Investigation on the foaming behaviors of NC-based gun propellants
2014-02-14YuxiangLIWeitaoYANGSanjiuYING
Yu-xiang LI,Wei-tao YANG,San-jiu YING*
School of Chemical Engineering,Nanjing University of Science and Technology,Nanjing 210094,China
Investigation on the foaming behaviors of NC-based gun propellants
Yu-xiang LI,Wei-tao YANG,San-jiu YING*
School of Chemical Engineering,Nanjing University of Science and Technology,Nanjing 210094,China
To prepare the porous NC-based(nitrocellulose-based)gun propellants,the batch foaming process of using supercritical CO2as the physical blowing agent is used.The solubilities of CO2in the single-base propellants and TEGDN(trimethyleneglycol dinitrate)propellants are measured by the gravimetric method,and SEM(scanning electron microscope)is used to observe the morphology of foamed propellants.The result shows that a large amount of CO2could be dissolved in NC-based propellants.The experimental results also reveal that the energetic plasticizer TEGDN exerts an important infuence on the pore structure.The triaxial tensile failure mechanism for solid-state nucleation is used to explain the nucleation of NC-based propellants in the solid state.Since some specifc foaming behaviors of NC-based propellants can not be explained by the failure mechanism,a solid-state nucleation mechanism which revises the triaxial tensile failure mechanism is proposed and discussed. Copyright©2014,China Ordnance Society.Production and hosting by Elsevier B.V.All rights reserved.
Solid-state foaming;Plasticization;Foamed propellant;Nucleation;Pore growth;Tensile failure mechanism
1.Introduction
Porous gun propellants have gained extensive attentions due to their fast burning characteristics.The penetration of burning gas into the pores in the porous propellants is believed to cause the considerably increased burning surface when the porous propellants are ignited.Moreover,the convective mode of heat transfer,which is far more effective than the conductive mode of energy transfer which governs the burning rates of most conventional propellants,is easily achieved because of the gas penetration.Because of the considerably increased burning surface and the stronger heat feedback from the burning zone to the unburned zone,the porous propellants exhibited ultra-high burning rates[1,2].Relative applications, such as traveling charge and caseless ammunition,can be realized using the porous propellants due to their fast burning characteristics.
Among the porous propellants,the foamed propellants based on polymer bonded nitramines[3,4]exhibit a bright application prospect due to the variable burning characteristics and the low vulnerability.The foamed propellants are prepared by chemical foaming process in which the polycondensation reaction of isocyanate and polyol produces polyurethane and CO2,and the generated CO2causes the formation of pore in the propellants.In addition to the foamed propellants based on polymer bonded nitramines,the VHBR(very high burning rate)propellants also show a ultra-high burning rate[5]. Further investigation on the VHBR propellants revealed that the porous structure and the presence of boron hydride saltsanions)play important role in the fast burning characteristics of VHBR propellants.
NC-based gun propellants have been widely used in the ammunition charge for several decades.Porous NC-based propellants have gained increasing attentions due to clean and fast burning characteristics.To prepare the porous NC-based propellants,the salt particulates are generally used as porogen.The salt particulates,such as KNO3,are usually premixed with the NC(nitrocellulose)powders,and the propellants containing salt particulates are prepared using theconventional method.The leaching process is carried out to leach out the salts in the propellants.Finally,the pores in the propellants are generated,and the pore size is directly related to the size of salt particulate.The concerns for this process are the lengthy leaching steps and the low porosity.
The supercritical fuid foaming process,differing from the leaching method,provides a feasible way to prepare the porous NC-based propellants with the advantage of green and simple process[6].In general,four steps are needed to prepare foamed materials:(a)dissolution of gas,(b)cell nucleation,(c)cell growth,and(d)stabilization of foam structures.However,two major properties of NC should be considered for the foaming of the NC-based propellants.The frst major property of NC is its high crystallinity.NC,which is prepared by nitrating cotton or wood cellulose fber,is generally aggregated in a state of fber with high degree of crystallinity(ca.50%).For the gas dissolving in the polymer, the crystallites are generally believed to be impenetrable for most non-reactive molecules including CO2,and CO2can only diffuse into the amorphous regions but not the crystalline regions[7].Thus,the high crystallinity of the NC-based propellantswould affectthe CO2sorption and then pore formation.The other major property of NC is its lack of any detectable phase transitions(i.e.melting or glass transition)below the thermal degradation temperature(ca. 183-190°C).Generally,the porous structure of polymers can be generated in the foaming process when the polymer system is melt and its crystallite structure is destroyed [8-11].But for NC-based propellants,the melt state of the propellants can not be reached to due to the low degradation temperature of NC.Therefore,the conventional foaming process of polymer melt can not be used to process the NC-based propellants.
