Effect of nitrogen fertilizer on distribution of starch granules in different regions of wheat endosperm
2014-03-13FeiXiongXurunYuLingZhouJingZhngYnpingJinDonglingLiZhongWng
Fei Xiong*,Xurun Yu,Ling Zhou,Jing Zhng,Ynping Jin,Dongling Li,Zhong Wng
aCollege of Bioscience and Biotechnology,Yangzhou University,Yangzhou 225009,China
1.Introduction
Among the cereals,wheat is the most widely grown in the world.Wheat starch is one of the primary food sources for humans,and the accumulation of starch in endosperm is a fundamental component of grain yield [1,2].Starch is stored in the wheat endosperm as discrete semicrystalline aggregates called starch granules (SGs) [3].Wheat SGs in mature grains are known to have a bimodal size distribution composed of larger A-type and smaller B-type SGs [4,5],which have been characterized structurally and evaluated for their functional properties [6].In addition,a trimodal size distribution of A-,B-and C-type SGs has been observed by some researchers[7–9].The distribution of SGs influences the starch-to-protein ratio in the endosperm,thereby affecting flour composition and quality[10].
Many studies have reported on SG development in wheat endosperm.A-type SGs are generally believed to be synthesized during the early stages of endosperm development and B-type SGs to develop during later stages [11].A-type SGs are formed about 4 days after anthesis(DAA),and then continue to enlarge to their maximum at about 19 DAA,with diameters approaching 25–50 μm[7,12].B-type SG formation begins at about 10–19 DAA[13],but these SGs do not enlarge until 21 DAA,with a diameter of only about 9 μm at maturity.The origin of B-type SGs has been debated during the history of starch research in wheat.Badenhuizen [14] demonstrated that B-type SGs are formed in mitochondria; however,many researchers have reported that B-type SGs form in vesicles budded off from outgrowths of A-type granules [15] or in protrusions emanating from A-type granules containing amyloplasts[9,13,16,17].
The development and distribution of SGs have been shown to be controlled largely by wheat genotype [18–20].Environmental factors,such as drought or temperature during grain filling,also affect wheat grain development,SG size and SG features[21].Tester et al.[22]reported that higher temperatures result in smaller SGs,but Hurkman et al.[23] reported that in conditions with high temperatures the proportion of A granules increases,while that of B granules decreases.Endosperm subjected to drought stress has lower numbers of B-type SGs per cell [24].After drought and temperature,nitrogen (N)nutrition,an indispensable nutrient for wheat production,is considered the third most important environmental factor influencing starch composition and properties [21,25,26].Blacklow and Incoll [27] showed that a moderate reduction in N leads to small increases in starch content in wheat.Increased N fertilization improves the ratio of A-type SGs while the ratios of B-type SGs in the endosperm of strong-gluten wheat cultivars decreases,but the opposite occurs in the medium-gluten and weak-gluten cultivars [28].Although N application during endosperm development greatly affects the distribution of SGs and the properties of starch,very little information is available on the microstructure of N-treated wheat relative to the distribution of SGs in different regions of the endosperm.Visualizing the microstructure of SGs from immature and mature kernels will potentially allow the exploration of the interior of SGs.In the present study,we used image analysis software to investigate the distribution of both A-and B-type SGs under N treatment.Based on these primary measurements,the reasons for variations in the distribution of SGs in different regions of wheat endosperm are discussed.
2.Materials and methods
2.1.Plant materials and N treatment
Wheat(Triticum aestivum L.)cv.Xumai 30,a widely grown hard red winter wheat,was provided by the National Wheat Improvement Center.The experiment was conducted in the research fields of the College of Bioscience and Biotechnology,Yangzhou University,Jiangsu,China from November 1,2011 to August 10,2012.The sowing date was 1 November,2011,and the wheat density was 2.4 × 106seedlings per hectare.The sandy loam soil [Typic Fluvaquent,Entisols (US taxonomy)]contains 12.58 g kg-1of organic material and 75.19,45.52 and 99.3 mg kg-1of available N,phosphorus and potassium,respectively.Plot dimensions were 4 × 5 m and plots were separated by an alley 1 m wide with plastic film inserted into the soil.Each of the treatment had three plots as repetitions in a complete randomized block.The treatment plots received 240 kg ha-1at the booting stage.The control plots received no N at the booting stage.All other field conditions and cultivation managements were kept uniform.During the period of wheat anthesis,the anthesis dates were recorded by dotting the glumes and hanging time tags on the wheat plants.Caryopses that bloomed on the same day but developed on different days for the two treatments were chosen for experimentation.Samples were harvested at 15 and 45 DAA.
2.2.Preparation of materials for light microscopy
First,2 mm cubic blocks were cut by cross-sectioning from wheat caryopses harvested at 15 DAA.The specimens were then fixed with 2.5% glutaraldehyde and 1% paraformaldehyde in a 0.05 mol L-1cacodylate buffer solution (pH 7.2) and post-fixation treatment in 1% osmic acid in a 0.15 mol L-1sodium cacodylate buffer solution (pH 7.2) for 3 h was applied.The blocks were washed,dehydrated through an ethanol series of 30%–100%,and embedded in Spurr's low-viscosity embedding medium.Sections of 1 μm thick were cut with a glass knife on a Leica Ultracut R (Leica Microsystems,Inc.,Wetzlar,Germany),and stained with 0.5% toluidine blue O for 5 min.The sections were visualized and photographed with a Leica Dmls microscope(Leica Microsystems,Inc.).To reflect the nature of caryopsis structure,the findings were compared and confirmed in numerous sections made from developing grains.Five representative regions of transverse sections of the endosperm were observed for every specimen:subaleurone in dorsal endosperm(SDE),center in dorsal endosperm(CDE),modified aleurone(MA),subaleurone in ventral endosperm (SVE),and center in ventral endosperm (CVE),using three replications and 20 micrographs representing ten blocks from different regions.
