21]. The combination of two or more high-risk features (Table 2) is strongly suggestive of AAD[20]. Up to 20% of these patients will suffer from syncope. Although acute aortic valve insufficiency and myocardial/cerebral ischemia are observed mainly in type A dissection, type B dissection could more often lead to paraplegia, acute renal insufficiency or even limb ischemia. In AADs, compromise of one or both renal arteries occurs in 5%-8% of cases, while mesenteric ischemia (ischemia of the large intestines) occurs 3%-5% of the time[21]. Approximately 30% of patients who present with ATBAD have a complicated dissection, making immediate treatment imperative to save the life or the limb of the patient[22]. Independent predictors of death in type B dissection have been jointly termed the ‘deadly triad’: hypotension/shock, absence of chest/back pain on presentation, and branch vessel involvement[23].
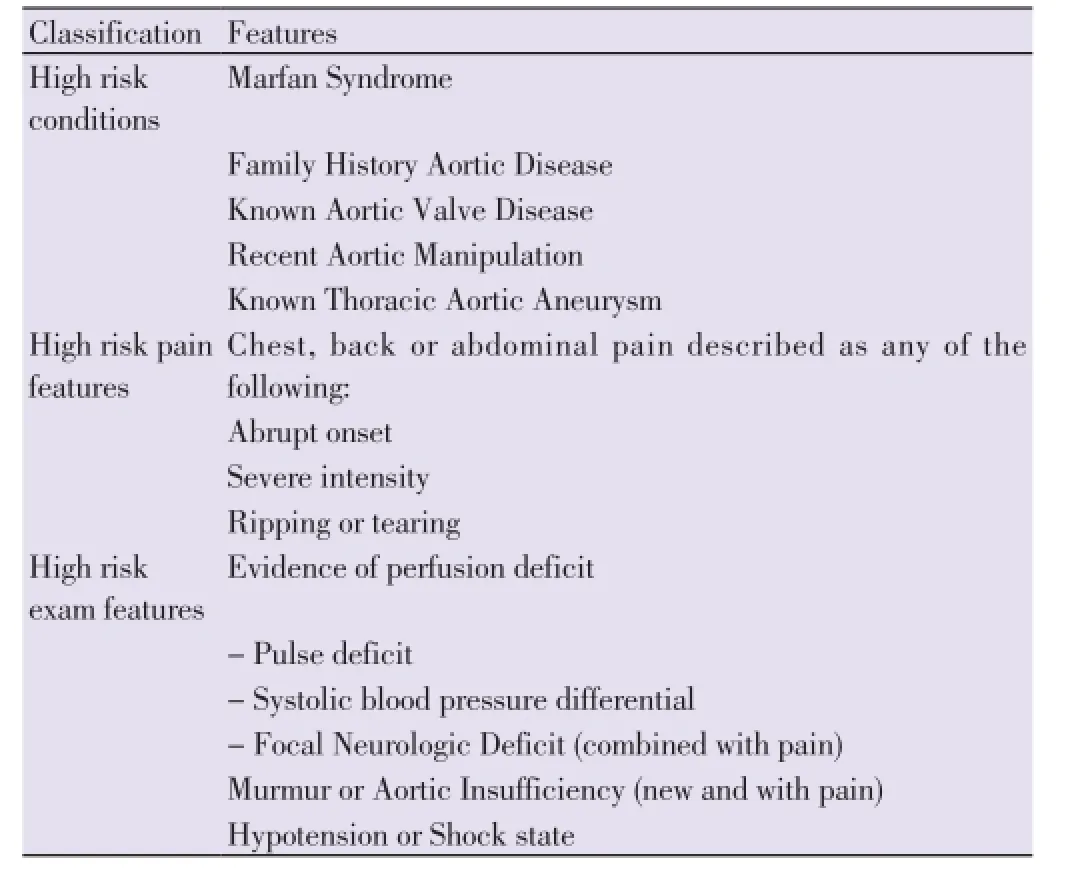
Table 2 High-risk features for aortic dissection.
