Diversification of primary gene pool through introgression of resistance to foliar diseases from synthetic amphidiploids to cultivated groundnut(Arachis hypogaea L.)
2014-03-13VrshKumriGowVinoTsiwlMnishPneyRmeshBhtNliniMllikrjunHriUphyyRjeevVrshney
Vrsh Kumri,M.V.C.Gow,Vino Tsiwl,Mnish K.Pney,Rmesh S.Bht,Nlini Mllikrjun,Hri D.Uphyy,Rjeev K.Vrshney,e,*
1.Introduction
Cultivated groundnut (Arachis hypogaea L.),also known as peanut,is grown on nearly 24 million hectares of land area globally with an annual production of 38 million tons(Mt)[1].Although it originated in South America,the vast majority of groundnut is produced in Asia (68%,23 Mt) and Africa (24%,8 Mt),whereas the remaining (8%,3.5 Mt) comes from North America,Caribbean countries,Europe and Oceania[1].Besides being a major source of vegetable oil and providing several confectionary preparations,this crop is also a principal source of nutrition by providing human dietary protein,oil/fat,and vitamins such as thiamine,riboflavin and niacin in parts of Asia and Africa [2].Additionally,it provides an important livestock feed along with improving soil fertility through contributing up to 60 kg ha-1of nitrogen to the soil [3].
Surmounting biotic and abiotic pressure along with the narrow genetic base of the cultivated gene pool has seriously reduced the crop potential and hampered the possibility of meeting future demands of continuously increasing human and animal populations [4,5].Control of drought stress and foliar diseases requires urgent attention in order to sustain productivity in the fields of resource-poor farmers.Foliar diseases such as late leaf spot (LLS) caused by Cercosporidium personatum and leaf rust caused by Puccinia arachidis are important diseases of groundnut in Africa,Asia,and the Americas [6,7].The extent of economic loss due to LLS [8]may be much higher than the reported global yield loss of 600 million US$.Disease management through application of fungicides is not a viable option for resource-poor farmers;also,fungicides may pollute the environment and ground water besides causing greater risk and damage to crop [7].Hence,the only eco-friendly approach is to equip popular cultivars with resistance genes that will ensure sustainable resistance against foliar fungal pathogens.
Molecular analysis has shown that cultivated groundnut possesses a narrow genetic base [9,10] due to a single hybridization event that occurred ~3500 years ago [11].The genus Arachis has a total of nine sections possessing different genomes.Earlier reports have indicated the existence of a large range of variability among these sections.However,this variability cannot be exploited in a direct way because of ploidy or genome differences among the species [12,13].In order to overcome the genetic bottleneck of restricted gene flow,the development of synthetic amphidiploids is an effective option to diversify the cultivated gene pool.To date,several synthetics have been developed by using different diploid species through colchicine-mediated genome duplication [14–17].These highly diverse synthetics provide an opportunity for introgression of some important traits to cultivated germplasm.However,limited success has been achieved so far in using the wild species as genetic resources for the development of resistant cultivars.Nevertheless,release of an Indian variety (GPBD 4) containing resistance to foliar diseases in chromosome segments from Arachis cardenasii is an example of success.GPBD 4 is an improved variety developed as a second cycle derivative of an interspecific cross and is grown in several states in India for its desirable traits such as foliar disease resistance and high yield.Because of its high levels of resistance,A.cardenasii Krapov.& W.C.Greg.is the most widely used wild species in groundnut breeding programs aimed at improving foliar disease resistance.However,it is always better to look for alternative sources of resistance in order to diversify the cultivated gene pool [4].Realizing the great potential of synthetic amphidiploids for enhancing the richness of the gene pool,this study was undertaken to broaden the genetic base of cultivated groundnut by introgressing resistance genes into five cultivated genotypes.We report the development of diverse genetic materials in groundnut with potential for several genetic and breeding applications.
2.Materials and methods
Synthetic amphidiploids ISATGR 278-18 [ICG 8138 (Arachis duranesis Kaprov.& W.C.Greg.) × ICG 13160 (Arachis batizocoi Kaprov.& W.C.Greg.)] and ISATGR 5B [ICG 8960 (Arachis magna Kaprov.,W.C.Greg.& C.E.Simpson) × ICG 8209 (A.batizocoi Kaprov.& W.C.Greg.)] with 2n = 2x = 40 were generated at ICRISAT (Hyderabad,India).Seeds from these amphidiploids were planted in a glasshouse at the University of Agricultural Sciences (UAS),Dharwad,India.Both amphidiploids were used to generate backcross populations with five elite varieties/genotypes,namely ICGV 91114,ICGS 76,ICGV 91278,JL 24,and DH 86 after making two backcrosses.
Flowers of cultivated genotypes were emasculated a day before pollination.Cross pollination was carried out before 10:00 a.m.on the following day by using the synthetic amphidiploids as pollen parents.Cotton swabs impregnated with gibberellic acid (GA3) (0.5 mL; 75 mg L-1) were wrapped around the base of pollinated pistils.Flowering was generally observed on recurrent parents about 45 days after sowing(DAS) and continued,allowing crossing for the next 30 days.The pods were harvested and percentages of crossed pods were calculated.In the next season,the F1plants were used as pollen parents for the first backcross to each recurrent parent.Pods of BC1F1generation from all crosses were harvested and grown in the next season.These plants were then used to make second backcrosses.The BC2F1s were grown and selfed thrice to produce BC2F4population after three seasons(Fig.1).