2.3.SEM observation of caryopsis structure
Mature grains were harvested at 45 DAA and fractured by applying slight pressure on the middle of the caryopsis with a razor blade.The sample thickness was ~3 mm.Caryopses were mounted with the fractured surface facing upwards on a specimen stub and sputter-coated with gold before viewing with a scanning electron microscope(XL30 ESEM,Philips,The Netherlands)at 20 kV to observe the distribution of SGs.
2.4.Determination of number and percentage of SGs
The samples at 15 DAA were used to determine the numbers and percentages of SGs.SGs observed in the image were first marked with a specified color using Photoshop CS4 software(Adobe,U.S.A.)and the image was then analyzed to determine the numbers and percentages of SGs using software Image-Pro Plus 6.0(Media Cybernetics,U.S.A.).Each treatment was represented by 4 kernels,5 sections were chosen in each kernel,and 10 images were acquired for each section.The number of starch granules including A-type (longest axis ≥10 μm) and B-type (longest axis <10 μm) was determined in an area of 0.04 mm2.
2.5.Statistical analysis
Data analysis was performed using Excel 2003 software(Microsoft,U.SA.).Statistical comparisons were performed using SPSS 19.0 (IBM,U.S.A.).The significance levels of differences were calculated for all measured traits,and the means compared at P <0.05.
3.Results
3.1.The size distributions of starch granules in different regions of endosperm
The morphology and number of SGs varied in the five representative regions of transverse sections of endosperm(Figs.1 and 2).Subaleurone cells (Fig.1C,F) contained more A-type SGs and protein bodies (PBs) than central endosperm(Fig.1D,G),while modified cells contained fewer PBs and thicker cell walls(Fig.1E).The diameters of SGs in SDE ranged from 1.5 to 25.5 μm,with peak values ranging from 1.5 to 14.5 μm(Fig.2A); two populations of SGs (Fig.2B) in CDE had peak diameters in the ranges of 2.5–7.5 μm and 16.5–21.5 μm,respectively.In comparison with other regions,fewer SGs were found in MA and with peak diameters ranging from 2.5 to 18.5 μm (Fig.2C).Fig.2D shows SGs in SVE with peak diameters ranging from 2.5 to 14.5 μm.Two populations of SGs(Fig.2E)in CVE had peak diameters ranging from 2.5 to 8.5 μm and 12.5 to 22.5 μm,respectively.The above results indicated that the size distribution of SGs in SDE was consistent with that in SVE,the size distribution in CDE was similar to that in CVE,but distribution of SGs in MA was significantly different from that in the other four regions.The total number of SGs including A-and B-type SGs in the five regions of the endosperm was in the order SDE >CDE >SVE >CVE >MA(Fig.2F).
