Degradation of Cry1Ab Protein Within Transgenic Bt Maize Tissue by Composite Microbial System of MC1
2014-03-07MengYaoGuWanrongYeLefuChenDongshengLiJingandWeiShi
Meng Yao, Gu Wan-rong, Ye Le-fu, Chen Dong-sheng Li Jing, and Wei Shi*
1Heilongjiang Academy of Land Reclamation Sciences, Harbin 150038, China
2College of Agriculture, Northeast Agricultural University, Harbin 150030, China
Degradation of Cry1Ab Protein Within Transgenic Bt Maize Tissue by Composite Microbial System of MC1
Meng Yao1,2, Gu Wan-rong2, Ye Le-fu2, Chen Dong-sheng1, Li Jing2, and Wei Shi1*
1Heilongjiang Academy of Land Reclamation Sciences, Harbin 150038, China
2College of Agriculture, Northeast Agricultural University, Harbin 150030, China
Environmental safety issues involved in transgenic plants have become the concern of researchers, practitioners and policy makers in recent years. Potential differences between Bt maize (ND1324 and ND2353 expressing the insecticidal Cry1Ab protein) and near-isogenic non-Bt varieties (ND1392 and ND223) in their influence on the composite microbial system of MC1 during the fermentation process were studied during 2011-2012. Cry1Ab protein in Bt maize residues didn't affect characteristics of lignocellulose degradation by MC1, pH of fermentation broth decreasing at initial stage and increasing at later stage of degradation. The quality of various volatile products in fermentation broth showed that no significant difference of residues fermentation existed between Bt maize and non-Bt maize. During the fermentation MC1 efficiently degraded maize residues by 83%-88%, and cellulose, hemicelluloses and lignin content decreased by 70%-72%, 72%-75% and 30%-37%, respectively. Besides that, no consistent difference was found between Bt and non-Bt maize residues lignocellulose degradation by MC1 during the fermentation process. MC1 degraded 88%-89% Cry1Ab protein in Bt maize residues, and in the fermentation broth of MC1 and bacteria of MC1 Cry1Ab protein was not detected. DGGE profile analyses revealed that the microbial community drastically changed during 1-3 days and became stable until the 9th day. Though the dominant strains at different fermentation stages had significantly changed, no difference on the dominant strains was observed between Bt and non-Bt maize at different stages. Our study indicated that Cry1Ab protein did not influence the growth characteristic of MC1.
Bt maize, degradation of Cry1AB protein, composite microbial system of MC1
Introduction
Global cultivated area of genetically modified plants reached 170.3 million hm2in 2012, and among which, plants expressing the Bacillus thuringiensis (Bt) protein was 108.2 million hm2, and where Bt Maize covered 37.6 million hm2(James, 2012). Bt maize is one of the dominant grown genetically modified crops in the world update. With Bt maize introduced into food and feed market, the necessity of elucidating origin and potential genetic modifications of the novel components, such as crystal protein Cry1Ab encoded by Bt gene led to numerous research activities to prevent risk to consumers (Wu, 2006). One particular concern is that transgenic plants may pose risks for non-target organisms (Conner et al., 2003).
Exposure of non-target soil organisms to Bt protein is potentially important, as the protein is expressed constitutively in all the parts of the plants, so that both plant residues remained after harvest and root exudates released during plant growth could contain Bt protein and be incorporated into the soil. The degradation of CryIAb proteins in soil has been determinedby immunology and bioassays with susceptible insect species, as well as degradation of Bt maize biomass. As the toxin, released to soil from Bt maize in root exudates, has been shown to degrade slowly and to accumulate in soil, it is desirable to assess the effects of Bt maize cultivation on non-target soil organisms, similar to the assessment of any other kinds of pesticide. However, such a positive effect had not been found in several previous studies investigating the effects of Cry1Ab plants or purified Cry1Ab on microorganisms (Clark and Coats, 2006), isopods, protozoa, nematodes, fungi, bacteria, algae, and earthworms (Koskella and Stozky, 2002). In contrast, some papers reported effects of the tissue from Bt transgenic maize and rice (Wu et al., 2004) on biological activities in the soil and changes in the microbial population associated with decomposing Bt plant leaves. Microbial properties of soil amended by a Bt maize hybrid expressing relatively high levels of Cry1Ab indicated a significantly reduced microbial community compared to its control (Raubuch et al., 2007). The observed effects could be caused by altered chemical composition of the plant tissues as a result of Bt gene construct insertion.