Both amphidiploids were evaluated for component traits of rust and late leaf spot (LLS) resistances using a detached leaf technique [18].On the 40th DAS,tetrafoliate leaves were excised from the pulvinous regions and arranged in plastic trays containing autoclaved sand in a randomized block design with two replications.In order to compare the disease severities,a susceptible check (variety “TMV 2”) was used for both the diseases.P.arachidis urediniospores and C.personatum conidia were initially produced on susceptible cultivar TMV 2 and harvested with a cyclone spore collector.The concentrations of the spore suspensions were set to 20,000 spores mL-1using a hemocytometer by adding sterile distilled water containing a few drops of Tween-80(polyoxyethylene sorbitan mono-oleate)in order to promote adhesion.Spore suspensions of both the pathogens were sprayed on to the leaves by using an atomizer,and the trays were kept in a growth chamber at 23–25 °C immediately after the inoculation to ensure leaf moisture during the night.Two weeks after inoculation,leaves were inspected for symptoms and time to sporulation.Damage due to rust and LLS was determined after 30 days based on these parameters.Cultivar TMV 2 was used as the susceptible check and cultivar GPBD 4 was used as resistant check in all disease screening experiments.
Plants of BC2F2generation generated from each of the nine crosses were screened for disease resistance during the rainy season of 2011 following the protocol of Subrahmanyam et al.[19].Seeds were treated with seed protectant and sown in the field with 45 cm and 10 cm inter-and intra-row spacing,respectively.The parental genotypes were sown once as controls and TMV 2 (susceptible variety for both diseases)was planted at every 10th row as well as a border around the field to maintain an effective inoculum load.Uniform inoculation across the field was performed in the evening of 45th DAS.Disease scoring for LLS and rust occurred on the 80th and 90th DAS using a 0–9 scale of disease severity(%)on the leaves for lesions and defoliation in the case of LLS,and on pustules and necrosis in the case of rust.Scores were as follows: (i) 1.0,no disease; (ii) 2.0,1%–5% severity,lesions/pustules on lower leaves; (iii) 3.0,6%–10% severity,lesions/pustules mostly on lower leaves and very few on middle leaves along with defoliation or necrosis of lower leaves;(iv) 4.0,11%–20% severity,lesions/pustules on lower and middle leaves but severe on lower leaves with defoliation/necrosis of some leaflets on lower leaves; (v) 5.0,21%–30%severity,lesions/pustules on all lower and middle leaves along with defoliation/necrosis of >50% lower leaves; (vi) 6.0,31%–40%severity,severe lesions/pustules on lower and middle leaves,few symptoms on top leaves along with extensive defoliation/necrosis of lower leaves and some middle leaves;(vii) 7.0,41%–60% severity,lesions/pustules present on all leaves but less severe on the top leaves along with complete defoliation/necrosis of lower leaves and some middle leaves;(viii) 8.0,61%–80% severity,lesions/pustules fully covering lower and middle leaves and severe lesions on top leaves along with some defoliation/necrosis of top leaves;and(ix)9.0,81%–100% severity,almost complete defoliation/necrosis for lower,middle and top leaves leaving bare stems.
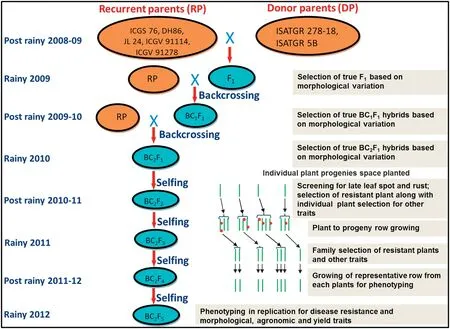
Fig.1-Development of introgression lines by backcrossing and self-pollination.
The final introgression lines were characterized for morphological traits such as plant height,leaf features (length,width,color,and shape),stem features(pigmentation,pubescence) secondary branching,flower color,growth habit and branching pattern.Growth habit was scored as “Erect” (main stem erect),“Decumbent-1” (completely spreading,primary branches at 90° angles with the main stem),“Decumbent-2”(semi spreading,primary branches at 60° to the main stem)and “Decumbent-3” (semi erect,primary branches at 45° to the main stem).Similarly,branching pattern was recorded as“Sequential” (flowers on main stems and primary branches,but not on secondary branches),“Irregular with flower on main stem” (flowers on main stems,primary branches and secondary branches),and “Irregular without flower on main stem”(no flowers on main stems,but present on primary and secondary branches).
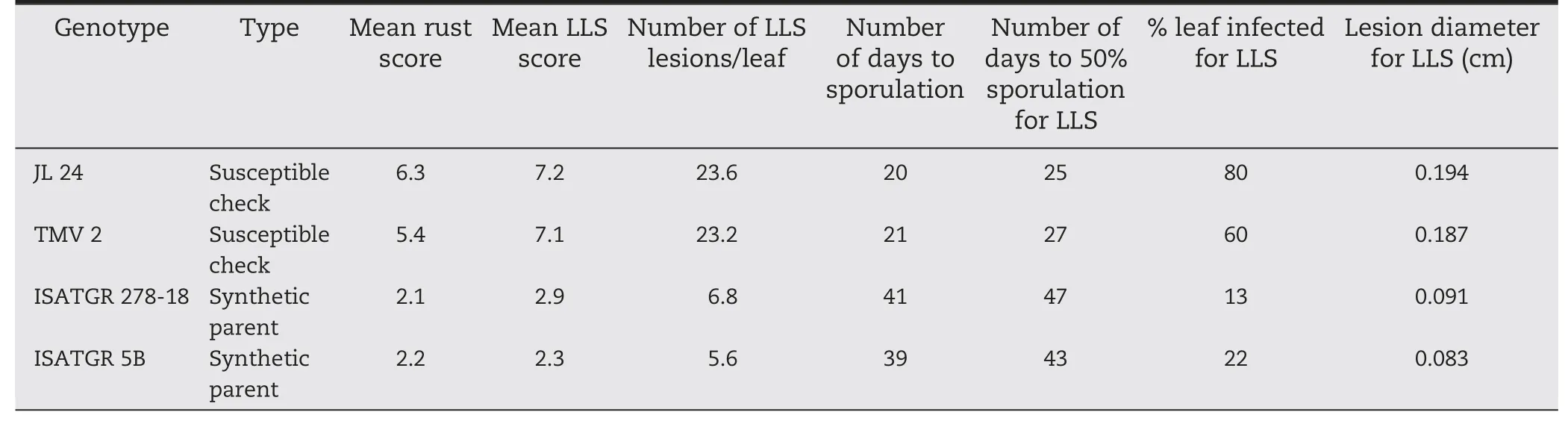
Table 1-Disease response scores for synthetic amphidiploids and cultivated parents used for introgression of disease resistance.