3.2.Effect of N on the distributions of SGs in different dorsal regions of endosperm
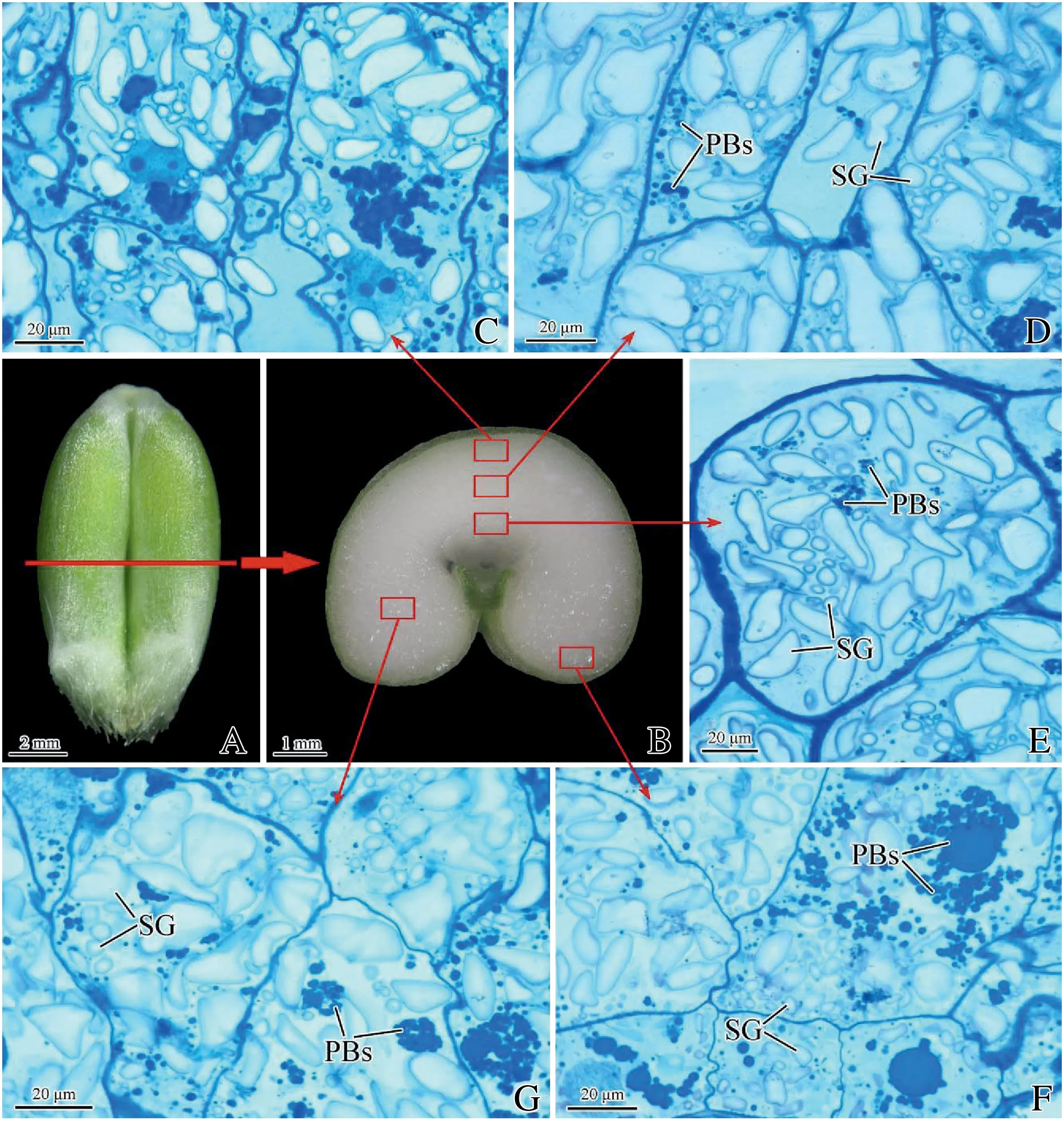
Fig.1-Micrographs of different regions of endosperm in control wheat c.v.Xumai 30 at 15 DAA.A:wheat caryopsis;B:transverse section of caryopsis;C:subaleurone of dorsal endosperm;D:center of dorsal endosperm;E:modified aleurone;F:subaleurone of ventral endosperm;G,center of ventral endosperm;PBs:protein bodies;SG:starch grain.
The application of N fertilizer altered the numbers,shapes,and distributions of SGs in dorsal regions of endosperm(Figs.3–6; Tables 1,2),but responses to N varied in different regions.
A-type SGs in control SDE appeared to have irregular shapes and smaller sizes (Fig.3A),whereas the SGs appeared ellipse-shaped and their sizes became larger under N treatment(Fig.3B).The number of B-type SGs under N treatment was significantly higher,by 33%,than that in the control(Table 1).
The N treated endosperm contained smaller,spherical B-type SGs(Fig.3D).N increased the number of B-type SGs in CDE but decreased the number of A-type SGs (Fig.3C,D).The number of B-type SGs under N treatment was higher by 52%than that in the control (Table 1).However,the number of A-type SGs under N treatment was significantly fewer,by 75%,than that in the control(Table 1).
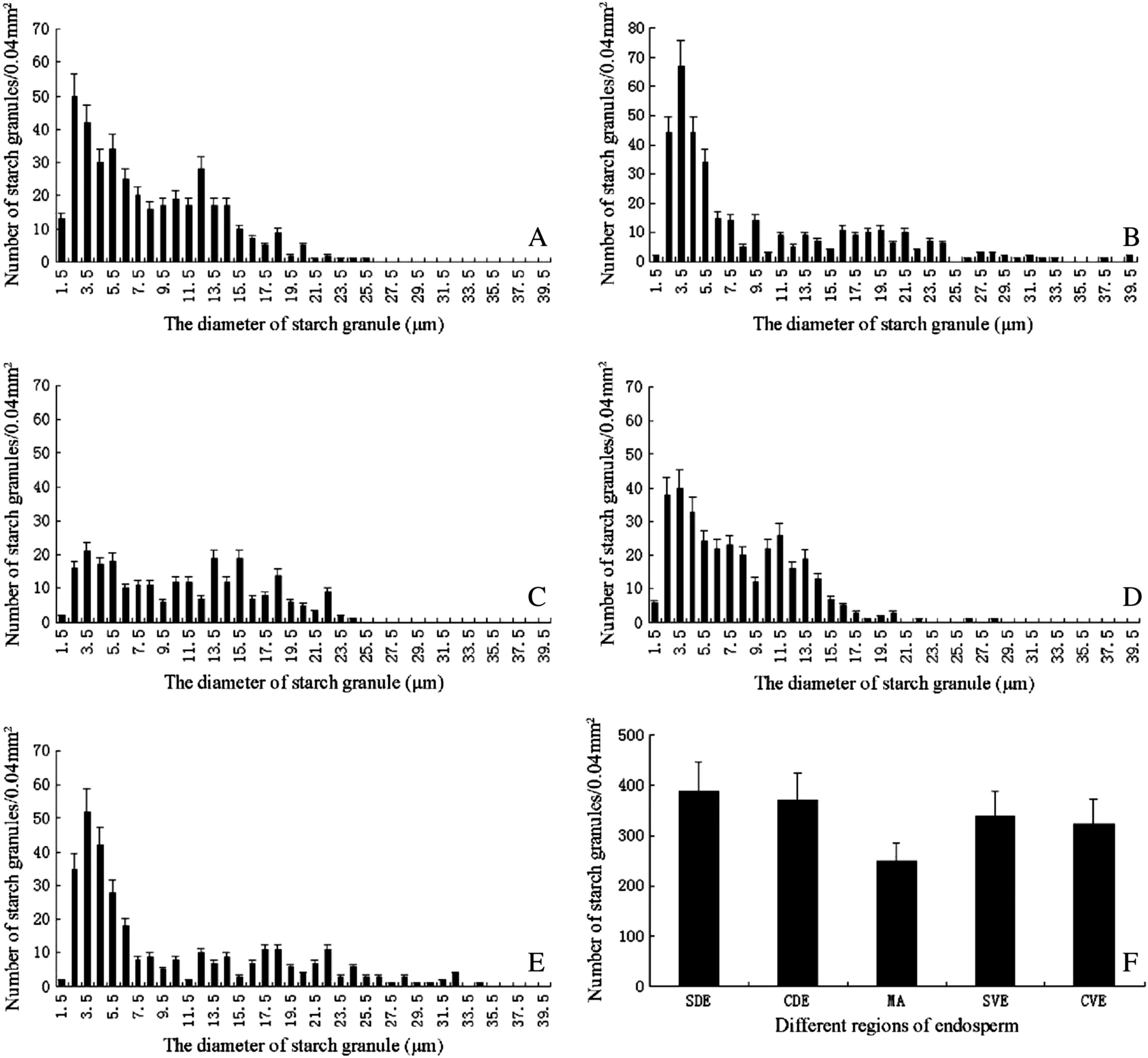
Fig.2-The number distribution of starch granules in different regions of wheat endosperm.A: subaleurone of dorsal endosperm(SDE);B,center of dorsal endosperm(CDE);C:modified aleurone(MA);D:subaleurone of ventral endosperm(SVE);E:center of ventral endosperm(CVE);F:comparison of number of starch granules in SDE,CDE,MA,SVE,and CVE.The number of starch granules was determined in an area of 0.04 mm2,each data point in the graphs represents the mean of 40 replicates.
The SGs in N-treated MA appeared to have spherical shapes and the spaces between the SGs became larger,while those in the control appeared to have irregular ellipse shapes and were arranged compactly (Fig.3E,F).N significantly decreased the number of A-and B-type SGs in MA,by 90% and 103%,respectively (Table 1).The results showed that N markedly affected the distribution of SGs in MA.
3.3.Effect of N on distribution of different ventral regions of endosperm