3. Diagnostic tools
Although imaging studies are sensitive and specific in general, timely diagnosis can be delayed because of variability in presenting symptoms and the relatively low frequency with which acute aortic syndromes are seen in the emergency setting. Therefore, utilization of proper diagnostic tools is imperative to set final diagnosis, although the indication for use of most imaging studies is of evidence level C.
3.1. Plain chest X-ray
Findings on plain chest radiographs of patients with aortic dissection are variable and often overlap those of patientswithout dissection. In an older study by Jagannathet al., a widened aortic knob, widened descending aorta, and widened mediastinum showed the greatest inter-observer agreement (P<0.001) although the overall inter-observer agreement was poor[24]. Although their utilization in the emergency setting is useful, this finding dictates that further definitive investigation should be undertaken. In another retrospective study by Laiet al., postero-anterior (PA) chest radiography showed a higher diagnostic accuracy compared to antero-posterior (AP) imaging[25]. However, according to the authors, a lower threshold for proceeding to a computed tomography (CT) evaluation is recommended in the elderly and patients with widened mediastinum in the AP X-ray. Additionally, data indicate a limitation of plain chest radiography in discriminating between AAD and other acute coronary syndromes[26].
3.2. D-dimers
D-dimers are cleavage products of fibrin that occur during plasmin-mediated fibrinolysis of blood clots[27]. In the emergency setting, their measurement in serum represents a valuable and cost-effective tool in the differential diagnosis of acute chest pain including the main lifethreatening entities: acute coronary syndrome, pulmonary embolism, and acute aortic syndrome[27]. However, because of limitations in specificity, d-dimer testing is only one component in the diagnosis of acute chest pain. It has been shown that a positive d-dimer test has a sensitivity of about 97%, a specificity of 56%, a positive predictive value of about 60%, and a negative predictive value of up to 96%[28]. Furthermore, d-dimer levels seem to correlate with the anatomic extension of the dissection as well[29]. In the recent meta-analysis of Shimonyet al.[30], the authors conclude that plasma levels <500 ng/mL is a useful screening tool to identify patients who do not have AAD. Therefore, serum d-dimers could be used to identify subjects who are unlikely to benefit from further aortic imaging.
3.3. Ultrasound assessment
The combination of different ultrasound techniques such as transthoracic, suprasternal, subcostal, and transesophageal ultrasonography has a high sensitivity and specificity in the diagnosis of aortic dissection[31]. Main goals of this examination are a) to confirm the diagnosis by visualizing the intimal membrane, b) to differentiate the true or false lumen, c) to detect the intimal tear, d) to determine the extent of the dissection and classify it, and e) to detect wall motion abnormalities or side branch involvement[31]. There are studies showing that the transesophageal approach can identify specific important elements of the dissection (such as false lumen thrombosis or visualization of flap in the aorta) more accurately than the transthoracic approach[32]. Recently, there have been studies highlighting the potential value of intravascular ulrtasonography (IVUS) in the diagnosis of AAD as well as aortic intramural hematoma[33].
3.4. Computed tomography/angiography (CT/CTA)
The widespread use of 3-dimensional imaging such as CT has increased dramatically in the last decades, and the incidence of diagnosed aortic dissection cases has increased as well. Current methods to risk stratify patients with type B aortic dissection, rely upon static imaging, usually CT angiography[34]. In a study by Sommeret al., spiral CT demonstrated a specificity of 100% compared to multiplanar transesophageal ultrasound (94%)[35]. Concerning timeresolved CT-angiography, data indicate that it is feasible at a reasonable effective radiation dose and adds significant diagnostic information with therapeutic consequences in patients with aortic dissection[36].