3.Results
3.1.Development of introgression lines
Disease screening was carried out for foliar disease responses(leaf rust and LLS)among the parental genotypes(ICGV 91114,ICGS 76,ICGV 91278,JL 24,DH 86,ISATGR 278-18,and ISATGR 5B).Both amphidiploids (ISATGR 278-18 and ISATGR 5B)showed high levels of resistance (disease scores 2.0–3.0) to both rust and LLS whereas the cultivated parental genotypes were susceptible(disease scores 6.0–7.0)(Table 1).Five crosses were achieved for ISATGR 278-18 (ICGV 91114 × ISATGR 278-18,ICGS 76 × ISATGR 278-18,ICGV 91278 × ISATGR 278-18,JL 24 × ISATGR 278-18,and DH 86 × ISATGR 278-18),and ISATGR 5B (ICGV 91114 × ISATGR 5B,ICGS 76 × ISATGR 5B,ICGV 91278 × ISATGR 5B,JL 24 × ISATGR 5B,and DH 86 ×ISATGR 5B).Peg formation began about 25 days after pollination.From the 597 buds pollinated,198 pods were harvested with percentage seed set ranging from 15 (DH 86 × ISATGR 278-18)to 47%(JL 24 × ISATGR 5B)(Table 2).
All 212 potential F1seeds from 198 pods were planted and examined for hybridity based on morphological attributes.A total of 51 plants from ten crosses were confirmed to be hybrids.Hybrids had a spreading growth habit along with distinctive leaf morphology,flower color and pod morphology similar to the synthetic parents.
True hybrids from all the nine crosses were used as pollen parents to make the first backcross with the respective recurrent parents (Tables 2 and 3).Of the 673 buds pollinated in all the backcrosses,293 mature pods were harvested.The mean percentage of seed set ranged from 38% (ICGV 91114 × ISATGR 5B) to 50% (DH 86 × ISATGR 278-18) (Table 2).The average percentage of seed set was higher in BC1F1generation (44.0%)than that achieved in F1generation(31.7%)plants.
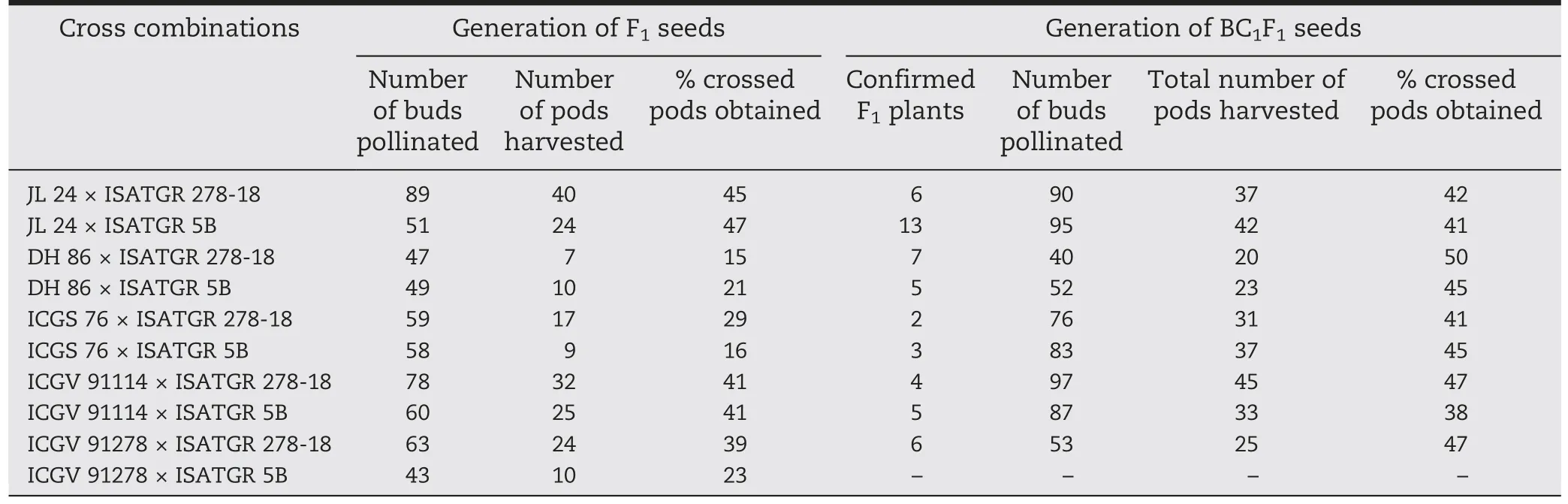
Table 2-Outcomes of attempted crosses between cultivated varieties and amphidiploids.
All 320 potential BC1F1seeds obtained from backcrossed plants were planted and subjected to phenotypic screening.A total of 84 BC1F1plants were confirmed for hybridity based on morphological traits and disease reaction(Table 4).Confirmed BC1F1plants were again backcrossed with the recurrent parents and BC2F1pods were harvested.In the next season,BC2F1seeds were planted and BC2F1plants were again confirmed by morphological characters and disease response.Selected BC2F1s in each of the seven crosses were selfed and the progenies were screened for reaction to rust and LLS during the rainy season of 2011.