Some microbial communities have potential to degrade Cry1Ab, one extra protein in the environment which might cause their proliferation and lead to a faster decomposition of Bt versus non-Bt maize (Zwahlen et al., 2007). Soil microorganisms will come into and directly contact with transgenic toxin when they are released from Bt maize and other crops as root exudates or from decomposing tissue (Saxena et al., 2002). However, a few studies reported that Bt toxin has degraded by the known microbial community. Lignocellulose is one of the most abundant structural materials in Bt maize, and can be degraded by microorganisms. A composite microbial system (MC1) with efficient and stable cellulose degradation characteristics was developed by a research team from China Agriculture University (Cui et al., 2002), and the constituent microbial community of the bacteria degraded rice straw by 60% within 4 days at 50℃ (Haruta et al., 2002).
Accordingly, in this study, degradation characteristics of MC1 system with Bt and non-Bt maize tissues were evaluated by immunology and bio-assays. For microbial community analysis, gradient-denaturing gradient gel electrophoresis (DGGE) of PCR-amplified 16S rDNA was used to find changes of MC1 working with Cry1Ab protein. Furthermore, we tested whether MC1 system was able to degrade Cry1Ab protein in the leaf residues of Bt maize.
Materials and Methods
Bacterial strains and culture conditions
The experiment was carried out during 2011-2012. MC1 was a highly efficient cellulose-degradation composite microbial system, isolated from compost heaps. MC1 was cultured in the peptone cellulose solution (PCS) composed of 5 g peptone, 10 g corn stalk, 1 g yeast extract, 3 g CaCO3, 5 g NaCl and 1 L H2O (pH 8.0). And it was maintained as frozen stock at –20℃ in 20% glycerol. Inoculated in PCS medium containing 1% maize stalk (w/v) for 3 days, MC1 was prepared for the subsequent experiment as inoculants. After inoculation MC1 (seed volume of 5%), the medium was cultured under static conditions at 50℃(Cui et al., 2002) for nine days, where sampling for analyzing was conducted each day.
Maize cultivars and their residues preparation
Maize cultivars used in this study, were the transgenic Bt hybrids ND1324 and ND2353, and their near isogenic line of non-transgenic commercial hybrids were ND1392 and ND2233 (National Maize Improvement Centre, Beijing, China). Two kinds of Bt maize contained a synthetic version of the same genes from Bacillus thuringiensis coding for the expression of the insecticidal endotoxin Cry1Ab. Maize hybrids were planted on a sandy loam soil in adjacent strip plots of 20 m2area on the experimental farm of China Agricultural University. At maturity stage, 10 plants were randomly collected from the plots. The maize plantswere air-dried and ground in using an analytical mill. Maize residues were submerged in 1.5% (w/v) NaOH for 24 h, then washed with water to pH of 7.0, and dried again at 80℃ before using.
pH and product measurements
The composite microbial of MC1 was inoculated in 400 mL PCS containing 4 g maize residues under static conditions at 50℃. pH was determined by using the compact pH meter (Model B-212, Horiba, Japan). The determination of volatile products was conducted by using GC-MS. On the 1st, 3rd, 5th, 7th, and 9th, samples obtained from the culture solution were filtered through an aperture of 0.22-μm and analyzed with GC-MS (model QP- 2010, Shimadzu, Japan) on line with a capillary column, CP-Chirasil-Dex CB (25 mm×0.25 mm). The column temperature was 60℃ (1 min)→100℃ (1 min), 7℃/min→195℃(2 min), 18℃/min; injector temperature, 190℃; ion source temperature, 200℃; carrier gas: He (60 kPa); rate of flow: 34 mL • min-1; splitter ratio: 1/20; voltage of detector: 0.7 kV; sample volume: 1 μL (Cui et al., 2002). The final results of the peaks were qualitatively analyzed by NIST database. Dilutions of the corresponding compounds were used as a standard to confirm the positions of the peaks and analyzed quantitatively.