In this work,considering the two major properties of NC mentioned above,the solid-state foaming was proposed and used to prepare the porous NC-based propellants.The socalled“solid-state”means that the foaming temperature is well below the melting temperature of the NC-based propellants and the propellants remain in the solid state when they are foamed.The foaming process of NC-based propellants was carried out in a water bath where the foaming temperature below 100°C can be achieved in this work.The glycerol bath which can heat the NC material to higher temperature is not recommended due to the low degradation temperature of NC and the diffculty in removing the residual glycerol.
In the foaming process of polymer with supercritical CO2,a key step and a crucial associated phenomenon are the plasticization of polymer induced by CO2sorption[12].Generally, the CO2-induced plasticization of polymer lowers the glass transition temperature(Tg)of the CO2/polymer system,facilitating the foaming process.Once the thermodynamic unstable state is achieved by either pressure decrease or temperature increase,the nucleation and pore growth would take place as long as the polymer remains in the rubbery state.After that, the polymer enters the glassy state as CO2escapes from the polymer matrix,and during this period,the produced porous structure is stabilized.This polymer foaming process has been applied to several amorphous or semi-crystalline polymers with low to moderate degrees of crystallinity,such as,PS, PVC,PC,PSF(amorphous polymer),PP,and PET(semicrystalline polymer).For the foaming of the NC-based propellants in the solid state,the plasticization of NC induced by CO2sorption would lower Tgof NC/CO2system to a certain degree.To further lower Tgof NC and increase the plasticization of NC,trimethyleneglycol dinitrate(TEGDN)which is an energetic plasticizer usually used in the propellants to improve the mechanical property at low temperature,was mixed with NC to produce the TEGDN propellants in this work.For preparation of porous NC-based propellants,the single-base propellants and TEGDN propellants were both foamed using supercritical CO2as the blowing agent in the solid state.The foaming behaviors of NC-based propellants foamed at temperature above and below Tgof NC-based propellants have been investigated and discussed.
To our knowledge,few researches on the foaming behaviors of NC-based propellants have been carried out till now.In this paper,the supercritical CO2foaming behaviors of NC-based propellants in the solid state,for the frst time,was investigated in detail.The foaming behavior and mechanism of NC-based propellants were also discussed and studied in this paper.
2.Experiment
2.1.Material
NC powders(nitrogen content,11.8-12.4%)and TEGDN NC tablet(nitrocellulose 67 wt-%,TEGDN 33 wt-%)prepared by Sichuan Nitrocell Co.,Ltd,CO2(purity>99.2%)prepared by the 55th Research Institute,China Electronics Technology Group Corporation,and ethanol and acetone made by A.P, Sinopharm Chemical Reagent Co.,Ltd were used in the experiment.
NC powders,ethanol and acetone were mixed and kneaded in a kneading machine for 3 h,and the mixture was then extrusion molded to prepare the single-base propellants.The single-base propellants were cut into the grains,and then dried in a drying oven at 40°C for 5 days so that the dissolved solvent in NC could be completely removed from the propellants.The TEGDN propellants with TEGDN mass contents of 20%,25%and 33%were also produced using this method, respectively.The single-base and TEGDN propellant grains have 5 mm in diameter and 10 mm in length.
2.2.Batch foaming process
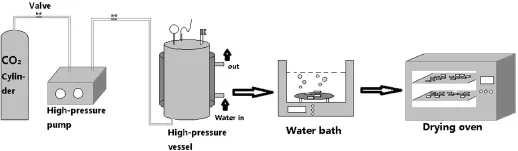
Fig.1.Schematic diagram of batch foaming process.