A-type SGs in SVE appeared ellipse-shaped and their size was larger under the N treatment than in the control(Fig.4A,B).N increased the number of A-type SGs in SVE (Table 2).The results were similar to those in SDE.

Fig.3-Micrographs of control(A,C,E)and nitrogen treatments(B,D,F)in different regions of endosperm.A,B:subaleurone of dorsal endosperm;C,D:center of dorsal endosperm;E,F:modified aleurone;PBs:protein bodies;SG:starch grain.
The size of A-type SGs in CVE was increased by N application (Fig.4C,D).Although N significantly increased the number of B-type SGs,by 39%,it significantly decreased the number of A-type SGs,by 130%,compared to the control(Table 2).The results were similar to those in CDE.
All results above corresponded well to observations made with the scanning electron microscope (Figs.5 and 6).These observations visually demonstrated the marked influence of N on the size and morphology of SGs and thus have potential implications for determining the structure and texture of wheat grain.
4.Discussion
Starch is stored in two types of granules,known as A-type and B-type SGs,having different physical,chemical and functional properties [17,29–31].Although numerous researchers have reported on the size distribution and development of SGs in wheat endosperm,most of them have focused on the whole grain;little information is available about the distribution of SGs in different regions of the endosperm under N treatment.This is the first cytological study on the effect of N on distribution of SGs in different regions of the endosperm.In the study we found that the number of SGs in SDE and SVE was higher than that in CDE and CVE,and that MA had the fewest SGs.The different distribution patterns of SGs based on their locations within the endosperm were probably caused by the different paths of development in endosperm[9]and the pathways that assimilate follow when transferred into SGs[11].Based on the location of SGs within the endosperm,cells followed several different paths of development.For example,starch formation was different in the subaleurone and central endosperm during endosperm development.
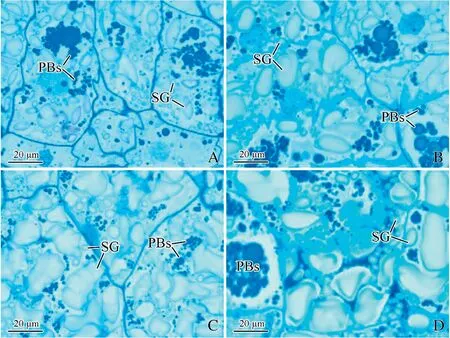
Fig.4-Micrographs of control(A,C)and nitrogen treatment(B,D)in different regions of endosperm.A,B:subaleurone of ventral endosperm;C,D:center of ventral endosperm;PBs:protein bodies;SG:starch grain.

Fig.5-SEM image of control(A,C,E)and nitrogen treatments(B,D,F)in different regions of mature endosperm.A,B:subaleurone of dorsal endosperm;C,D:center of dorsal endosperm;E,F:modified aleurone;PBs:protein bodies;SG:starch grain.