3.5. Magnetic resonance imaging (MRI)
Although MRI shows a high sensitivity and specificity for the diagnosis of AAD, this technique is not usually available in all institutions, and the examination can be very difficult in unstable patients[35]. MRI study allows multiplanar study of the lesions without contrast medium, and best visualization of sub-endothelial bleeding in dissections without intimal lesion[37]. Therefore, Liuet al. support that 3D contrast-enhanced MR angiography with post-processing is a fast, accurate, and noninvasive technique that may prove to be the optimal imaging modality in medically stable patients with aortic dissection[38].
3.6. 18F-flurodeoxyglucose positron emission tomography (18F-FDG PETCT)
This is a novel imaging technique, with preliminary data showing that it can visualize atherosclerotic plaques and that it has prognostic value concerning risk for rupture and progression of dissection[39,40]. Additionally, Reepset al. have found that this imaging tool could probably differentiate acute from chronic aortic dissection in unclear cases[41]. However, more studies are needed to clarify its role in clinical scenario.
4. Treatment strategies
4.1. Conservative therapy
Patients suffering from acute distal aortic dissection are at significantly lower risk of early death from complications of the dissection than are those with proximal dissection[42]. Therefore, aggressive medical treatment has been recommended since almost 50 years and has been advocated by many authors[43]. The main goal is the reduction of systolic blood pressure (goal levels: 100-120 mmHg) anddiminution of the rate of left ventricular ejection (dP/dt). Primary concern is also the relief from pain using mostly morphine regimens[43]. The combination of beta-blockers along with another antihypertensive agent is usually recommended. However, it is suggested that nitrates should be given after beta-blockage, in order to avoid immediate vasodilatation, secondary catecholamine release, increase of left ventricular contractility and subsequent extension of dissection. This intensive medical therapy seems to reduce mortality in type B aortic dissection[44]. Promising results have been reported with angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) as well[45], although some authors have not found a significant prognostic value[42].
Heart rate reduction has been thought to be an important aspect of medical treatment as well. Kodamaet al.conclude that tight heart rate reduction improved the outcome in patients with AAD who were conservatively managed[42]. A ‘complication-specific’ approach has been suggested, including medical management with ‘anti-impulse therapy’for uncomplicated acute descending dissections[46]. Following initial stabilization with intravenous antihypertensives, most patients will require long-term antihypertensive treatment. As recommended by the latest guidelines, acute thoracic aortic dissection involving the descending aorta should be managed medically unless lifethreatening complications develop (such as malperfusion syndrome, progression of dissection, enlarging aneurysm, inability to control blood pressure or symptoms; Level of Evidence: B)[20].
However, uncertainty still remains regarding the optimal management strategy for uncomplicated acute type B dissection. Best medical treatment is associated with a considerable risk for disease progression towards complicated dissection or aneurysm degeneration of the affected segment with an estimated incidence of almost 40%[47]. Furthermore, long-term outcomes after primary conservative treatment have been associated with a very high complication rate[48].
4.2. Open repair
Despite aggressive antihypertensive treatment, hospital mortality after primary conservative treatment is still high and a substantial proportion of patients require surgery during initial hospitalization[49]. Main goal of surgical management in ATBAD is the prevention of aortic rupture or the treatment of serious complications (Table 3). Carrelet al. underline that early surgery will be needed in the following cases, even for uncomplicated type B dissection: i) younger patients with 5 cm diameter of the aorta at initial evaluation, ii) those with Marfan syndrome, iii) patients with limited false aneurysm or retrograde dissection into the aortic arch, and iv) those with poor medical compliance or uncontrollable proximal hypertension[49].

Table 3 Major complications of acute aortic dissection.
Perioperative mortality for patients treated for aortic dissection ranges from 5% to 10% and could reach 70% in complicated cases[50]. Independent prognostic factors for perioperative mortality in open repair include the presence of cardiac tamponade, the location of the intima tear, the duration of surgery, the presence of renal/visceral ischemia, renal dysfunction and the presence of pulmonary disease[51]. Although data reveal a superiority of endovascular techniques regarding early and midterm mortality, Moulakakiset al.conclude in their recent meta-analysis that open repair still has a significant role as endovascular repair is not applicable in all patients and there are still concerns regarding the durability of this technique[48]. However, the absence of randomized trials comparing endovascular with open repair treatments in complicated type B acute dissection remains a limitation.