The solid-state batch foaming process using CO2as the blowing agent was carried out to foam the NC-based propellants.The complete batch foaming process is shown in Fig.1.In a typical experiment,the single-base propellant grains were placed in a high-pressure vessel,and a certain amount of CO2was then purged into it after closing the vessel. The amount of CO2purged was controlled by a liquid pump, and the saturation temperature is controlled by a water bath. CO2was sorbed by NC at the saturation pressure of 10 MPa and the saturation temperature of 40°C for saturation time of 6 h which is long enough to form the uniform CO2/NC system. After the sorption,a rapid venting of the vessel(<30 s)was carried out,and the propellant grains were then immediately immersed in the boiling water bath for foaming the propellants.After 10 min,the foamed propellant grains were taken out of the water bath and then dried in the drying oven for 48 h to get rid of the residual water in the foamed propellant grains.
With the same foaming batch process,the TEGDN propellants with TEGDN mass contents of 20%,25%and 33% saturated at 10 MPa and 40°C for 6 h are foamed at foaming temperature of 100°C,respectively.
2.3.CO2solubility measurement
The CO2solubility measurement was carried out by the gravimetric method[13].Like the CO2sorption in the batch foaming process shown in Fig.1,the propellant grains were placed in the vessel and then saturated in the CO2atmosphere. CO2was sorbed by the propellant grains at the saturation pressure of 10 MPa and the saturation temperature of 40°C for 2 h.After the saturation time,a rapid venting of the vessel (<30 s)was carried out,and the grains were immediately transferred to an analytical balance for measuring the amount of CO2dissolved in the propellant grains.The solubilities of CO2in the single-base propellant grains at the saturation pressure of 10 MPa and the saturation temperature of 40°C for the saturation of 4 h,6 h,8 h and 10 h were also measured using this method,respectively.
The amount of CO2dissolved in the TEGDN propellants with TEGDN mass content of 33%at the saturation pressure of 10 MPa and the saturation temperature of 40°C for the saturation of 2 h,4 h,6 h,8 h and 10 h were measured using this method,respectively.All the measurements were carried out at room temperature(25°C).msis the mass of CO2/NC system when the propellant grains are immediately transferred from high-pressure vessel to analytical balance.The relative sorbed CO2amount,q,results from the quotient of the CO2mass and the initial mass of propellant grains,mo,q=(ms-mo)/mo.
2.4.Morphology characterization
The scanning electron microscope(SEM,QUANTA FEG 250,FEI)was used to observe the inner structures of foamed propellants.Prior to analysis,the samples were fractured after freezing in the liquid nitrogen,and then they were coated with gold for scanning.To further investigate the pore morphology of the foamed TEGDN propellants,the fractures in the transverse section and longitudinal section of the propellant grain were observed,respectively.
3.Results and discussion
3.1.CO2solubility in NC-based propellants
Fig.2 shows the relative amounts of CO2dissolved in the single-base propellants and TEGDN propellants at the saturation temperature of 40°C and the saturation pressure of 10 MPa for saturation of 2 h,4 h,6 h,8 h and 10 h.The curve of relative mass of sorbed CO2in the single-base propellants shows that a large amount of CO2can be dissolved in the NC-based propellants,which suggests that the NC-based propellants is possible to be foamed by supercritical CO2due to the CO2sorption by the amorphous region of NC.It can be also clearly seen from the curve that the amount of sorbed CO2increases with saturation time,the equilibrium state is reached when NC-based propellant is saturated for 6 h,and a little increase in sorbed CO2mass in the single-base propellants is observed after saturation of 6 h.This CO2sorption behavior in the single-base propellants indicates that the uniform CO2/NC system can be generated by immersing the single-base propellants in the supercritical CO2for 6 h.
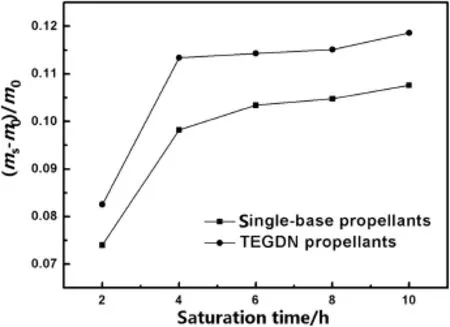
Fig.2.Relative CO2solubilities in the NC-based propellants.