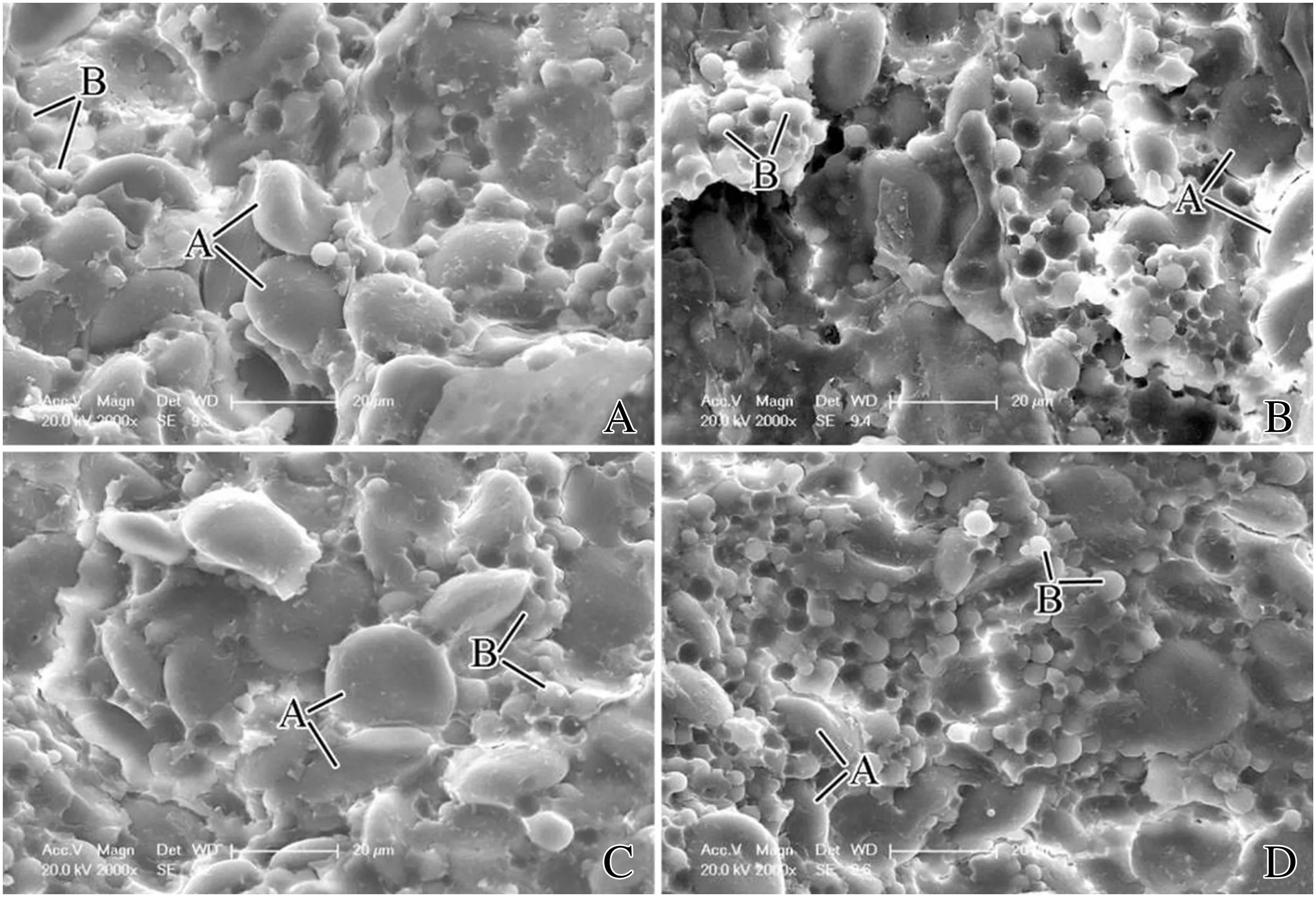
Fig.6-SEM image of control(A,C)and nitrogen treatment(B,D)in different regions of endosperm.A,B:subaleurone of ventral endosperm;C,D:center of ventral endosperm;PBs:protein bodies;SG:starch grain.
The nutrient transport tissues in wheat caryopses include the main vascular bundle,chalaza,nucellar projection,modified aleurone,and aleurone cells [32].Nutrients from vascular bundles are unloaded into the endosperm cavity.The tissues involved in nutrient transfer are the chalaza and nucellar projection.Following uptake from the cavity,there are two pathways into the endosperm(Fig.7):1)via modified aleurone and starchy endosperm tissue,and 2) via aleurone around the endosperm [11].In the present study,we inferred that the sucrose from modified aleuronic cells first accumulated in the outer cells of endosperm,then in the inner cells of endosperm,and finally in modified cells.At the same time,the aleuronic cells also absorbed sucrose from the apoplast and the sucrose was transported from the ventral to the dorsal region.The aleurone began to store aleurone grains and spherosomes,which might hinder nutrient transfer;accordingly,in the later development of caryopses,the modified aleurone became the main pathway of nutrient transfer.Based on the functions of aleurone and modified aleurone,the number of SGs accumulating in different region of endosperm was in the order subaleurone >central endosperm >modified aleurone.The subaleurone in dorsal endosperm had more SGs than the ventral endosperm,probably owing to their proximity and the availability of additional sucrose from modified aleurone.

Table 1-Distribution of starch grains of control and nitrogen-treated wheat in subaleurone of dorsal endosperm (SDE),center of dorsal endosperm (CDE),and modified aleurone(MA).