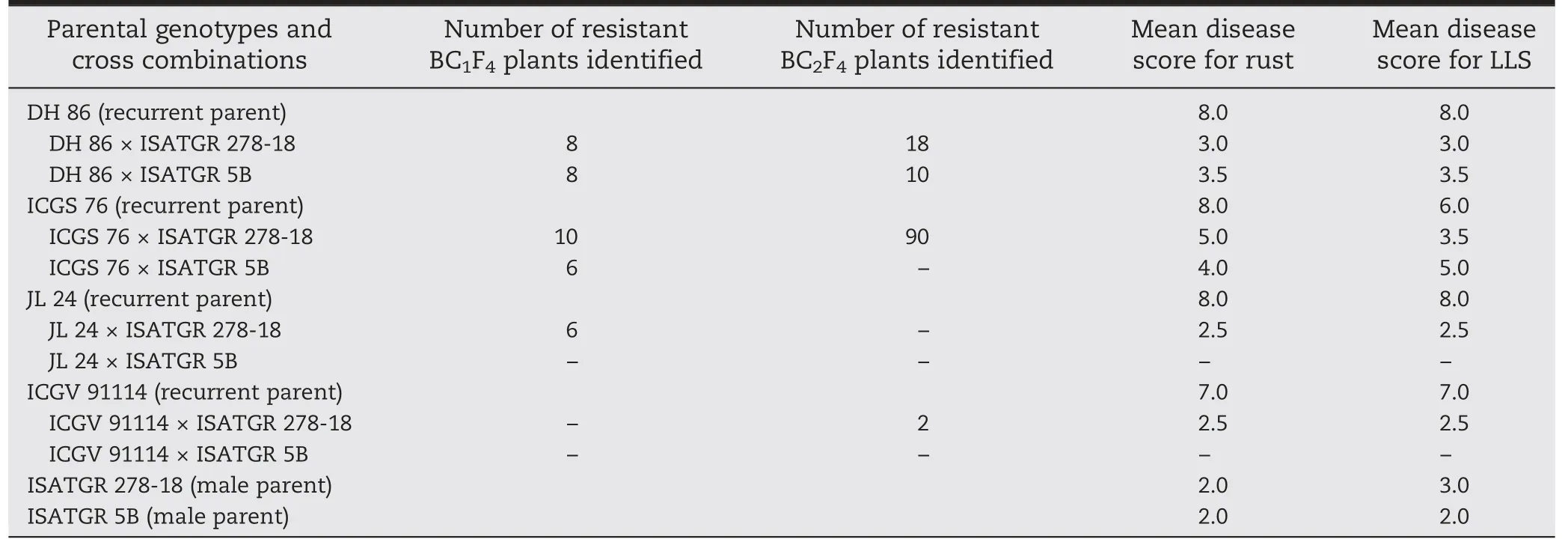
Table 3-Numbers of backcross introgression lines possessing resistance to rust and late leaf spot (LLS) and their mean disease scores(0-9 scale).
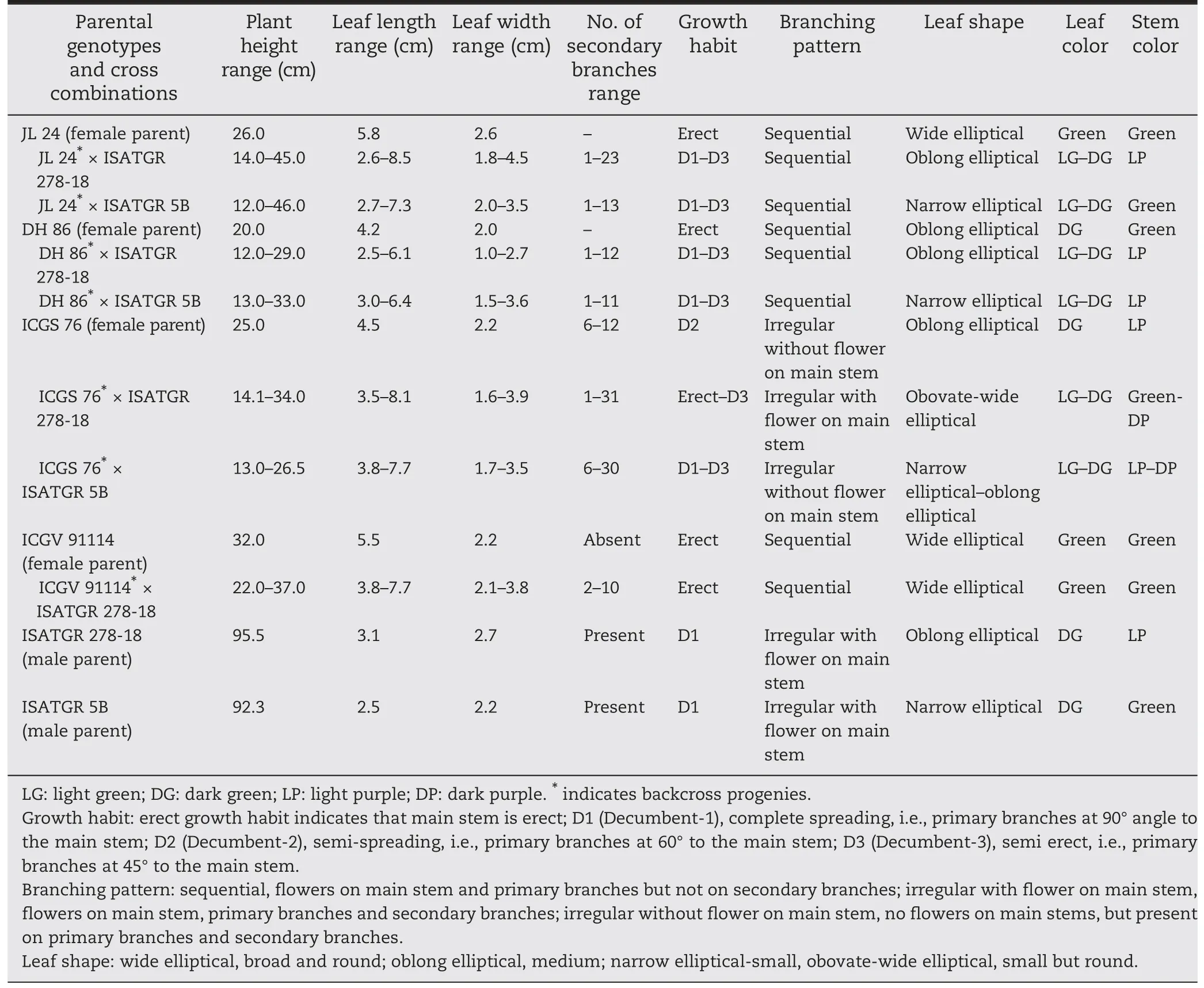
Table 4-Morphological descriptions of parental genotypes and introgression lines.