As shown in Fig.2,similar CO2sorption behavior is observed in the TEGDN propellants,a large amount of CO2can be dissolved in the NC matrix plasticized by TEGDN, and the equilibrium state of the CO2/NC system is reached by immersing the TEGDN propellants in CO2for 4 h.Besides,the mass value of CO2sorbed by the TEGDN propellants,which is higher than that sorbed by single-base propellants in the same condition,can be clearly seen from Fig.2.The differences in the amount of CO2solubilities in the two propellants are attributed to the plasticizer TEGDN. TEGDN increased the NC molecule chain mobility and the free volume of NC matrix,and this plasticization effect facilitated the CO2sorption by NC.Therefore,the more amount of CO2is dissolved in the TEGDN propellants.The increased chain mobility and free volume of NC also facilitated the rapid formation of the equilibrium state for the CO2/NC system.
3.2.Pore structure
The single-base propellants were saturated in the supercritical CO2at 10 MPa and 40°C for 6 h and foamed by solid-state batch foaming process.The inner morphology of the foamed single-base propellants is shown in Fig.3.Except the voids which are believed to be caused by pulling out the NC fbers from the fracture surface of single-base propellant grain when the grain is fractured,few pores are generated in the NC matrix.The assumption that a large amount of CO2can be absorbed by the NC materials has been proved in the above experiment of CO2solubility measurement.Besides, the unstable state of the CO2/NC system is also achieved by rapid venting and heating in the batch foaming process,and based on these conditions,this phenomenon that few pores are generated in the single-base propellants can be only attributed to high Tgof CO2/NC system[8,9].Though the plasticization induced by CO2would lower Tgof CO2/NC system,the foaming temperature(i.e.100°C)is still much lower than Tgof CO2/NC system for the rigid property of NC molecule.Thus,NC still remains in a glassy state when it is foamed in boiling water,and NC molecules can not gain the enough chain mobility to nucleate and grow,and this eventually causes the failure of pore formation in the single-base propellants.
The morphology of unprocessed TEGDN propellants is shown in Fig.4a,and the morphology of TEGDN propellants, foamed at 100°C,with TEGDN mass content of 33%is shown in Fig.4b and c.Fig.4b shows the morphology of the transverse section in the propellant grain,and Fig.4c shows the morphology of longitudinal section in the propellant grain. As it can be seen from Fig.4,the massive pores with pore size of ca.15 μm are generated in the foamed TEGDN propellants. Besides,the pore exhibited a circular shape in the transverse section of propellant grain,whereas the pore exhibited a cylinder shape in the longitudinal section of propellant grain.The massive pores generated in the TEGDN propellants proved that the solid-state foaming of NC-based propellants using supercritical CO2is feasible.
In contrast with the foaming behaviors of single-base propellants,the TEGDN propellants exhibit a good foaming performance in the same foaming conditions.It is the difference in the matrix properties between the two propellants that cause the different foaming behaviors.The plasticizer TEGDN is believed to increase the molecule chain mobility and then lower Tgof NC[14].The lowering of Tgof the NC matrix by the addition of a large amount of TEGDN enables the NC molecules to gain enough chain mobility for nucleating and growing when TEGDN propellants are foamed at 100°C,and eventually the circular pores are stabilized and formed[8,11].
The pores of cylinder shape are believed to be caused by the distributed NC fbers in the NC matrix.When the cylinders are foamed in the boiling water,according to the classical nucleation theory[8],the interfaces between the NC fber and surrounded matrix could be the preferential cell nucleation sites during foaming,and these distributed long NC fbers in the matrix serves as the nucleation agent.Thus,the pores grow along the interface between the NC fber and its surrounded matrix,and the cylindrical pores are fnally generated.
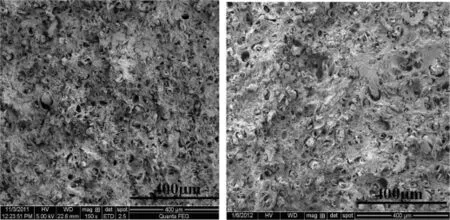
Fig.3.Inner morphology of foamed single-base propellants.