Two recent meta-analyses seem to lead to controversial results. Luebkeet al. suggest that the use of endovascular treatment in complicated type B aortic dissection leads to favourable early outcomes with lower neurologic and vascular complications, although there were no sufficient data for long-term outcomes[52]. However, Zhanget al. conclude that endovascular treatment reduces shortterm mortality, although it does not improve postoperative complications or long-term mortality significantly[53].
4.3. Endovascular repair
Endovascular repair may be of particular value in patients with significant co-morbid conditions (older age, substantial cardiac, pulmonary or renal dysfunction) who would be considered poor or non-candidates for open surgery[20]. Data indicate that patients who are not considered candidates for open surgery but who have undergone endovascular grafting have substantially poorer long-term outcomes than patients who are reasonable candidates for open operation and are treated with endografts[20]. Endovascular treatment of aortic dissection includes three major therapeutic approaches:1) placement of an aortic endograft, 2) fenestration and 3) stenting of aortic branches[54]. The main goal of endovascular management is to seal the tear of the intima and to cause the thrombosis of the false lumen. This will have a positive effect on aorta remodeling during the dissection and will decrease the risk of rupture[54].
Regarding the comparison between the two interventional methods, there are no firm data conclusively demonstrating that the prevalence of spinal cord ischemic injury (lower extremity paralysis or paresis) is less for endovascular approaches than for open surgical repair. Similarly, there are no firm data indicating that overall medical care costs are lower with endovascular repair[20,55]. Furthermore, some patients are not suitable candidates for endovascular grafting procedures. Absence of suitable “landing zones”above and below the aneurysm (usually 2 to 3 cm of normal diameter aorta without circumferential thrombus) as well as landing zone width exceeding the recommended width for the largest available endovascular grafts (generally 10% to 15% larger than the width of the aorta) are also contraindications[55]. Finally, lack of vascular access sites as well as severe atherosclerosis and intraluminal thrombus of the aorta may increase the risk of peripheral embolism during manipulation of guidewires and catheters[56].
Regarding prognosis, Desaiet al. conclude in their study that delayed intervention appears to lead to lower complication risk after thoracic endovascular aneurysm repair (TEVAR) in patients who are stable enough to wait[57]. Additionally, Wilkinsonet al. found that early aortic repair for complicated type B dissection leads to high mortality and re-intervention rates, with results of TEVAR being similar with that of open repair[58]. However, Tanget al. showed that emergency endovascular repair of complicated acute dissection within 24 h is associated with good results and decreases mortality[59].
The recent report of IRAAD underlines that TEVAR is associated with lower 5-year mortality than medical therapy for ATBAD, although more randomized trials with longterm follow-up are needed[60]. However, Hannaet al. found recently that TEVAR is associated with excellent short-term outcomes after acute dissection, with durable and sustained results over long-term follow-up[61]. Studies so far suggest that endovascular techniques may shift the risk of patients with acute complicated type B dissection from high to lower mortality, comparable to that seen in uncomplicated distal dissection[62]. The IRAAD database suggests a better outcome in patients treated with stent graft for acute dissection compared to open surgical repair, lowering short-term mortality to the level of medically managed uncomplicated type B dissection[62].
As aforementioned, there still remains a debate regarding the indicated strategy for uncomplicated type B aortic dissection. Recently, one year results of the ADSORB trial were published, concerning whether endovascular repair could be applied in uncomplicated ATBAD[63]. Conservative treatment of such cases is followed by a high 30-day mortality and intervention rate within 4 years. Therefore, endovascular treatment plus optimal medical therapy was compared with best medical treatment only in this multicenter randomized trial. Although early death and neurologic complications rates were low for both groups, 1-year aortic remodeling was better in the first group[63].
