热带亚热带土壤氮素反硝化研究进展
2014-02-09续勇波XUZhihong蔡祖聪
续勇波,XU Zhihong,蔡祖聪
1. 云南农业大学烟草学院,云南 昆明 650201;2. Environmental Futures Centre and School of Biomolecular and Physical Sciences, Griffith University, QLD 4111, Australia;3. 南京师范大学地理科学学院,江苏 南京210097
热带亚热带土壤氮素反硝化研究进展
续勇波1*,XU Zhihong2,蔡祖聪3
1. 云南农业大学烟草学院,云南 昆明 650201;2. Environmental Futures Centre and School of Biomolecular and Physical Sciences, Griffith University, QLD 4111, Australia;3. 南京师范大学地理科学学院,江苏 南京210097
热带亚热带独特的土壤性质可能使得反硝化机理有别于温带土壤。文章综述了热带亚热带地区土壤氮素生物反硝化的研究进展,试图更好地了解该地区土壤反硝化在全球氮(N)循环以及在全球环境变化和生态系统响应互作中的角色。热带亚热带土壤反硝化强度普遍较温带地区弱,且随着土地利用方式和耕作管理措施的不同而呈现较大的时空变异性。影响土壤水分状况和土壤碳(C)、N转化特性和速率的因素即为区域和农田尺度上的反硝化影响因素。湿润型热带亚热带土壤由于含有丰富的氧化物而致使土壤氧化还原势较高,这也是导致该地区土壤反硝化势较温带地区较低的关键土壤因素之一。然而土壤pH值不是该地区土壤反硝化势较低的主要限制因素。有机C矿化过程较土壤全氮含量和土壤C/N比在决定湿润型亚热带土壤反硝化势方面更为重要。愈来愈多的证据表明热带亚热带土壤反硝化的生态环境效应不同于温带地区,热带亚热带地区土壤反硝化对全球变暖的贡献应综合考虑其对其它温室气体(如CH4,CO2)排放和氮沉降的影响。热带亚热带土壤生态系统具有一些防止土壤氮素反硝化损失的机制和保氮策略。然而,热带亚热带生态系统对全球变化的响应机制及其生物地球化学调控机制仍然不清楚,这些研究对于反硝化和其它同时发生的氮转化过程模型的精确构建至关重要。
反硝化;环境效应;氮淋失;保氮策略;氧化亚氮
反硝化是指将硝态氮或亚硝态氮还原成一氧化氮(NO)、一氧化二氮(N2O)和氮气(N2)的过程,是氮循环中重要的转化过程和环节。是除了厌氧铵氧化(Anammox)之外唯一能将陆地或水生生态系统中活性氮(Nr)转化为惰性的N2的途径(Galloway 等, 2004)。Galloway等的研究表明全球尺度上关于Nr产生的研究数据远远多于Nr转化为N2的数据(Galloway等, 2008)。反硝化速率和产物的关键控制因素研究对于量化人类活动对陆地生态系统氮循环的影响以及控制和缓解氮污染引起的严重环境问题至关重要(Boyer等, 2006)。
关于土壤反硝化过程、反硝化在生态系统氮损失中的贡献、影响反硝化的因素和其环境效应方面已有众多研究(Wijler和Delwiche, 1954; Šimek等, 2000; Šimek和Cooper, 2002; Hofstra和Bouwman, 2005; Dannenmann等, 2008)。然而土壤反硝化研究主要集中在温带地区恒电荷土壤(Hofstra和Bouwman, 2005),极少有量化研究热带和亚热带地区可变电荷土壤反硝化的报道(Pu等, 2001; Pu等, 2002),因此,影响热带亚热带地区土壤反硝化速率和产物的主要土壤因素和机理尚不十分清楚。