Fig.4.Morphology of foamed TEGDN propellants with 33%TEGDN mass content.
3.3.Infuence of plasticizer on cell formation
The TEGDN propellants,saturated in the supercritical CO2at 10 MPa and 40°C for 6 h,with TEGDN mass contents of 20%and 25%were then foamed at 100°C,respectively.The morphologies of the foamed TEGDN propellants with TEGDN mass contents of 20%and 25%are shown in Figs.5 and 6.In contrast with the circular pores in the TEGDN propellants with TEGDN content of 33%,the small cracks are formed in the TEGDN propellants with TEGDN content of 20%when they are foamed at 100°C.Compared with the massive circular pores in the TEGDN propellants with TEGDN content of 33%, the smaller population of pores is generated in the TEGDN propellants with TEGDN content of 25%,as shown in Fig.6.
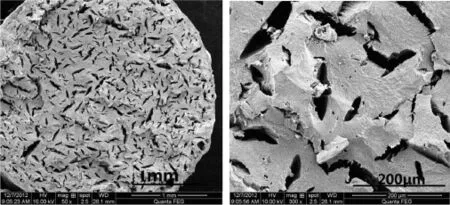
Fig.5.Morphology of foamed TEGDN propellants with 20%TEGDN mass content.
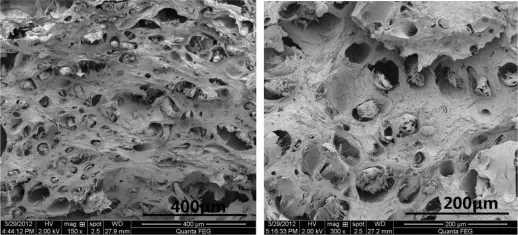
Fig.6.Morphology of foamed TEGDN propellants with 25%TEGDN mass content.
In the past couple of decades,most of the researches aimed at the foaming behaviors of the polymer melt,the cracks in the foamed polymers were seldom found and studied.Except the failure mechanism presented in Refs.[12],the investigation on the solid-state foaming behaviors of the polymer in the vicinity of Tgare seldom carried out.According to the mechanism of solid-state cell nucleation proposed by Holl[12],the volume dilation phenomenon(polymer swelling)is caused by the hydrostatic tensile stress in the polymer matrix.When the polymer is heated to the foaming temperature which is below the melting temperature of the gas/polymer system,the polymer remains in the solid state,but the polymer strength decreases due to the increased molecule chain mobility.Minor fractures are then generated when the increased triaxial stress exceeds the polymer strength,and once the fractures are activated,gas leaves the gas-polymer mixture to fll the growing cells.This gas depletion would reduce the local state of polymer swelling as the growing fractures absorb the dissolved CO2.Finally,the fractures are stabilized when the driving force for fracture growth disappears.
For the NC-based propellants foaming,the crack structure of the foamed TEGDN propellants with TEGDN content of 20%is believed to be generated due to the increased triaxial tensile stress in exceed of the strength propellant matrix.The cracks in the foamed propellants also suggest that the foaming temperature(ca.100°C)is in the vicinity of Tgof TEGDN propellants with TEGDN content of 20%.When the propellants with TEGDN mass content of 20%are placed in a boiling water bath,and the material strength decreases due to the heating effect.Then,the tensile stress in the matrix exceeds the matrix strength,the minor fractures are formed and then grow as the dissolved CO2in the matrix goes into the cracks.When the driving force for fracture growth disappears, the cracks are fnally stabilized.
As for the foaming performance of TEGDN propellants with TEGDN contents of 25%and 33%,the circular pores are attributed to the lower Tgof TEGDN propellants induced by the increased plasticizer content.As the content of plasticizer TEGDN increases,the stronger plasticization effect increases the chain mobility of NC and then lowers its Tgto less than 100°C.Thus,NC is foamed above Tgwhen the TEGDN propellants are immersed in the boiling water,and the circular pores are fnally formed in the propellants.The differences in the pore diameters and pore densities of TEGDN propellants with TEGDN contents of 25%and 33%under the same foaming parameter are attributed to the different NC molecule chain mobilities caused by addition of TEGDN.Higher content of TEGDN in NC enables the NC to gain better chain mobility.Therefore,the bigger pore diameter and higher pore density are achieved in the TEGDN propellants with TEGDN content of 33%[8,11].