3.2.Evaluation and identification of introgression lines with foliar disease resistance
Hybrids in different generations (F1,BC1F1,and BC2F1) were scored for rust and LLS response and those possessing resistance for components of response compared to the respective susceptible parents were selected (Fig.2).After each backcross,the plants were selfed to obtain segregating backcross F2s(BC1F2,BC2F2),which were selfed twice to obtain BC1F4and BC2F4backcross progenies.These were then subjected to phenotyping and several lines with high levels of resistance to rust and LLS compared to the susceptible parents were selected.The numbers of resistant plants in each cross,generation,and range of disease scores were recorded (Table 3).Among the BC2F4introgression lines,very high frequencies of resistant lines (90 of 164) were selected from the cross ICGS 76 × ISATGR 278-18 followed by 18 lines(out of 52) from DH 86 × ISATGR 278-18.No resistant plants were detected in JL 24 × ISATGR 5B and ICGV 91114 ×ISATGR 5B.
A few morphological variants that were phenotypically similar to the amphidiploid parents for traits such as growth habit,plant height,leaf morphology (shape and size) and color,flowers on main stem,flower color,peg pattern,stem pubescence,stem pigmentation,testa color,number of primary and secondary branches,and pod constriction/reticulation were recovered in the selected backcross lines(Tables 4,5,Figs.2,and 3).Line AB-ICGS76-25-3 showed dense stem pubescence and a high number of secondary branches.Line AB-ICGS76-73-6 produced broad leaves,AB-ICGS76-1-4 had narrow leaves,AB-ICGS76-10-1 had deep constrictions and reticulations in pods,and AB-ICGS76-7-1 showed high resistance to both diseases along with erect growth habit(Tables 5,6,and Fig.2).

Fig.2-Variation in morphological characteristics and disease resistance in the susceptible female parent and resistant BC2F4 lines from the cross ICGS 76 × ISATGR 278-18.This figure shows variability between cultivated parents and introgression lines for stem pigmentation,pubescence,growth habit,and branching pattern.
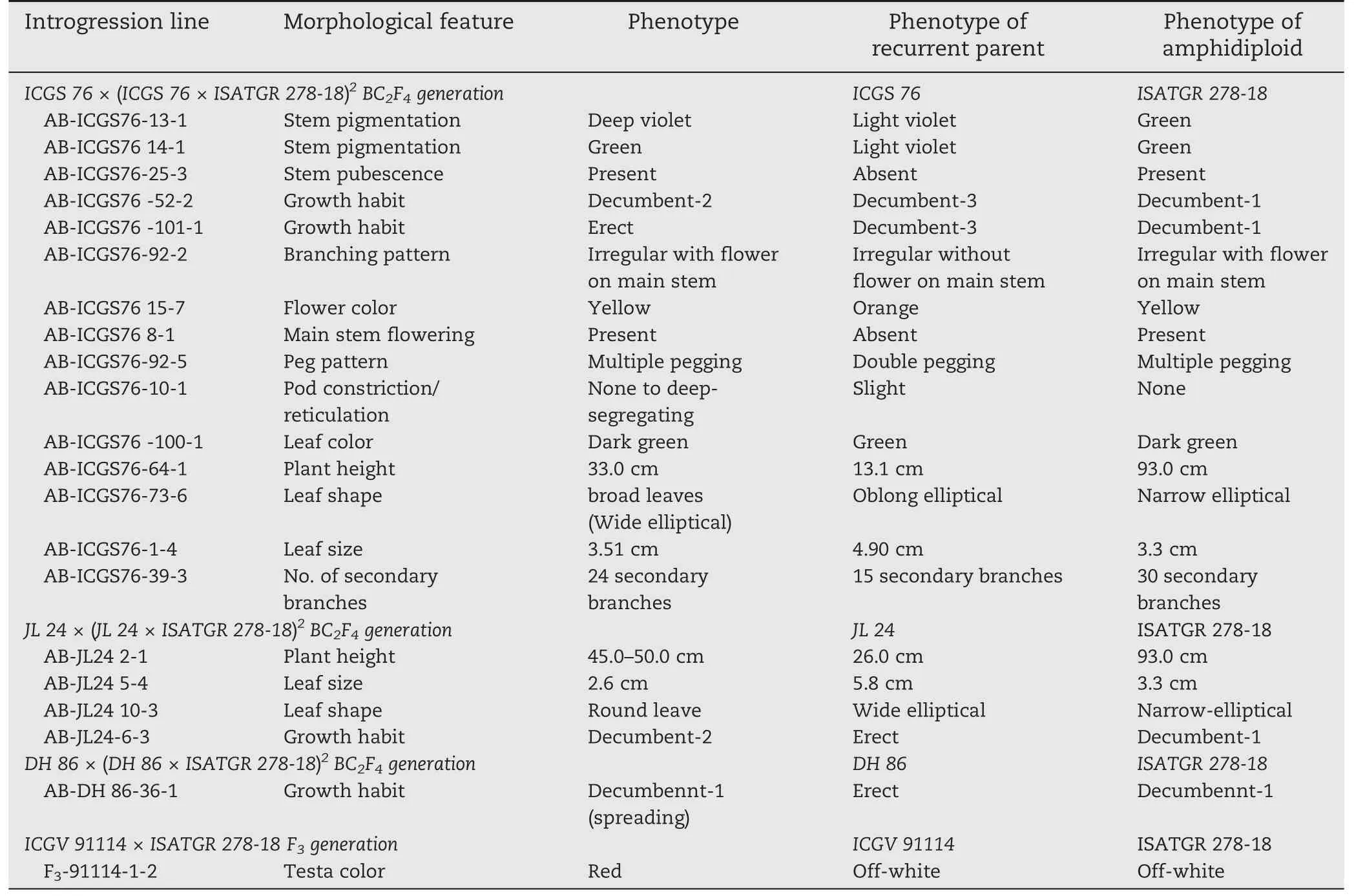
Table 5-Morphological trait features of introgression lines and their parental genotypes.