5. Conclusions
Conservative management with optimal medical therapy remains the first line strategy for patients with uncomplicated acute type B aortic dissection. Open repair is indicated only for complicated cases. Endovascular repair shows promising results in selected patients with increased perioperative risk, when there are no contraindications. There are promising results regarding the utilization of endovascular repair even in uncomplicated cases, although more data on long-term outcomes are needed. The decision on optimal strategy should always be based on the individual characteristics and risk factors of each patient.
Conflict of interest statement
The authors report no conflict of interest.
Acknowledgements
There were no acknowledgements.
[1] Coady MA, Ikonomidis JS, Cheung AT, Dake MD, Chaikof EL, Cambria RP, et al. Surgical management of descending thoracic aortic disease: open and endovascular approaches: a scientific statement from the American Heart Association. Circulation 2010; 121: 2780-2804.
[2] Lissin LW, Vagelos R. Acute aortic syndrome: a case presentation and review of the literature. Vasc Med 2002; 7(4): 281-287.
[3] Carpenter SW, Kodolitsch YV, Debus ES, Wipper S, Tsilimparis N, Larena-Avellaneda A, et al. Acute aortic syndromes: definition, prognosis and treatment options. J Cardiovasc Surg (Torino) 2014; 55(2 Suppl 1): 133-144.
[4] Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: part I: from etiology to diagnostic strategies. Circulation 2003; 108: 628-635.
[5] Mehta RH, Manfredini R, Hassan F, Sechtem U, Bossone E, Oh JK, et al. Chronobiological patterns of acute aortic dissection. Circulation 2002; 106: 1110-1115.
[6] Hughes GC, Andersen ND, McCann RL. Management of acutetype B aortic dissection. J Thorac Cardiovasc Surg 2013; 145(3 Suppl): S202-S207.
[7] Ijaz AK, Chandra KN. Clinical, diagnostic, and management perspectives of aortic dissection. Chest 2002; 122: 311-328.
[8] Braverman AC, Thompson R, Sanchez L. Diseases of the aorta. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s heart disease. 9th ed. Philadelphia: Elsevier; 2011. [9] Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M, et al. Gender-related differences in acute aortic dissection. Circulation 2004; 109: 3014-3021.
[10] Hata M, Sezai A, Niino T, Yoda M, Wakui S, Unosawa S, et al. Prognosis for patients with type B acute aortic dissection: risk analysis of early death and requirement for elective surgery. Circ J 2007; 71: 1279-1282.
[11] Jost D, Schachner T, Czuprin C, Richter G, Hupp T. [Traumatic aortic rupture and concomitant type B aortic dissection after skiing accident]. Unfallchirurg 2014; 117(1): 72-74. German.
[12] Fikar CR, Koch S. Etiologic factors of acute aortic dissection in children and young adults. Clin Pediatr (Phila) 2000; 39: 71-80.
[13] Schoenhoff FS, Jungi S, Czerny M, Roost E, Reineke D, Matyas G, et al. Acute aortic dissection determines the fate of initially untreated aortic segments in Marfan syndrome. Circulation 2013; 127(15): 1569-1575.
[14] Dean JH, Woznicki EM, O’Gara P, Montgomery DG, Trimarchi S, Myrmel T, et al. Cocaine-related aortic dissection: lessons from the International Registry of Acute Aortic Dissection. Am J Med 2014; 127(9): 878-885.
[15] Trimarchi S, Jonker FH, Froehlich JB, Upchurch GR, Moll FL, Muhs BE, et al. Acute type B aortic dissection in the absence of aortic dilatation. J Vasc Surg 2012; 56(2): 311-316.
[16] Trimarchi S, Jonker FH, Hutchison S, Isselbacher EM, Pape LA, Patel HJ, et al. Descending aortic diameter of 5.5 cm or greater is not an accurate predictor of acute type B aortic dissection. J Thorac Cardiovasc Surg 2011; 142(3): e101-e107.