3.4.Solid-state nucleation mechanism
The solid-state nucleation mechanism which revises the tensile failure mechanism was proposed based on the above foaming behaviors of NC-based propellants in this paper.The solid-state nucleation mechanism is shown in Fig.7.
The nucleation process begins with a gas-free polymer,as shown in Fig.7a.When the polymer is placed in a pressurized environment and initially experiences a hydrostatic compression generated by the surrounded high pressure gas,as shown in Fig.7b.As gas diffuses into the polymer,the gas-polymer mixture expands and the volume expansion is observed,as shown in Fig.7c.The gas-saturated polymer is removed from the high pressure environment and returned to atmospheric pressure which would result in triaxial tensile stress in the polymer,as shown in Fig.7d.Then,the polymer is heated, the gas/polymer strength decreases while still remaining in the solid state.The foaming behaviors of the gas/polymer system depend on its Tgand the foaming temperature.When the foaming temperature is well above Tgof polymer system which is still in a solid state,the polymer molecule gains enough chain mobility for nucleation and pore growth due to relatively low matrix strength in the rubbery state,and the massive circular pores would be generated in the polymer system,as shown in Fig.7e.If the foaming temperature is in the vicinity of Tgof gas/polymer system,meanwhile,the tensile stress in the matrix exceeds the material strength,the minor fractures would then generated in the polymer matrix. Once the nucleation is activated,gas dissolved in the matrix would leave the matrix to fll the growing cracks,and the gas depletion would reduce the state of hydrostatic tension.As the driving force for nucleation and fracture growth decreases,the cracks would start to stabilize,as shown in Fig.7f.If the material strength can sustain the tension stress in the matrix the minor fractures would not be formed due to the excellent material strength when the foaming temperature is in thevicinity of Tgof the gas/polymer system.In this case,the dissolved gas would run out of the polymer matrix slowly while the polymer remains in a swollen state.When the completely dissolved gas gets rid of the polymer,the polymer swelling phenomenon would disappear,besides,few pores or cracks are fnally generated in the matrix,as shown in Fig.7g.
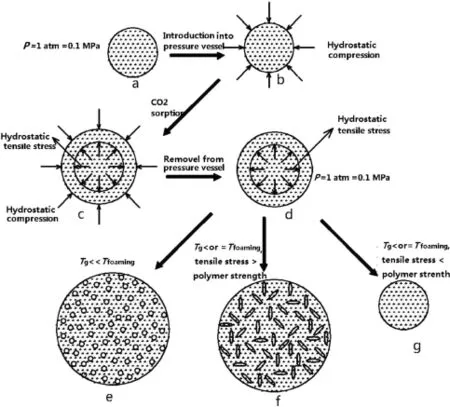
Fig.7.Equilibrated states to the nucleation in the gas/polymer mixture.
As for the foaming behaviors of NC-based propellants,Tgof TEGDN propellants decreases to less than 100°C due to the addition of a large amount of TEGDN into NC.Thus,the massive circular pores in the TEGDN propellants with TEGDN mass content of 33%are generated,as illustrated in Fig.7e,when TEGDN propellants were foamed at 100°C which is well above Tgof CO2/NC system.As the TEGDN content deceases,Tgof the NC system would increase.The cracks in the TEGDN propellants with TEGDN content of 20%are attributed to the decreased material strength when TEGDN propellants are foamed at 100°C which is in the vicinity of Tgof NC with TEGDN content of 20%.The tensile stress in the matrix exceeds the material strength at 100°C,the minor fracture then nucleates and grows,the cracks in the matrix are fnally formed,as illustrated in Fig.7f.For the single-base propellants,the phenomenon that few pores are generated is attributed to Tgof NC matrix higher than foaming temperature and the excellent material strength at 100°C.In that case,the nucleation takes place,as illustrated in Fig.7g.
4.Conclusions
The solid-state batch foaming process was used to prepare the porous NC-based propellants and frst investigated in detail in this work.To investigate the foaming behaviors of NC-based propellants in the solid state,the solubilities of CO2in the NC-based propellants were measured.Besides,the mechanism for the nucleation of the NC-based propellants in the solid state was also proposed in this paper.The test data indicate that:
(1)A relatively large amount of CO2could be dissolved in the NC-based propellants.