N is the most important nutrient affecting grain quality,especially in PB accumulation,but little information is available on the effects of N on the distribution of SGs.In the present study we found that N markedly influenced the distribution of SGs.However,our results show some disagreement with those of previous research on the effects of N on SGs in wheat endosperm.Gu et al.[28]reported that increasing N fertilization increased the proportion of A-type SGs and decreased that B-type SGs in strong-gluten wheat,but that the effects were the opposite in weak-and medium-gluten wheat and,moreover,that increasing N fertilization decreased amylose contents.In contrast,Li et al.[33] suggested that N in the range of 0–240 kg ha-1improved the proportion of B-type SGs and amylose and amylopectin contents,but that excess nitrogen decreased starch content.The results obtained in the present study showed that N applied at 240 kg ha-1at the booting stage increased the number of B-type SGs in different regions of the endosperm,in agreement with Li et al.[33].The difference in the results may have resulted not only from the cultivars selected and the periods of N application,but from the methods of measurement or calculations used by the software.
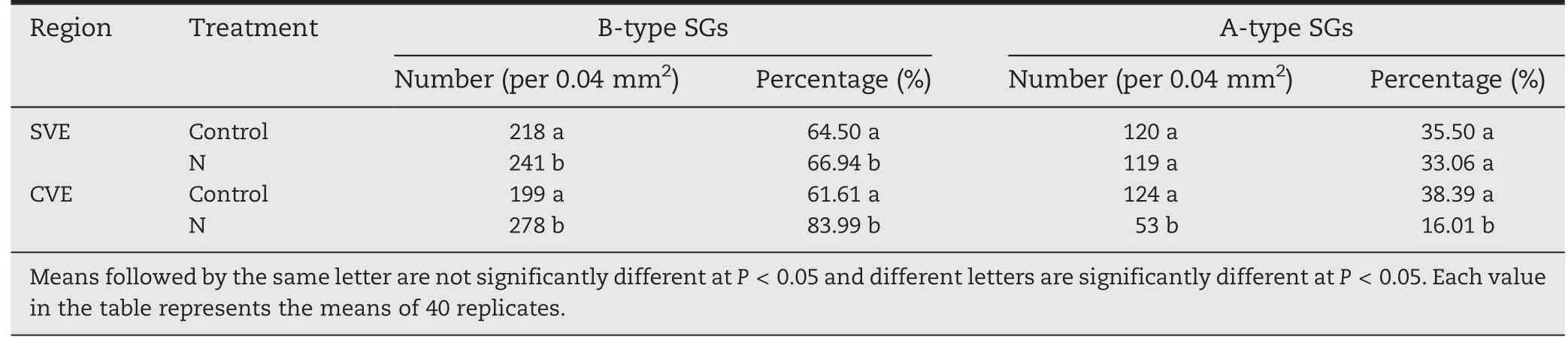
Table 2-The distribution of starch grains of control and nitrogen-treated wheat in subaleurone of ventral endosperm(SVE)and the center of ventral endosperm(CVE).
A-type starch granules generally have higher amylase contents than do smaller granules [34].Thus,N fertilizer not only affects distribution of A-type and B-type but also affects the content and proportion of starch in wheat grains.In this study,we analyzed the distribution of SGs in different regions of endosperm and their response to N.We speculate that in practice the distribution of A-to B-type SGs is regulated by the timing and amount of N fertilizer applied.However,only one variety of wheat,cv.Xumai 30,a hard red winter wheat,was observed in this study.Although Xumai 30 is widely grown in agricultural production,other varieties should be studied in the future.This study also did not address the effect of N on starch granules and its relation to the starch component of SGs,questions that await further study.
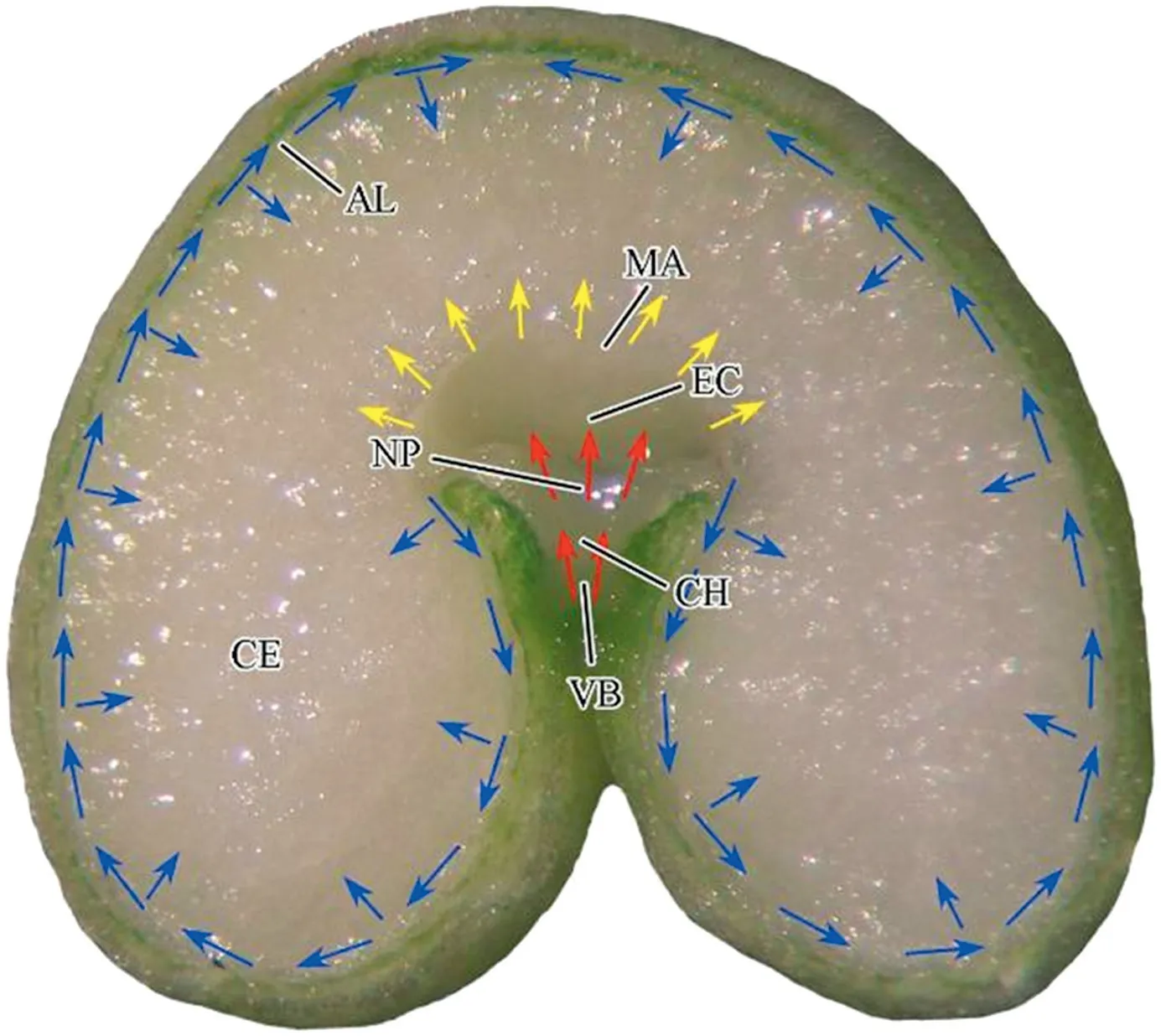
Fig.7-Schematic representation of the pathway of assimilate transfer from vascular bundles to the starchy endosperm in the developing wheat grain.EC:endosperm cavity;AL:aleurone;CH:chalaza;CE:central endosperm;ME:modified aleurone;NP:nucellar projection;VB:vascular bundle.Red,yellow and blue arrows show the flow of sucrose into the endosperm cavity via apoplast,starchy endosperm via modified aleurone,and central endosperm via aleurone,respectively.