[17] Kimura N, Yamaguchi A, Noguchi K, Adachi K, Adachi H, Ino T. Type B aortic dissection associated with Salmonella infection. Gen Thorac Cardiovasc Surg 2007; 55(5): 212-216.
[18] Rodríguez-Caulo EA, Velázquez CJ, García-Borbolla M, Barquero JM. Mega-aorta syndrome development in giant cell arteritis. A same entity? Ann Vasc Surg 2011; doi: 10.1016/ j.avsg.2011.07.006.
[19] Barbetseas J, Alexopoulos N, Brili S, Aggeli C, Chrysohoou C, Frogoudaki A, et al. Atherosclerosis of the aorta in patients with acute thoracic aortic dissection. Circ J 2008; 72(11): 1773-1776.
[20] Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010; 121(13): e266-e369.
[21] Svennson LG, Crawford ES. Aortic dissection and aortic aneurysm surgery: clinical observations, experimental investigations and statistical analysis. Part II. Curr Probl Surg 1992; 29: 913-1057.
[22] Criado FJ. Aortic dissection: a 250-year perspective. Tex Heart Inst J 2011; 38(6): 694-700.
[23] Suzuki T, Mehta RH, Ince H, Nagai R, Sakomura Y, Weber F, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation 2003; 108(Suppl 1): 312-317.
[24] Jagannath AS, Sos TA, Lockhart SH, Saddekni S, Sniderman KW. Aortic dissection: a statistical analysis of the usefulness of plain chest radiographic findings. AJR Am J Roentgenol 1986; 147(6): 1123-1126.
[25] Lai V, Tsang WK, Chan WC, Yeung TW. Diagnostic accuracy of mediastinal width measurement on posteroanterior and anteroposterior chest radiographs in the depiction of acute nontraumatic thoracic aortic dissection. Emerg Radiol 2012; 19(4): 309-315.
[26] Hartnell GG, Wakeley CJ, Tottle A, Papouchado M, Wilde RP. Limitations of chest radiography in discriminating between aortic dissection and myocardial infarction: implications for thrombolysis. J Thorac Imaging 1993; 8(2): 152-155.
[27] Hahne K, Lebiedz P, Breuckmann F. Impact of d-dimers on the differential diagnosis of acute chest pain: current aspects besides the widely known. Clin Med Insights Cardiol 2014; 8(Suppl 2): 1-4.
[28] Weber T, Högler S, Auer J, Berent R, Lassnig E, Kvas E, et al. D-dimer in acute aortic dissection. Chest 2003; 123(5): 1375-1378.
[29] Ohlmann P, Faure A, Morel O, Petit H, Kabbaj H, Meyer N, et al. Diagnostic and prognostic value of circulating D-Dimers in patients with acute aortic dissection. Crit Care Med 2006; 34(5): 1358-1364.
[30] Shimony A, Filion KB, Mottillo S, Dourian T, Eisenberg MJ. Meta-analysis of usefulness of d-dimer to diagnose acute aortic dissection. Am J Cardiol 2011; 107(8): 1227-1234.
[31] Erbel R. Role of transesophageal echocardiography in dissection of the aorta and evaluation of degenerative aortic disease. Cardiol Clin 1993; 11(3): 461-473.
[32] Penco M, Paparoni S, Dagianti A, Fusilli C, Vitarelli A, De Remigis F, et al. Usefulness of transesophageal echocardiography in the assessment of aortic dissection. Am J Cardiol 2000; 86: 53-56.
[33] Hu W, Schiele F, Meneveau N, Seronde MF, Legalery P, Bonneville JF, et al. The potential value of intravascular ultrasound imaging in diagnosis of aortic intramural hematoma. J Geriatr Cardiol 2011; 8(4): 224-229.
[34] Clough RE, Zymvragoudakis VE, Biasi L, Taylor PR. Usefulness of new imaging methods for assessment of type B aortic dissection. Ann Cardiothorac Surg 2014; 3(3): 314-318.