热带亚热带地区雨量丰沛、气温较高,土壤高度风化、土壤脱硅、富含铁铝氧化物(Qafoku等, 2004)。据报道亚热带地区土壤无定型铁氧化物含量显著高于温带地区土壤(Zhang等, 2009),使得相似土壤水分含量条件下,亚热带地区土壤氧化还原势较高(丁昌璞, 2008)。此外,未经人为干扰的湿润热带亚热带土壤大多数都酸性较强(Xu和Cai, 2007),为继承性肥力较低的氧化土(Oxisols)和老成土(Ultisols),富含可变电荷矿物质且微生物区系以真菌为主(Sanchez等, 1989)。显然热带亚热带土壤的化学性质与温带地区的恒电荷土壤有诸多不同特点(徐仁扣等, 2014),这些独特性质有可能使其反硝化机理有别于其它地区土壤。
关于温带地区土壤反硝化的速率、影响因素和研究方法等已有详细综述(Payne, 1981; Tiedje, 1988; Nieder等, 1989; Aulakh等, 1992),因此本文首先简要概述热带亚热带地区土壤反硝化的一般特征,讨论不同研究尺度上影响反硝化速率和产物的主要环境因素和机理;其次综述热带亚热带土壤反硝化的环境效应,分析热带亚热带土壤防止和降低反硝化氮损失的保氮策略;最后讨论了热带亚热带地区反硝化的研究展望。
1 热带亚热带土壤氮素反硝化的一般特性
1.1 热带亚热带土壤氮素反硝化势通常较低
湿润型热带亚热带地区高温高湿的气候特点使人认为反硝化可能是该地区农业生态系统氮素损失的重要途径和机制,然而一些证据表明反硝化也许不是大多数热带土壤氮素损失的主要途径。例如,通过测定加入K15NO3后培养试验中NO, N2O和N2产生量而计算的反硝化速率表明中国热带亚热带森林土壤显著低于东部温带地区森林土壤(Zhang等, 2009)。Xu和Cai也报道了湿润型亚热带土壤以NO3--N还原速率表征的反硝化势普遍较低(Xu和Cai, 2007)。其它一些研究表明热带森林土壤和农用旱地土壤反硝化速率低于或相当于相应温带地区土壤(Nieder等, 1989; Aulakh等, 1992; Bowden, 1986; Robertson和Tiedje, 1988; Groffman和Tiedje, 1989; Griffiths等, 1993),表明热带亚热带地区土壤反硝化通常不是氮损失主要途径。
酸性热带亚热带土壤反硝化势较低的原因可能为:(1)热带亚热带土壤氧化物含量较高使得土壤氧化能力较强,进而提高了土壤氧化还原势,从而抑制了反硝化(Zhang等, 2009);(2)有机C和矿质N含量较低不足以维持足够的反硝化微生物数量和活性(Xu和Cai, 2007; Wang和Cai, 2008);(3)土壤pH值较低,远远低于大多数反硝化菌生长和活性发挥的适宜pH值范围(6~8)(Aulakh等, 1992),也可能是该地区土壤反硝化势较低的原因之一;(4)澳大利亚农用土壤上自然条件下土壤水分较难以达到饱和状态或者在土壤水分达到饱和状态时矿质态15N的淋失都导致反硝化含15N气态产物排放量较低(Pu等, 2002)。
1.2 热带亚热带土壤反硝化势变异较大