The research was supported by the National Natural Science Foundation (31171482),Jiangsu Natural Science Foundation(BK2011445),Jiangsu Graduate Innovation Project(CXLX12-0910),and the Priority Academic Program Development from Jiangsu Government,China.
[1] M.Thitisaksakul,R.C.Jiménez,M.C.Arias,D.M.Beckles,Effects of environmental factors on cereal starch biosynthesis and composition,J.Cereal Sci.56(2012)67–80.
[2] B.Svihus,A.K.Uhlen,O.M.Harstad,Effect of starch granule structure,associated components and processing on nutritive value of cereal starch: a review,Anim.Feed Sci.Technol.122 (2005) 303–320.
[3] P.Calvert,Biopolymers: the structure of starch,Nature 389(1997) 338–339.
[4] B.K.Patel,K.Seetharaman,Effect of heating rate on starch granule morphology and size,Carbohydr.Polym.65(2006)381–385.
[5] R.F.Tester,J.Karkalas,X.Qi,Starch-composition,fine structure and architecture,J.Cereal Sci.39(2004) 151–165.
[6] J.Zeng,G.Li,H.Gao,Z.Ru,Comparison of A and B starch granules from three wheat varieties,Molecules 16(2011)10570–10591.
[7] D.B.Bechtel,I.Y.Zayas,L.Kaleikau,Y.Pomeranz,Size distribution of wheat starch granules during endosperm development,Cereal Chem.67 (1990) 59–63.
[8] M.O.Raeker,C.S.Gaines,P.L.Finney,T.Donelson,Granule size distribution and chemical composition of starches from 12 soft wheat cultivars,Cereal Chem.75 (1998)721–728.
[9] D.B.Bechtel,J.D.Wilson,Amyloplast formation and starch granule development in hard red winter wheat,Cereal Chem.2(2003) 175–183.
[10] Z.M.Dai,Y.P.Yin,M.Zhang,W.Y.Li,S.H.Yan,R.G.Cai,Z.L.Wang,Distribution of starch granule size in grains of wheat grown under irrigated and rainfed conditions,Acta Agron.Sin.34 (2008) 795–802(in Chinese with English abstract).
[11] Z.Wang,Y.J.Gu,W.F.Li,G.Chen,H.Y.Shi,X.H.Chen,Development of wheat endosperm and pathway of nutrient entering the endosperm,Acta Agron.Sin.5(1998)536–543(in Chinese with English abstract).
[12] M.S.Peng,M.Gao,M.Båga,P.Hucl,R.N.Chibbar,Starch-branching enzymes preferentially associated with A-type starch granules in wheat endosperm,Plant Physiol.124 (2000) 265–272.
[13] M.L.Parker,The relationship between A-type and B-type starch granules in the developing endosperm of wheat,J.Cereal Sci.3 (1985) 271–278.
[14] N.P.Badenhuizen,Structure,properties and growth of starch granules,in: W.Ruhland (Ed.),Encyclopedia of Plant Physiology,VI,Springer Verlag,1958,pp.137–153.
[15] M.S.Buttrose,Ultrastructure of the developing wheat endosperm,Aust.J.Biol.16(1963) 305–317.