[35] Sommer T, Fehske W, Holzknecht N, Smekal AV, Keller E, Lutterbey G, et al. Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging. Radiology 1996; 199: 547-552.
[36] Meinel FG, Nikolaou K, Weidenhagen R, Hellbach K, Helck A, Bamberg F, et al. Time-resolved CT angiography in aortic dissection. Eur J Radiol 2012; 81(11): 3254-3261.
[37] Gualdi GF, Volpe A, Polettini E, Di Biasi C, Trasimeni G, Melone A. [Aortic dissection: the role of diagnostic imaging, with special reference to magnetic resonance imaging and its implications in the pathogenesis]. Clin Ter 1993; 142(6): 539-544. Italian.
[38] Liu Q, Lu JP, Wang F, Wang L, Tian JM. Three-dimensional contrast-enhanced MR angiography of aortic dissection: a pictorial essay. Radiographics 2007; 27(5): 1311-1321.
[39] Kimihiko K, Akiko N, Noriyuki K, Hisashi U, Tetsuo F, Toyoaki M. Uptake of 18F-FDG in acute aortic dissection: a determinant of unfavorable outcome. J Nuclear Med 2010; 51: 674-681.
[40] Ogawa M, Ishino S, Mukai T, Asano D, Teramoto N, Watabe H, et al. 18F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med 2004; 45: 1245-1250.
[41] Reeps C, Pelisek J, Bundschuh RA, Gurdan M, Zimmermann A, Ockert S, et al. Imaging of acute and chronic aortic dissection by 18F-FDG PET/CT. J Nucl Med 2010; 51(5): 686-691.
[42] Kodama K, Nishigami K, Sakamoto T, Sawamura T, Hirayama T, Misumi H, et al. Tight heart rate control reduces secondary adverse events in patients with type B acute aortic dissection. Circulation 2008; 118: 167-170.
[43] Suzuki T, Isselbacher E, Nienaber C, Pyeritz R, Eagle K, Tsai T, et al. Type-selective benefits of medications in treatment of acute aortic dissection. Am J Cardiol 2012; 109: 122-127.
[44] Tefera G, Acher CW, Hoch JR, Mell M, Turnipseed WD. Effectiveness of intensive medical therapy in type B aortic dissection: a single-center experience. J Vasc Surg 2007; 45: 1114-1118.
[45] Hackam DG, Thiruchelvam D, Redelmeier DA. Angiotensinconverting enzyme inhibitors and aortic rupture: a populationbased case-control study. Lancet 2006; 368: 659-665.
[46] Elefteriades JA, Lovoulos CJ, Coady MA, Tellides G, Kopf GS, Rizzo JA. Management of descending aortic dissection. Ann Thorac Surg 1999; 67(6): 2002-2005.
[47] Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders T, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 2009; 120(25): 2519-2528.
[48] Moulakakis KG, Mylonas SN, Dalainas I, Kakisis J, Kotsis T, Liapis CD. Management of complicated and uncomplicated acute type B dissection. A systematic review and metaanalysis. Ann Cardiothorac Surg 2014; 3(3): 234-246.
[49] Carrel T, Nguyen T, Gysi J, Kipfer B, Sigurdsson G, Schaffner T, et al. [Acute type B aortic dissection: prognosis after initial conservative treatment and predictive factors for a complicated course]. Schweiz Med Wochenschr 1997; 127(36): 1467-1473. German.
[50] Apostolakis E, Baikoussis N, Georgiopoulos M. Acute type B aortic dissection: the treatment strategy. Hellenic J Cardiol 2010; 51: 338-347.
[51] Miller DC, Mitchell RS, Oyer PE, Stinson EB, Jamieson SW, Shumway NE. Independent determinants of operative mortality for patients with aortic dissection. Circulation 1984; 70: 153-164.