热带亚热带土壤反硝化的另一个特点是随着土地利用方式和耕作管理措施的不同反硝化势变异很大,在淹水厌氧充氮气培养试验条件下从不发生反硝化作用(如某些旱地土壤)到11 d内并加入200 mg N kg-1的NO3--N完全消耗完都有可能发生(如某些稻田土壤)(Xu和Cai, 2007)。无论是田间还是实验室土壤培养试验条件下对反硝化绝对量的量化研究都非常缺乏。表1中例举了不同土壤生态系统因植被类型和农业耕作管理措施的不同反硝化氮素损失率变异很大的特性(反硝化氮素损失率占施入15N标记氮肥的1%~53%)(Mahmood等, 2000; Aulakh等, 2001; Weier, 1994; Weier等, 1991, 1993; Bacon和Freney, 1989; Mahmood等, 2001; Houlton等, 2006)。
北半球热带亚热带土壤大多数(>70%)氮素反硝化损失都发生在6月至8月,该期间土壤温度较高、季风性降雨量丰沛,表现出反硝化除了具有上述极大的空间变异性外还具有明显的时间变异性。土壤质地、土壤含水率和NO3--N含量是影响田间原位反硝化速率时间变异性的主要原因(Mahmood等, 2000)。
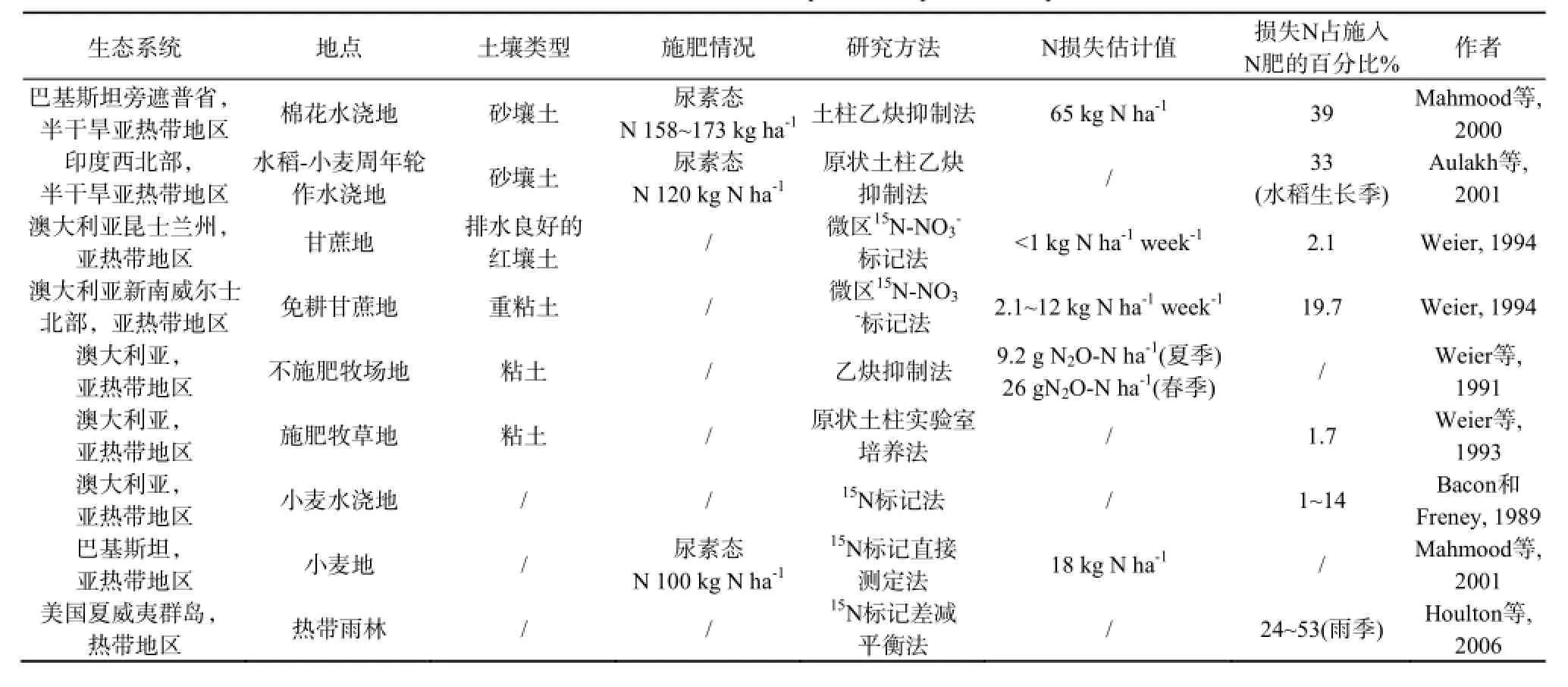
表1 热带亚热带土壤氮素反硝化损失估计值示例Table 1 Estimated denitrification losses in tropical/subtropical soils in published studies
2 热带亚热带土壤反硝化的主要影响因素
2.1 区域和田间尺度影响因素
在区域尺度上研究反硝化应关注土壤类型(土壤质地、自然渗透能力)对土壤水分含量的影响和植物群落类型对土壤中反硝化菌C和NO3--N有效性的影响。总的说来降雨量是区域尺度上反硝化的主要控制因素,其次是土壤有效态C和NO3--N含量(Pu, 1996)。
正如象温带地区那样,当降雨量不是反硝化主要影响因素时,热带亚热带土壤反硝化与土壤C和N转化的特性和速率密切相关(Griffiths等, 1993)。研究大田尺度和区域尺度上的反硝化影响因素需要研究影响土壤C和N转化的因素,如土地利用方式、作物类型、残茬管理方式、有机肥投入、养分管理策略、间套作和耕作收获强度等。凡是能促进土壤C和N积累和厌氧微生物活性的土地利用方式和耕作管理措施均会促进反硝化的进行。
在中国的亚热带土壤反硝化研究表明,反硝化势受土壤母质和土地利用方式的深刻影响(Xu和Cai, 2007)。我们的研究表明反硝化受土壤母质的影响,花岗岩母质发育的土壤反硝化势显著高于第四纪红粘土和第三季红砂岩,土壤有机C和N的有效性是决定3种母质间反硝化势差异的关键因素。土地利用方式对反硝化势的影响表现为稻田土壤反硝化势显著高于林地、旱地、茶园和灌丛土壤,且掩盖了不同土壤母质对反硝化势的影响。稻田土壤反硝化势高的原因主要为淹水的耕作管理措施促进了有机C和N的积累以及可进行厌氧呼吸的微生物的生长和活性的发挥(Xu和Cai, 2007)。
亚热带森林枯落物管理也影响反硝化,如,澳大利亚亚热带南洋杉林地采伐后的残枝和枯落物堆积条带微区域的反硝化15N损失占加入15N肥料的百分比大于条带微区域之间的反硝化15N损失,但这种影响效应2年后将差异不显著(Pu等, 2001)。在水稻-小麦轮作系统土壤可持续生产力的培育中合理的作物残茬和绿肥管理措施是一种环境友好的耕作管理措施,不会显著增加大气中N2O排放负荷(Aulakh等, 2000a, 2001)。然而过量施用N和C将极大地增加N2O排放量。澳大利亚粮食主产区残茬还田已进行了连续10年,在田间原位和实验室土壤培养试验中加入15N后2~4周该土壤反硝化氮损失(施入15N的91%~95%)较不进行秸秆还田(秸秆移出田外且焚烧)的甘蔗地土壤反硝化氮损失(施入15N的51%~85%)高(Pu, 1996)。半干旱亚热带土壤添加不同C/N比有机肥同样也会影响反硝化气态N损失,土壤反硝化氮素累积损失量随施入的有机肥含氮量升高和C/N比降低而呈增加趋势,即绿肥>家禽粪肥>压滤泥浆>牲畜粪肥(Aulakh等, 2000b)。