Weight and components of residual solid cellulosic substrate measurements
Residual solid substrates were washed with water to remove non-solid materials, and then dried at 50℃ for three days before weighing. Residual solid cellulosic substrates were assayed as the followings, the precipitate was washed with acetic-nitric reagent and then with water to remove non-cellulosic materials. With an uninoculated medium as the control, the residual substrates were determined using method described by Tailliez et al (1989). Residual solid cellulosic substrates of the maize residues were crushed into pieces, screened through a 1-mm cribble and each 0.5 g sample was transferred into a special pocket then analyzed by fiber analysater.
Cry1Ab protein quantification
Content of Cry1Ab protein in maize residue powder from different varieties was measured by using a commercial enzyme-linked immunosorbent assay (ELISA) quantification kit (EnviroLogix, Portland, ME, USA). Cry1Ab protein was extracted from 0.5 g air-dried Bt maize residues powder with 1.5 mL extraction buffer. The suspensions were centrifuged at 2 000×g for 5 min and resulting supernatants were used for quantification of Cry1Ab protein. Level of Cry1Ab protein was determined using a spectrophotometer. Standard curve, dilution factors, positive and negative controls, and calculations were done following the kit protocol (EnviroLogix, Portland, ME, USA). Three replicates were measured for each sample.
Microbial community research using PCRDGGE
DNA extraction was carried out on the 1st, 3rd, 5th, 7th, and 9th day; 7 mL fermentation broth was centrifugated at the speed of 15000 r • min-1for 20 min; supernate was decanted carefully to obtain the sediment. An extraction buffer was used to preserve the sediment at –20℃. Extraction of DNA was carried out using the benzyl chloride method (Fukumori et al., 1989).
16s rDNA PCR amplification was performed using GeneAmp PCR System (Model 9700, Applied Biosystems, USA). The primers used for DGGE were 357FGC, 5'-CCTACGGGAGGCAGCAG-3' (Escherichia coli positions, 341-357), which was attached to a GC clamp (5'-CGCCCGCCGCGC GCGGCGGGCGGGGCGGGGGCACGGGGGG-3') at the 5'-terminus, and 517R, 5'-ATTACCGCGGCTG CTGG-3' (E. coli positions, 517-534) (Muyzer et al., 1993). Initial DNA denaturation was performed at 95℃for 10 min, followed by 30 cycles of the denaturation at 93℃ for 1 min, annealing at 48℃ for 1 min, and elongating at 72℃ for 1 min and 30 s, followed by a final elongation step at 72℃ for 5 min. The productswere examined by electrophoresis on 2% agarose gel. DGGE analysis of PCR products was performed by DcodeTM system (Bio-Rad Laboratories, Hercules, CA) as described by Muyzer et al. (1993) and Haruta et al (2002). Samples were applied to a 1-mm-thick, 6%-12% (w/v) polyacrylamide gradient gels in 0.5× TAE electrophoresis buffer (20 mmol • L-1Tris-HCl pH 8.3, 10 mmol • L-1acetic acid, 0.5 mmol • L-1EDTA), with 20%-60% denaturant gradient (where 100% was defined as 7 mol • L-1urea with 40% formamide). Electrophoresis was performed at constant voltage of 200 V and temperature of 61℃ for 5 h. After that V3 region bands of 16S rDNA on the gel were stained with SYBR Green I (Molecular Probe, Eugene, Ore.) and photographed. Bands on DGGE gel were observed under UV 302 nm using Alpha Imager 2200 Imaging System (Alpha Innotech, USA).
Statistical analysis
Differences of pH for fermentation broth, residual solid weight, cellulosic weight of solids, degradation products, toxin content and enzyme activities were expressed as means and compared statistically by Tukey's t-test at 5% level with SPSS 11.5 (SPSS for Windows, Version 11.5, USA). Differences between values at P>0.05 were considered as not significant difference.
Results
Changes in pH during degradation of Bt and non-Bt maize residues
pH of the fermentation broth with Bt and non-Bt maize residues was measured during the degradation process (Fig. 1). Irrespective of different maize residues of the media, pH decreased from 8.0 to approximate 6.0, during the initial stage of the process (until the 3rd day). After that pH increased and reached a value of 8.3 on the 9th day. Moreover, no difference of pH was detected between control and Bt maize (P>0.05).