(2)The porous NC-based propellants can be prepared by using CO2as the physical blowing agent.
(3)The plasticizer TEGDN plays an important role in the pore formation and pore structure in the NC-based propellants.
(4)The foaming temperature,Tgof the propellants and the material strength of the propellants determine whether circular pores or cracks in the NC-based propellants can be generated or not.
Acknowledgment
This project is funded by the priority academic program development of Jiangsu Higher Education Institutions.
[1]Kuo KK,Vichnevetsky R,Summerfeld M.Theory of fame front propagation in porous propellant charges under confnement.AIAA J 1973;11(4):444-51.
[2]Frolov YV,Korostelev V.Combustion of gas-permeable porous systems. Propellants Explos Pyrotech 1989;14(4):140-9.
[3]B¨ohnlein-Mauß J,Eberhardt A,Fischer TS.Foamed propellants.Propellants Explos Pyrotech 2002;27(3):156-60.
[4]B¨ohnlein-Mauß J,Kr¨ober H.Technology of foamed propellants.Propellants Explos Pyrotech 2009;34(3):239-44.
[5]James TB,Edward BF.Combustion mechanism of very high burning rate (VHBR)propellant.Report ARL-CR-242.USA:Army Research Laboratory;1995.
[6]Su J,Ying S,Xiao Z,Xu F.Research on titanium-containing microporous propellants with rapid burning rate.Propellants Explos Pyrotech 2013;38(4):533-40.
[7]Jiang X-L,Liu T,Xu Z-M,Zhao L,Hu G-H,Yuan W-K.Effects of crystal structure on the foaming of isotactic polypropylene using supercriticalcarbon dioxide asafoaming agent.JSupercritFluid 2009;48(2):167-75.
[8]Colton J,Suh NP.The nucleation of microcellular thermoplastic foam with additives:Part I:Theoretical considerations.Polym Eng Sci 2004;27(7):485-92.
[9]Goel SK,Beckman EJ.Nucleation and growth in microcellular materials: supercritical CO2 as foaming agent.AIChE J 1995;41(2):357-67.
[10]Kazarian S.Polymer processing with supercritical fuids.Polym Sci Ser CC/ C VYSOKOMOLEKULIARNYE SOEDINENIIA 2000;42(1):78-101.
[11]Colton J,Suh N.The nucleation of microcellular thermoplastic foam with additives:Part II:experimental results and discussion.Polym Eng Sci 2004;27(7):493-9.
[12]Holl M,Kumar V,Garbini J,et al.Cell nucleation in solid-state polymeric foams:evidence of a triaxial tensile failure mechanism.J Mater Sci 1999;34(3):637-44.
[13]Muth O,Hirth T,Vogel H.Investigation of sorption and diffusion of supercritical carbon dioxide into poly(vinyl chloride).J Supercrit Fluid 2001;19(3):299-306.
[14]Ceccorulli G,Pizzoli M,Scandola M.Effect of a low-molecular-weight plasticizer on the thermal and viscoelastic properties of miscible blends of bacterial poly(3-hydroxybutyrate)with cellulose acetate butyrate. Macromolecules 1993;26(25):6722-6.
Received 24 December 2013;accepted 9 July 2014 Available online 22 July 2014
*Corresponding author.
E-mail address:yingsanjiu@126.com(S.J.YING).
Peer review under responsibility of China Ordnance Society.
http://dx.doi.org/10.1016/j.dt.2014.07.002
2214-9147/Copyright©2014,China Ordnance Society.Production and hosting by Elsevier B.V.All rights reserved.
杂志排行
Defence Technology的其它文章
- Optimization of process parameters of the activated tungsten inert gas welding for aspect ratio of UNS S32205 duplex stainless steel welds
- Indirect robust control of agile missile via Theta-D technique
- Numerical simulation of base fow with hot base bleed for two jet models
- Analysis on the resistive force in penetration of a rigid projectile
- Numerical simulation of detonation of an explosive atmosphere of liquefed petroleum gas in a confned space
- Combined proportional navigation law for interception of high-speed targets