[16] S.M.J.Langeveld,R.Wijk,N.Stuurman,J.W.Kijne,S.Pater,B-type granule containing protrusions and interconnections between amyloplasts in developing wheat endosperm revealed by transmission electron microscopy and GFP expression,J.Exp.Bot.51(2000) 1357–1361.
[17] C.X.Wei,J.Zhang,Y.F.Chen,W.D.Zhou,B.Xu,Y.P.Wang,J.M.Chen,Physicochemical properties and development of wheat large and small starch granules during endosperm development,Acta Physiol.Plant.5 (2012) 905–916.
[18] L.G.Briarty,C.E.Hughes,A.D.Evers,The developing endosperm of wheat: a stereological analysis,Ann.Bot.44(1979) 641–658.
[19] D.G.Peterson,R.G.Fulchner,Variation in Minnesota HRS wheats:starch granule size distribution,Food Res.Int.34(2001) 357–363.
[20] W.Y.Li,S.H.Yan,Y.P.Yin,Y.Li,T.B.Liang,F.Gu,Z.M.Dai,Z.L.Wang,Comparison of starch granule size distribution between hard and soft wheat cultivars in eastern China,Agric.Sci.China 8(2008) 907–914.
[21] F.M.Dupont,S.B.Altenbach,Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis,J.Cereal Sci.38(2003)133–146.
[22] R.F.Tester,W.R.Morrison,R.H.Ellis,J.R.Pigott,G.R.Batts,T.R.Wheeler,J.I.L.Morison,P.Hadley,D.A.Ledward,Effects of elevated growth temperature and carbon dioxide levels on some physicochemical properties of wheat starch,J.Cereal Sci.22(1995) 63–71.
[23] W.J.Hurkman,K.F.McCue,S.B.Altenbach,A.Korn,C.K.Tanaka,K.M.Kothari,E.L.Johnson,D.B.Bechtel,J.D.Wilson,O.D.Anderson,F.M.DuPont,Expression of genes for starch biosynthesis is regulated by high temperature in developing wheat endosperm,Plant Sci.164 (2003) 873–881.
[24] A.Fábián,K.Jäger,M.Rakszegi,B.Barnabás,Embryo and endosperm development in wheat (Triticum aestivum L.)kernels subjected to drought stress,Plant Cell Rep.30(2011)551–563.
[25] D.R.Kindred,T.M.O.Verhoeven,R.M.Weightman,J.S.Swanston,R.C.Agu,J.M.Brosnan,R.Sylvester-Bradley,Effects of variety and fertiliser nitrogen on alcohol yield,grain yield,starch and protein content,and protein composition of winter wheat,J.Cereal Sci.48(2008) 46–57.
[26] X.Y.Wang,M.R.He,F.Li,Y.H.Liu,H.H.Zhang,C.G.Liu,Coupling effects of irrigation and nitrogen fertilization on grain protein and starch quality of strong-gluten winter wheat,Front.Agric.China 3(2008) 274–280.
[27] W.Blacklow,L.Incoll,Nitrogen stress of winter wheat changed the determinants of yield and the distribution of nitrogen and total dry matter during grain filling,Funct.Plant Biol.8(1981) 191–200.
[28] F.Gu,R.G.Cai,Y.P.Yin,M.Zhang,Y.S.Li,Z.L.Wang,Effects of nitrogen application rates on starch composition and pasting properties of high quality wheat,plant nutrition and fertilizer science,Plant Nutr.Fert.Sci.16(2010) 41–50(in Chinese with English abstract).
[29] W.R.Morrison,Lipids in cereal starches:a review,J.Cereal Sci.8(1988) 1–15.
[30] R.P.Ellis,M.P.Cochrane,M.F.B.Dale,C.M.Duffus,A.Lynn,I.M.Morrison,R.D.M.Prentice,J.S.Swanston,S.A.Tiller,Starch production and industrial use,J.Sci.Food Agric.77(1998) 289–311.
[31] A.B.Soulaka,W.R.Morrison,The amylose and lipid content,dimensions,and gelatinization characteristics of some wheat starches and their A-and B-granule fractions,J.Sci.Food Agric.36(1985) 709–718.
[32] R.D.Thompson,G.Hueros,H.Becker,Development and functions of seed transfer cells,Plant Sci.5 (2001) 775–783.
[33] W.Y.Li,J.C.Lu,S.H.Yan,X.Q.Shi,C.Y.Zhang,Z.Wang,Effects of nitrogen application rate on starch granules size distribution and processing qua1ity of winter wheat,J.Triticeae Crops 2(2012)297–302(in Chinese with English abstract).
[34] C.X.Wei,J.Zhang,Y.F.Chen,W.D.Zhou,B.Xu,Y.P.Wang,J.M.Chen,Physicochemical properties and development of wheat large and small starch granules during endosperm development,Acta Physiol.Plant.32 (2010)905–916.
杂志排行
The Crop Journal的其它文章
- Genome-wide analysis of the WRKY transcription factor gene family in Gossypium raimondii and the expression of orthologs in cultivated tetraploid cotton
- Development of highly glyphosate-tolerant tobacco by coexpression of glyphosate acetyltransferase gat and EPSPS G2-aroA genes
- SSR genetic linkage map construction of pea (Pisum sativum L.) based on Chinese native varieties
- Rank correlation among different statistical models in ranking of winter wheat genotypes,
- Effects of exogenous ABA application on post-anthesis dry matter redistribution and grain starch accumulation of winter wheat with different staygreen characteristics
- Genetic characterization and linkage disequilibrium mapping of resistance to gray leaf spot in maize(Zea mays L.)