Fig. 1 Changes of pH value during degradation of maize residues
Comparison of degradation rate and solid residue composition between Bt and non-Bt maize
Degradation rate was determined on day 0, 1, 3, 5, 7, and 9, respectively (Fig. 2). From the curve for degradation rate, the maize residues were degraded most expeditiously, during the initial stage of process, and the degradation ratio was 59%-67% on the 3rd day. Finally, the degradation ratio was 83%-88% on the 9th day. The degradation rates were similar among Bt and non-Bt groups (Fig. 2A), and no significant differences (P>0.05) were observed. As shown in Fig. 2, the dry weight of the maize residues decreases by 83%-88% after fermentation for nine days, where cellulose, hemicelluloses and lignin decrease by 70%-72% (Fig. 2B), 30%-37% (Fig. 2C) and 72%-75%, respectively (Fig. 2D). In the initial phase (until the 3rd day), the cellulose, hemicelluloses and lignin degradation amounts increased sharply in each system. However, in the latter phase (until the 9th day), cellulose, hemicelluloses and lignin degradation amounts increased obviously lower than those in the initial phase. In the end, cellulose, hemicelluloses and lignin degradation amounts were not different (P>0.05) between Bt and non-Bt maize.
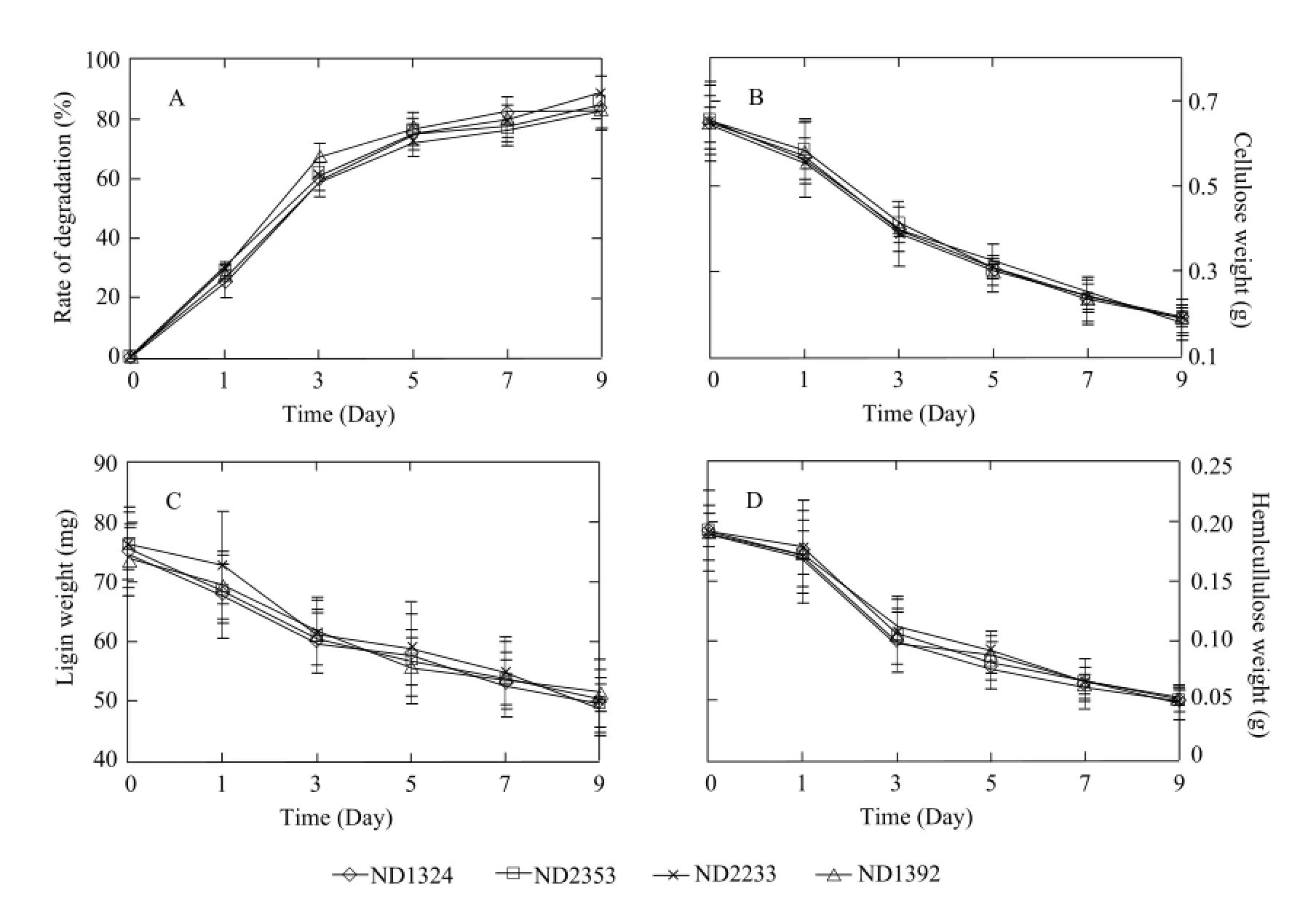
Fig. 2 Comparison of Bt and non-Bt maize residue composition during degradation
Analysis of volatile products for Bt and non-Bt maize residues
Maize residues could produce organic compounds of the low molecular weight through degradation by MC1. Results of qualitative analyses of the main volatile products are shown in Fig. 3. Five volatile products, ethanol, acetic acid, methanol, propanoic acid, and butanoic acid, were determined in the fermentation broth. Contents of the five compounds increased rapidly at the initial stage of process (until the 3rd day); however, at the latter phase (until the 9th day) decreased gradually (Fig. 3). Contents of the methanol and ethanol in the fermentation broth increased fleetly and reached the peak on the 3rd day (except for the methanol of ND1324). Contents of methanol and ethanol in the fermentation