不同的耕作和作物类型对土壤反硝化有显著影响。澳大利亚东部粮食作物主产区免耕土壤反硝化潜势高于传统耕作土壤,2种耕作方式均是长期休闲土壤的4~5倍((Pu, 1996; Islam, 1992)。不同作物-牧草轮作系统施入15N的反硝化损失率为休闲-豆科牧草(86%)>鹰嘴豆-小麦(58%)>紫花苜蓿-小麦(55%)>小麦连作(46%)>苜蓿-小麦(26%)>长期休闲(9%)(Islam, 1992)。研究结果表明反硝化氮素损失潜势受土壤水分含量、新施入的作物秸秆、施肥位置和氮源的影响。
绝大多数反硝化氮损失研究都集中在不同单作农业生态系统中,仅有很少研究报道轮作和间套作系统中反硝化途径在氮损失中的重要性。Mahmood等的结果表明半干旱亚热带气候条件下小麦-玉米轮作水浇地系统中反硝化氮损失量很大(Mahmood等, 2005),这一结果意味着有必要采取合理施肥和养分管理措施来降低氮损失。
热带地区土壤反硝化同样受不同植被类型下土壤水分和氮素有效性差异的强烈影响。大田尺度上研究反硝化影响因素必须考虑时间变异性。就非农业土地利用方式而言,热带森林土壤反硝化与林地所处的演替阶段有密切关系,森林演替初期(森林砍伐后几周内)和晚期(从未砍伐的原始森林)土壤反硝化势较森林演替中期(森林砍伐后2~25年)高得多(Robertson和Tiedje, 1988)。这和温带地区森林土壤反硝化研究结果一致,表明森林演替中期大多数植被处于氮同化旺盛生长时期,其对土壤水分和NO3--N的竞争也许是抑制了该时期反硝化进行的关键因素(Robertson和Tiedje, 1988; Groffman和Tiedje, 1989; Goodroad和Keeney, 1984; Myrold, 1988; Schmidt等, 1988)。
就农业土地利用方式而言,同样具有反硝化的时间变异性。反硝化随作物生长发育阶段的推进而增强,表明旺盛生长的植株根系分泌物中C有效性增加进而促进了反硝化进行。小麦-玉米轮作水浇地系统中作物不同生育期反硝化氮损失量不同,反硝化损失主要发生在有大量NO3--N累积的灌溉期(Mahmood等, 2005),因此土样采集时期应考虑不同作物生长季之间和干湿季交替之间(Groffman, 1995)。
2.2 影响热带亚热带土壤反硝化的土壤理化性质
影响土壤反硝化速率的因素很多,包括土壤含水率、NO3--N含量、溶解性有机C(DOC)、C和N有效性、氧气分压,土壤温度、微生物活性、土壤呼吸速率等(Griffiths等, 1993; Mahmood等, 2000; Pu, 1996; Mahmood等, 2005; Maag和Vinther, 1999)。已有很多研究证实反硝化速率和这些因素之间的密切相关性,包括温带地区(Šimek等, 2000; Pu等, 2001, 2002; Heinen, 2006; Attard等, 2011)和热带亚热带地区(Xu和Cai, 2007; Aulakh等, 2000a, 2000b, 2000c; Islam, 1992; Mahmood等, 2005; Jia等, 2010; Fellows等, 2011),然而关于湿润型热带亚热带地区反硝化势较弱的原因至今尚无定论。