[52] Luebke T, Brunkwall J. Outcome of patients with open and endovascular repair in acute complicated type B aortic dissection: a systematic review and meta-analysis of case series and comparative studies. J Cardiovasc Surg (Torino) 2010; 51(5): 613-632.
[53] Zhang H, Wang ZW, Zhou Z, Hu XP, Wu HB, Guo Y. Endovascular stent-graft placement or open surgery for the treatment of acute type B aortic dissection: a meta-analysis. Ann Vasc Surg 2012; 26(4): 454-461.
[54] Hinchliffe RJ, Halawa M, Holt PJ, Morgan R, Loftus I, Thompson MM. Aortic dissection and its endovascular management. J Cardiovasc Surg 2008; 49(4): 449-460.
[55] Aljabri B, Al WK, Abner D, Mackenzie KS, Corriveau MM, Obrand DI, et al. Patient-reported quality of life after abdominal aortic aneurysm surgery: a prospective comparison of endovascular and open repair. J Vasc Surg 2006; 44: 1182-1187.
[56] Qin YL, Deng G, Li TX, Wang W, Teng GJ. Treatment of acute type-B aortic dissection: thoracic endovascular aortic repair or medical management alone? JACC Cardiovasc Interv 2013; 6(2): 185-191.
[57] Desai ND, Gottret JP, Szeto WY, McCarthy F, Moeller P, Menon R, et al. Impact of timing on major complications after thoracic endovascular aortic repair for acute type B aortic dissection. J Thorac Cardiovasc Surg 2014; doi: 10.1016/ j.jtcvs.2014.10.105.
[58] Wilkinson DA, Patel HJ, Williams DM, Dasika NL, Deeb GM. Early open and endovascular thoracic aortic repair for complicated type B aortic dissection. Ann Thorac Surg 2013; 96(1): 23-30.
[59] Tang JD, Huang JF, Zuo KQ, Hang WZ, Yang MF, Fu WG, et al. Emergency endovascular repair of complicated Stanford type B aortic dissections within 24 hours of symptom onset in 30 cases. J Thorac Cardiovasc Surg 2011; 141(4): 926-931.
[60] Fattori R, Montgomery D, Lovato L, Kische S, Di Eusanio M, Ince H, et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc Interv 2013; 6(8): 876-882.
[61] Hanna JM, Andersen ND, Ganapathi AM, McCann RL, Hughes GC. Five-year results for endovascular repair of acute complicated type B aortic dissection. J Vasc Surg 2014; 59(1): 96-106.
[62] Fattori R, Tsai TT, Myrmel T, Evangelista A, Cooper JV, Trimarchi S, et al. Complicated acute type B dissection: is surgery still the best option? A report from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol Interv 2008; 4: 395-402.
[63] Brunkwall J, Kasprzak P, Verhoeven E, Heijmen R, Taylor P, ADSORB Trialists. Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic remodelling: 1 year results of the ADSORB trial. Eur J Vasc Endovasc Surg 2014; 48(3): 285-291.
ment heading
10.1016/S2221-6189(14)60058-5
*Corresponding author: George Galyfos, 2 Nikis Street, Kifisia, 14561, Athens, Greece. (georgegalyfos@hotmail.com)
Tel.: +30-213-2086243
Fax.: +30-210-7707574
#These authors contributed equally in this work.
Type B aortic dissection
Conservative management
Endovascular repair
杂志排行
Journal of Acute Disease的其它文章
- Acute and sub-acute toxicity study of Clerodendrum inerme, Jasminum mesnyi Hance and Callistemon citrinus
- Time-critical AMI Detection: A novel and fast technique using the 12-lead ECG
- Epidemiological survey on scorpionism in Gotvand County, Southwestern Iran: an analysis of 1 067 patients
- The acute effect of the antioxidant drug “U-74389G” on red blood cells levels during hypoxia reoxygenation injury in rats
- Successful treatment of lower urinary tract obstruction with peritonealamniotic and vesicoamniotic shunting
- Simvastatin-induced Toxic Epidermal Necrolysis