broth decreased gently until the 9th day (Fig. 3A and B). Although there was little difference between Bt and non-Bt maize residues in the contents of methanol and ethanol during the degradation, no significant difference (P>0.05) was observed among the maize varieties. Contents of acetic acid, propanoic acid, and butanoic acid in the fermentation broth increased sharply to the maximum on the 3rd day, and declined gradually thereafter (Fig. 3C, D and E). Generally, there was no significant difference between Bt and non-Bt maize for the contents of acetic acid, propanoic acid and butanoic acid, during the whole degradation process.
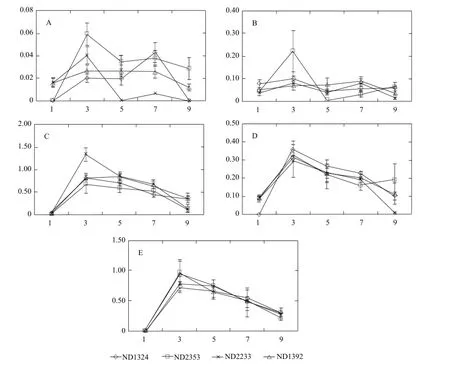
Fig. 3 Quantitative analyses of major volatile products of Bt and non-Bt maize residues during degradation by GC-MS
Cry1Ab protein degradation in Bt maize residues
Incubation of Bt maize residues with MC1 for 3 days, Cry1Ab protein content was significantly reduced, and the degradation ratio was 42%-44% (Table 1). Then, degradation rate of Cry1Ab protein decreased slightly and the degradation ratio was 88%-89% until the 9th day. However, Cry1Ab protein content of the non-Bt maize residues was not detected. On the other hand, Cry1Ab protein content was determined respectively in the fermentation broth of Bt, non-Bt maize and MC1 of bacteria, and no Cry1Ab protein was observed.
Analysis of MC1 during degradation
To investigate changes of the population and composition of MC1 during degradation, samples of days 1, 3, 5, 7, and 9 of the fermentation broth of different maize residues were analyzed by PCRDGGE (Fig. 4). The number of DNA bands was six, and strain of the genetic relationship represented by each DGGE band was (1) Clostridium thermosuccinogenes; (2) uncultured β-Proteobacterium WKB04; (3) Brevibacillus sp. Riau; (4) uncultured Brevibacillus sp. KL-13-4-10; (5) Brevibacillus sp. Riau; (6) Pseudoxanthomounas taiwanenis. Appearance and disappearance of the bands in DGGE pattern indicated importantshifts in the microbial community structure of MC1. On different days during degradation, no difference of bands between Bt and non-Bt maize residues was detected.