湿润型热带亚热带土壤有效态有机C、土壤水分、质地、氧气分压以及其它一些土壤理化性质与温带地区土壤相比并无显著差异,且温度也不是反硝化的限制因素,因此,这些土壤理化性质不会是热带亚热带地区土壤反硝化较弱的主要控制因素。如上所述,土壤pH对反硝化的影响尚存在争论(Šimek和Cooper, 2002),Wijler和Delwiche认为最适宜反硝化进行的pH范围为7.0~8.0(Wijler 和Delwiche, 1954)。许多湿润型热带亚热带森林土壤呈酸性且高度风化(Bennema等, 1970),然而一些关于中国湿润型亚热带土壤反硝化的研究表明土壤pH值和反硝化速率之间并无显著相关性,表明pH值不是该地区土壤反硝化势较弱的原因(Zhang等, 2009; Xu和Cai, 2007)。
土壤氧化还原势是造成不同气候带反硝化速率和N2O/N2比值存在差异的关键因素(Zhang等, 2009)。在相似的土壤水分含量条件下,中国湿润型热带亚热带森林土壤氧化还原势显著高于温带森林土壤(丁昌璞, 2008),湿润型热带亚热带土壤氧化还原势较高从而抑制了反硝化,尽管土壤有机C(SOC)在厌氧培养过程中对氧化还原势的降低起重要作用,但土壤氧化能力较SOC在降低氧化还原势方面作用更大(Zhang等, 2009)。
此外,反硝化酶的合成和活性受环境变化影响较大(Parkin, 1990),Pett-Ridge等,发现某热带土壤厌氧培养3周后反硝化速率显著升高(Pett-Ridge等, 2006)。反硝化菌的生长和酶的合成在含有丰富氧化物且氧化还原势较高的湿润型热带亚热带土壤上可能会受到抑制。Xu和Cai的研究也证实湿润型亚热带反硝化菌丰富度较低(Xu和Cai, 2007)。氧化物含量丰富的土壤氧化还原势较高,这也许是中国湿润型热带亚热带土壤反硝化势较低的主要原因之一(Zhang等, 2009)。然而关于土壤氧化物如何直接影响反硝化微生物的生长、酶合成和活性有待于进一步研究。
SOC矿化是反硝化的主要驱动力(Šimek等, 2000; Griffiths等, 1993; Heinen, 2006; Drury等, 1998; Mathieu等, 2006),SOC矿化较土壤全N和C/N比在决定湿润型亚热带土壤反硝化势方面更为重要(Xu和Cai, 2007)。反硝化是消耗电子的异养还原过程,土壤有机质的矿化不仅为反硝化还原NO3--N提供电子(Ahn, 2006; McLain和Martens, 2006),而且可降低土壤氧化还原势,因此湿润型热带亚热带土壤氧化物含量高对反硝化的抑制作用可通过增加土壤有机质含量得到缓解,Xu和Cai的研究结果已证实了这一点,他们的结果表明亚热带土壤反硝化势和土壤SOC含量呈显著正相关(Xu和Cai, 2007)。
反硝化可能受土壤铁氧化物含量及其不同价态铁氧化物之间相互转化的影响,这种影响有可能是生物化学过程(Kumaraswamy等, 2006; Li等, 2012)或者化学反硝化过程(Philips等, 2003),这些方面的研究有待进一步深入。
3 热带亚热带土壤反硝化的环境效应