4.Discussion
Enriching the primary gene pool is necessary for groundnut,which has a very narrow genetic base.Only limited success has been achieved by using wild relative species for improving cultivated groundnut germplasm.GPBD 4 is a good example of an improved variety that was developed as a second cycle derivative of an interspecific cross.Synthetics may be another effective way for bringing useful genes from wild relatives.In this direction,several synthetics are now available by using different diploid species and these need to be utilized for improving the cultivated gene pool [14–17].Thus,in this study,highly diverse synthetics were used to introgress disease resistance in five cultivars.As a result,foliar disease(leaf rust and LLS) resistance was introgressed into one or more of the genetic backgrounds of ICGV 91114,ICGS 76,ICGV 91278,JL 24,and DH 86 using two synthetic resistance sources namely ISATGR 278-18 and ISATGR 5B (Table 3).Seed setting percentage improved with repeated backcrossing.The presence of phenotypic traits from the donor synthetics enabled confirmation of hybrids as crossing in groundnut can be very difficult.In later generations,the presence of one or more of these traits still enabled confirmation of backcross hybrids.
Backcrossed introgression lines in different generations were scored for rust and LLS response and lines possessing disease resistance were identified.Of the 10 attempted combinations,resistant derivatives were obtained in high frequencies for ICGS 76 × ISATGR 5B and DH 86 × ISATGR 278-18.Unfortunately,no resistant plant could be recovered from JL 24 × ISATGR 5B and ICGV 91114 × ISATGR 5B.It is clearly evident that the frequency and level of resistance to both diseases were higher among crosses involving ISATGR 278-18 compared to ISATGR 5B.Thus,ISATGR 278-18 appears to be a potentially better source of disease resistance and other agronomic traits for further diversifying the primary gene pool of groundnut.Besides disease resistance,the synthetic derivatives also showed a high level of variation in morphological traits and several backcross lines were selected for those traits (Tables 5 and 6).Due to abnormal pairing during meiotic division in synthetic amphidiploids,arising of different types of allelic combinations in the segregating backcrossed populations was reported [20].Thus,the introgression lines are of importance and need further evaluation,as they might harbor currently undetected genes useful for the improvement of groundnut.Seeds of the introgression lines are available on request from the University of Agricultural Sciences(UAS),Dharwad(Ramesh S.Bhat).
Several wild species from the section Arachis had been successfully crossed with A.hypogaea and fertile hybrids[14–16] and various backcross introgression lines were obtained [21].Earlier Arachis glabrata Benth.from section Rhizomatosae was crossed with A.hypogaea by using in vitro techniques[22]and traits of interest such as resistance to late leaf spot and groundnut viral diseases such as peanut mottle virus (PMV),peanut stripe virus (PSTV),and peanut bud necrosis virus (PBNV) were transferred to elite genotype [23].Simpson et al.[24] developed TxAG-6,an amphidiploid[A.batizocoi K9484 × (A.cardenasii GKP10017 × Arachis diogoi GKP10602)] with resistance to early and late leaf spot (caused by Cerospora arachidicola S.Hori and Phaeoisariopsis personata Berk.& M.A.Curtis,respectively).With an objective of improving resistance,TxAG-6 was then used to generate a backcross population (78 progeny) and used to create a linkage map of RFLP markers [25].A similar study reported development of amphidiploid AiAd (A.ipaensis × A.duranensis)[26].This amphidiploid was extensively used for developing backcross populations by using cultivated tetraploid cultivar Fleur 11 as the recurrent parent and analyzed in different generations (BC1F1,BC2F1,BC3F1,BC2F2,and BC4F3) for linkage mapping[27]and QTL analysis[28,29]of various agronomic and yield traits.

Fig.3-Variation in morphology,disease reaction and pod features in introgression lines derived from synthetic amphidiploids.This figure shows variability between cultivated parents and introgression lines for peg formation,flower color,foliar disease resistance and pod features.
In summary,several introgression lines possessing disease resistance and other important traits were developed by backcross breeding using two synthetic amphidiploids(ISATGR 5B and ISATGR 278-18) and five cultivs (ICGV 91114,ICGS 76,ICGV 91278,JL 24,and DH 86).In order to assess and harness the full potential of these lines for other important traits,further phenotyping of the lines for a range of traits is required.Thus,these introgression lines possess disease resistance and several other traits useful for future genetic enhancement of groundnut such as improved pod yield,superior oil quality and resistance to biotic and abiotic constraints.