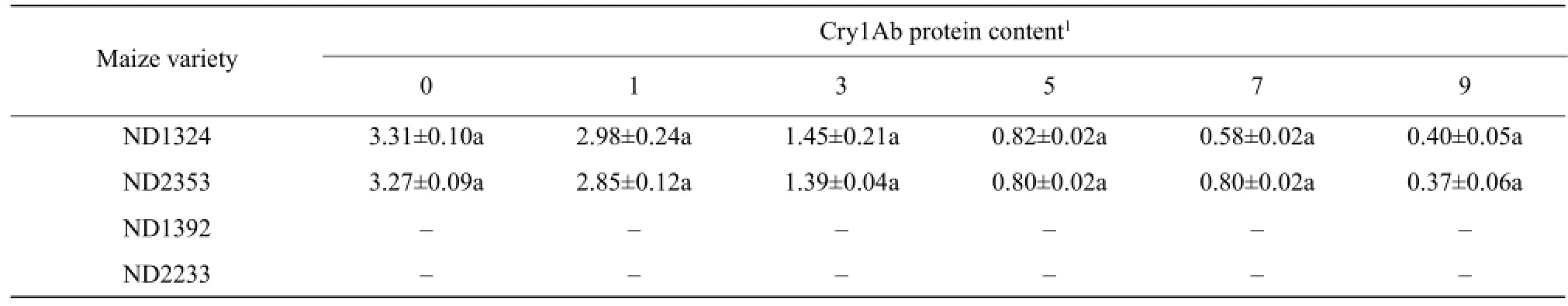
Table 1 Cry1Ab protein degradation by MC1 in Bt and non-Bt maize residues
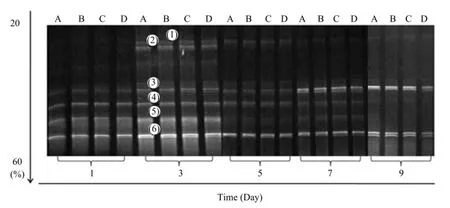
Fig. 4 DGGE profiles of 16S rDNA fragments of MC1 during degradation
Discussion
It has been well documented that physicochemical and microbiological properties of MC1 became relatively stable until the 9th day during the degradation (Guo et al., 2008). During the fermentation process, pH decreased rapidly to approximate 6.0 after being inoculated within three days when cellulose was strongly degraded, and then increased slowly to 8.3 until most cellulose was degraded. Similar pH profiles were reported in the cases of rice and wheat straw fermentation by MC1 (Cui et al., 2002). In this study, no significant difference of pH and volatile products of fermentation broth between Bt and non-Bt maize residues was found. For these evidence, we primarily concluded that existence of Cry1Ab protein didn't alter characteristics of MC1. Moreover, no difference of lignin content was detected between Bt and non-Bt maize. Cry1Ab protein did not inhibit the decomposition rate of maize residues or reduce the degradation amounts of cellulose, hemicelluloses and lignin.
DGGE analysis showed that bacterial community structure in the fermentation process changed dra-stically during the beginning three days and became stable until the 9th day. Although different strains dominated in different fermentation stages, no difference was observed between Bt and non-Bt maize at different stages. This indicated that Cry1Ab protein did not influence growth of MC1. Based on previous studies (Babendreier et al., 2007) and the results shown, it was logical to conclude that Cry1Ab plants or purified Cry1Ab had no positive or negative effects on microorganisms.
Conclusions
This study presented Cry1Ab protein could be degraded by MC1 and had no direct influence on the bacterial characteristics in maize residues. Investigation of degradation ability of MC1 revealed that no consistent differences existed between Bt maize and corresponding non-Bt maize residues. Dominant strains at different fermentation stages by population genetic analyses varied intensively, but no difference was found between Bt and non-Bt maize residues. Our results could confirm that Bt transgenic maize had no positive or negative effects on microorganism and offered implication of the means evaluating potential non-target effects of the transgenic crops.
Babendreier D, Joller D, Romeis J, et al. 2007. Bacterial community structures in honeybee intestines and their response to two insecticidal proteins. FEMS Microbiol Ecol, 59(3): 600-610.
Clark B W, Coats J R. 2006. Subacute effects of transgenic Cry1Ab Bt corn litter on the earthworm Eisenia fetida and the springtail Folsomia candida. Environ Entomol, 35(4): 1121-1129.
Conner A J, Glare T R, Nap J P. 2003. The release of genetically modified crops into the environment-part II. Overview of ecological risk assessment. Plant J, 33(1): 19-46.
Cui Z J, Li M D, Piao Z, et al. 2002. Selection of a composite microbial system MC1 with efficient and stability cellulose degradation bacteria and its function. Environmental Science, 23(3): 36-39.
Fukumori F, Kudo T, Sashihara N, et al. 1989. The third cellulase of alkalophilic Bacillus sp. strain N-4: evolutionary relationships within the cel gene family. Gene, 76(2): 289-298.
Guo P, Wang X F, Zhu W B, et al. 2008. Degradation of corn stalk by the composite microbial system of MC1. Journal of Environmental Sciences, 20(1): 109-114.
Haruta S, Cui Z, Huang Z, et al. 2002. Construction of a stable microbial community with high cellulose-degradation ability. Apply Microbiology Biotechnology, 59(4/5): 529-534.
James C. 2012. Global status of commercialized biotech/GM crops: 2012. ISAAA Briefs no. 34. ISAAA, Ithaca, NY.
Koskella J, Stozky G. 2002. Larvicidal toxins from Bacillus thuringiensis subspp, kurstaki, morrisoni (strain tenebrionis), and israelensis have no microbicidal or microbiostatic activity against selected bacteria, fungi, and algae in vitro. Canadian Journal of Microbiology, 48(3): 262-267.
Muyzer G, Waal E C D, Uitterlinden A G. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction amplified genes coding for 16S rRNA. Applied and Environmental Microbiology, 59(3): 695-700.
Raubuch M, Roose K, Warnstorff K, et al. 2007. Respiration pattern and microbial use of field grown transgenic Bt maize residues. Soil Biol Biochem, 39(9): 2380-2389.
Saxena D, Flores S, Stotzky G. 2002. Bt toxin is released in root exudates from 12 transgenic corn hybrids representing three transformation events. Soil Biol Biochem, 34(1): 133-137.
Tailliez P, Girard H, Millet J, et al. 1989. Enhanced cellulose fermentation by an asporogenous and ethanol-tolerant mutant of Clostridium thermocellum. Appl Environ Microbiol, 55(1): 207-211.
Wu F. 2006. An analysis of Bt corn's benefits and risks for national and regional policymakers considering Bt corn adoption. Int J Technology and Globalisation, 2(1): 115-136.
Wu W X, Q F Ye, H Min, et al. 2004. Bt transgenic rice straw affects the culturable microbiota and dehydrogenase and phosphatase activities in a flooded paddy soil. Soil Biol Biochem, 36(2): 289-295.
Zwahlen C, Hilbeck A, Nentwig W. 2007. Field decomposition of transgenic Bt maize residue and the impact on non-target soil invertebrates. Plant Soil, 300(1/2): 245-257.
S722.7
A
1006-8104(2014)-04-0010-08
Received 7 March 2014
Meng Yao (1983-), female, assistant researcher, engaged in the research of microbiology and research management. E-mail: mengyao830922@163.com
* Corresponding author. Wei Shi, professor, supervisor of Ph. D student, engaged in the research of plant high yield production and macro agriculture. E-mail: weishi5608@163.com
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Aggregation of Diesel Contaminated Soil for Bioremediation
- Effect of Plasticizers on Properties of Rice Straw Fiber Film
- Effect of Daidzein on Ileum Microflora Biodiversity in Hy-Line Variety Brown Layers
- Effect of Dietary Alanyl-glutamine Supplementation on Growth Performance,Development of Intestinal Tract,Antioxidant Status and Plasma Non-specific Immunity of Young Mirror Carp (Cyprinus carpio L.)