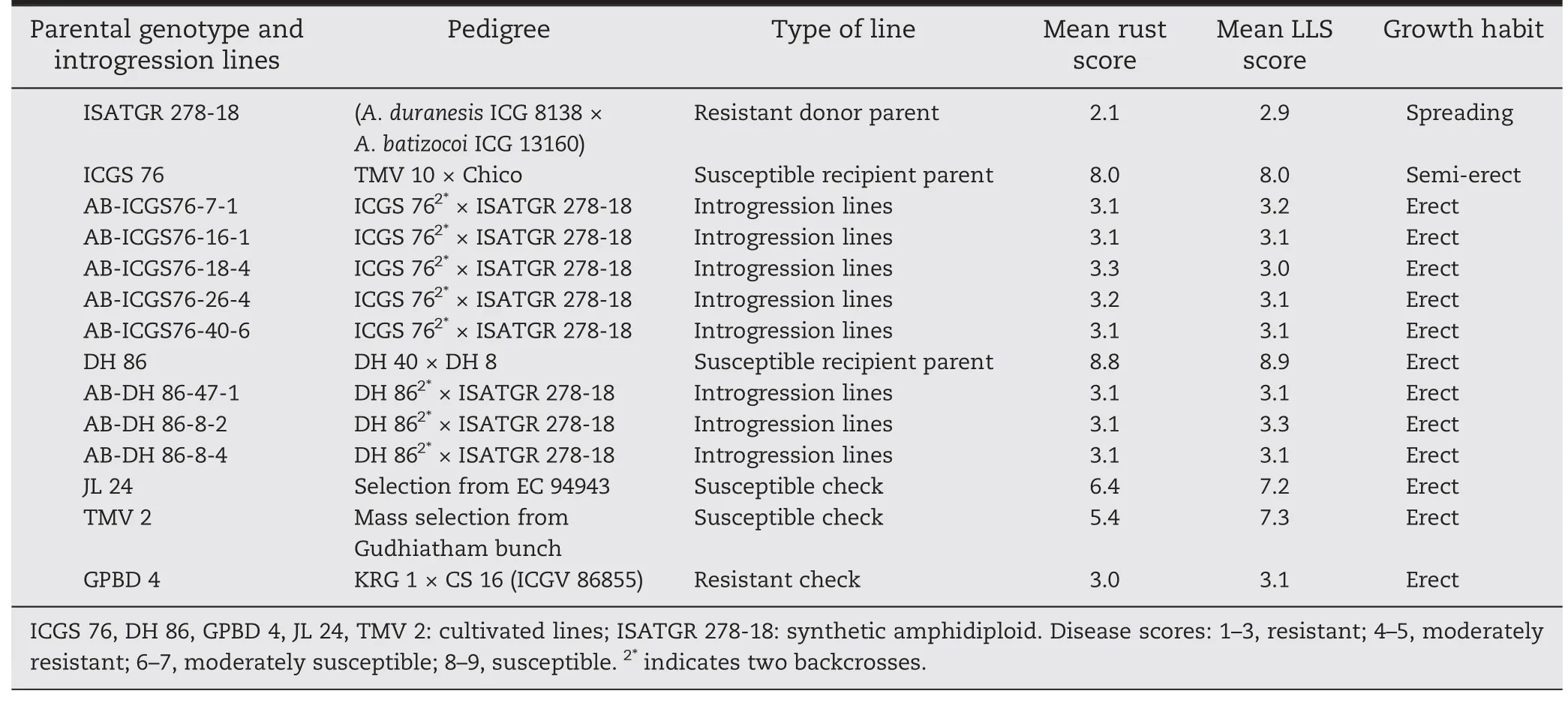
Table 6-Foliar disease resistance among a synthetic amphidiploid parent,second backcross introgression lines and controls.
The research presented in this article is a contribution from research projects sponsored by the Department of Biotechnology (DBT),Government of India,to UAS-Dharwad and ICRISAT.This work was undertaken as part of the CGIAR Research Program on Grain Legumes.
[1] FAOSTAT,Available at:http://faostat.fao.org/2012.
[2] G.P.Savage,J.I.Keenan,The composition and nutritive value of groundnut kernels,in:J.Smartt(Ed.),The Groundnut Crop.A Scientific Basis for Improvement,Chapman &Hall,UK,1994.
[3] J.Sprent,Nitrogen fixation,in:J.Smartt(Ed.),The Groundnut Crop.A Scientific Basis for Improvement,Chapman & Hall,UK,1994.
[4] R.K.Varshney,S.M.Mohan,P.M.Gaur,N.V.P.R.Gangarao,M.K.Pandey,A.Bohra,S.L.Sawargaonkar,A.Chitikineni,P.K.Kimurto,P.Janila,K.B.Saxena,A.Fikre,M.Sharma,A.Rathore,A.Pratap,S.Tripathi,S.Datta,S.K.Chaturvedi,N.Mallikarjuna,G.Anuradha,A.Babbar,A.K.Choudhary,M.B.Mhase,C.Bharadwaj,D.M.Mannur,P.N.Harer,B.Guo,X.Liang,N.Nadarajan,C.L.L.Gowda,Achievements and prospects of genomics-assisted breeding in three legume crops of the semi-arid tropics,Biotechnol.Adv.31(2013)1120–1134.
[5] M.K.Pandey,E.Monyo,P.O.Akins,X.Liang,P.Guimarães,S.N.Nigam,H.D.Upadhyaya,P.Janila,X.Zhang,B.Guo,D.R.Cook,D.J.Bertioli,R.Michelmore,R.K.Varshney,Advances in Arachis genomics for peanut improvement,Biotechnol.Adv.30(2012)639–651.
[6] P.Janila,S.N.Nigam,M.K.Pandey,P.Nagesh,R.K.Varshney,Groundnut improvement: use of genetic and genomic tools,Front.Plant Sci.4(2013) 23,http://dx.doi.org/10.3389/fpls.2013.00023.
[7] A.K.Singh,V.K.Mehan,S.N.Nigam,Sources of resistance to groundnut fungal and bacterial diseases: an update and appraisal,Information Bulletin No.50,Patancheru 502 324,International Crops Research Institute for the Semi-Arid Tropics,Andhra Pradesh,India,1997,ISBN 92-9066-367-7.48(Order code IBE 050).
[8] S.L.Dwivedi,J.H.Crouch,S.N.Nigam,M.E.Ferguson,A.H.Paterson,Molecular breeding of groundnut for enhanced productivity and food security in the semi-arid tropics:opportunities and challenges,Adv.Agron.80(2003) 153–221.
[9] T.Halward,H.Y.Stalker,E.Larue,G.Kochert,Use of single primer DNA amplifications in genetic studies of peanut(Arachis hypogaea L.),Plant Mol.Biol.18(1991) 315–325.
[10] M.S.Hopkins,A.M.Casa,T.Wang,S.E.Mitchell,R.E.Dean,G.Kochert,S.Kresovich,Discovery and characterization of polymorphic simple sequence repeats (SSRs) in cultivated peanut (Arachis hypogaea L.),Crop Sci.39(1999) 1243–1247.
[11] G.Kochert,H.T.Stalker,M.Gimenes,L.Galgaro,K.Moore,RFLP and cytogenetic evidence for the progenitor species of allotetraploid cultivated peanut,Arachis hypogaea(Leguminosae),Am.J.Bot.83 (1996) 1282–1291.
[12] S.R.Milla,T.G.Isleib,H.T.Stalker,Taxonomic relationships among Arachis sect.Arachis as revealed by AFLP markers,Genome 48 (2005) 1–11.
[13] R.Koppolu,H.D.Upadhyaya,S.L.Dwivedi,D.A.Hoisington,R.K.Varshney,Genetic relationships among seven sections of genus Arachis studied by using SSR markers,BMC Plant Biol.10(2010) 15.
[14] H.T.Stalker,J.S.Dhesi,D.Parry,J.H.Hahn,Cytological and interfertility relationships of Arachis,Am.J.Bot.8(1991)238–246.
[15] N.Mallikarjuna,S.Pande,D.R.Jadhav,D.C.Sastri,J.N.Rao,Introgression of disease resistance genes from Arachiskempff mercadoi into cultivated groundnut,Plant Breed.123 (2004)573–576.
[16] N.Mallikarjuna,D.R.Jadhav,K.R.Kranthi,S.Kranthi,Influence of foliar chemical compounds on the development of Spodoptera litura (Fab.) on interspecific derivatives of groundnut,J.Appl.Entomol.128 (2004) 321–328.
[17] N.Mallikarjuna,S.Senthilvel,D.Hoisington,Development of new sources of tetraploid Arachis to broaden the genetic base of cultivated groundnut (Arachis hypogaea L.),Genet.Resour.Crop.Evol.58(2011) 889–907.
[18] H.A.Melouk,D.Banks,A method of screening peanut genotypes or resistance to Cercospora leafs pots,Peanut Sci.5(1978) 112–114.
[19] P.Subrahmanyam,J.H.Williams,D.Mcdonald,R.W.Gibbons,The influence of foliar diseases and their control by selective fungicides on a range of groundnut(Arachis hypogaea L.) genotypes,Ann.Appl.Biol.104 (1984)467–476.
[20] N.Mallikarjuna,S.Srikanth,R.K.Vellanki,D.R.Jadhav,K.Das,H.D.Upadhyaya,Meiotic analysis of the hybrids between cultivated and synthetic tetraploid groundnuts,Plant Breed.131 (2012) 135–138.
[21] C.E.Simpson,Use of wild Arachis species/introgression of genes into A.hypogaea L,Peanut Sci.28(2001) 114–117.
[22] N.Mallikarjuna,D.C.Sastri,Morphological,cytological and disease resistance studies of intersectional hybrid between Arachis hypogaea L.and A.glabrata Benth,Euphytica 126(2002)161–167.
[23] N.Mallikarjuna,Wide hybridization in important food legumes,in:P.K.Jaiwal,R.P.Singh (Eds.),Improvement strategies of Leguminosae biotechnology,Kluwer Academic Publishers,U.S.A.,2003,pp.155–170.
[24] C.E.Simpson,J.L.Starr,S.C.Nelson,K.E.Woodard,O.D.Smith,Registration of TxAG-6 and TxAG-7 peanut germplasm,Crop Sci.33(1993) 1418.
[25] M.D.Burow,C.E.Simpson,J.L.Starr,A.H.Paterson,Transmission genetics of chromatin from a synthetic amphidiploid to cultivated peanut (Arachis hypogaea L.):broadening the gene pool of a monophyletic polyploid species,Genetics 159 (2001) 823–837.
[26] A.P.Favero,C.E.Simpson,J.F.M.Valls,N.A.Vello,Study of the evolution of cultivated peanut through crossability studies among Arachisipaensis,A.duranensis,and A.hypogaea,Crop Sci.46(2006) 1546–1552.
[27] D.Fonceka,T.Hodo-Abalo,R.Rivallan,I.Faye,M.N.Sall,O.Ndoye,A.P.Favero,D.J.Bertioli,J.C.Glaszmann,B.Courtois,J.F.Rami,Genetic mapping of wild introgressions into cultivated peanut: a way toward enlarging the genetic basis of a recent allotetraploid,BMC Plant Biol.9 (2009) 103.
[28] D.Fonceka,H.A.Tossim,R.Rivallan,H.Vignes,I.Faye,O.Ndoye,M.C.Moretzsohn,D.J.Bertioli,J.C.Glaszmann,B.Courtois,J.F.Rami,Fostered and left behind alleles in peanut:interspecific QTL mapping reveals footprints of domestication and useful natural variation for breeding,BMC Plant Biol.12(2012)26.
[29] D.Fonceka,H.A.Tossim,R.Rivallan,H.Vignes,E.Lacut,F.de Bellis,I.Faye,O.Ndoye,S.C.M.Leal-Bertioli,J.F.M.Valls,D.J.Bertioli,J.C.Glaszmann,B.Courtois,J.F.Rami,Construction of chromosome segment substitution lines in peanut(Arachis hypogaea L.)using a wild synthetic and QTL mapping for plant morphology,PLoS One 7 (2012) 11.
杂志排行
The Crop Journal的其它文章
- Effect of nitrogen fertilizer on distribution of starch granules in different regions of wheat endosperm
- Induced defense responses in rice plants against small brown planthopper infestation
- Overexpression of GmDREB1 improves salt tolerance in transgenic wheat and leaf protein response to high salinity
- Impacts of nighttime post-anthesis warming on rice productivity and grain quality in East China
- Establishment of the integrated applied core collection and its comparison with mini core collection in soybean(Glycine max)
- Genome-wide analysis of the WRKY transcription factor gene family in Gossypium raimondii and the expression of orthologs in cultivated tetraploid cotton