上述表明热带亚热带地区土壤反硝化研究甚少,影响反硝化的因素几乎和温带地区土壤相同,但是热带亚热带地区土壤反硝化的生态环境效应可能不同。热带亚热带可变电荷土壤硝化和反硝化作用能显著影响离子态养分的吸附和淋失,硝化作用伴随着质子化过程,而反硝化伴随着去质子化过程。森林砍伐后硝化作用受到促进,土壤电荷接近甚至低于电荷零点,使得可交换态离子可能从根区淋失。这一假设已被证实,如哥斯达黎加低地的人工幼林矿质养分存在普遍的N缺乏、铝毒和K/Mg比例失衡的现象(Zech等, 1997);Qian和Cai的研究也证明酸性亚热带土壤硝化作用是控制NO3--N淋失的重要因素,随硝化作用增强NO3--N淋失加剧。阳离子交换量较低、可交换盐基阳离子缺乏的土壤,NH4+-N和H+有可能作为NO3--N的伴随离子而淋失以平衡土壤正负电荷,导致不同土层pH值的改变(Qian和Cai, 2007)。
热带亚热带土壤反硝化势较低对NO3--N还原意义重大,当NO3--N不能被有效还原,残留在土壤中的NO3--N可能通过NO3--N淋失和渗透对环境造成威胁,特别是当气温较高、降雨量大的同时施用过量硝态氮肥时。在澳大利亚亚热带湿润夏季,二代轮作的南洋杉林地育林期间施入的NO3--N尽管有相当一部分经反硝化(6%~26%)和固持(14%~35%)而损失,但氮损失主要归因于NO3--N淋失(32%~53%)(Pu等, 2002; Pu等, 2005)。
3.2 热带亚热带气候条件下反硝化对温室气体排放和还原的影响
反硝化作为大气N2O(一种温室气体)和NO(参与对流层臭氧光化学反应的重要气体)排放源无论是在大田、区域尺度还是全球尺度上都越来越受到关注。尽管湿润型热带亚热带森林土壤自养硝化作用和反硝化作用较弱,但在气候因素、土壤特性、较快的氮循环速率等综合作用下,气态氮氧化物损失较温带森林土壤高,且是全球最大的N2O和NO自然排放源(Xu和Cai, 2007; Lashof和Ahuja, 1990); (Matson和Vitousek, 1990; Davidson和Kingerlee, 1997; Stehfest和Bouwman, 2006; Zhao等, 2007)。Veldkamp(1998)等认为热带农田生态系统N2O和NO排放量远远高于温带地区农业土壤,可能也是全球N2O和NO主要排放源,尽管这一科学假设还没有得到直接证据。
众多研究表明,不同土地利用方式下气候因素和土壤理化特性(如土壤pH、NO3--N和DOC含量、氧气分压、土壤氧化还原势)的相互作用影响N2O产生底物的有效性,进而影响反硝化过程中N2O还原酶酶活性和N2O占总气态含氮产物的比率(Wolf和Brumme, 2003; Manconi等, 2006; Chapuis-Lardy等, 2007; Dannenmann等, 2008; Xu等, 2012)。但是影响热带亚热带地区土壤和温带地区土壤反硝化N2O/N2比差异的因素和机理尚需进一步研究。
厌氧培养条件下,热带亚热带地区森林土壤反硝化过程中产生的NO占总气态含氮产物的比率和N2O占总气态含氮产物的比率通常远远高于温带森林土壤(Keller等, 1983; Keller等, 1986; Parsons等, 1993; Zhang等, 2009)。土壤氧化能力也许是影响热带亚热带土壤反硝化速率的关键因素,因为土壤氧化物含量高的土壤氧化能力强,即土壤氧化还原势高,从而抑制了土壤反硝化的进行,使得NO和N2O占总气态含氮产物的比率增加(Zhang等, 2009)。
尽管众多文献报道了热带亚热带地区不同土地利用方式或土地利用方式的改变对N2O排放的影响,但是很少有对这些生态系统下N2O排放途径进行区分的研究。可控实验室培养条件下用15N示踪法研究N2O不同排放途径,结果表明,40%~52%WFPS(土壤含水空隙率)的好氧条件下,反硝化是中国亚热带酸性森林土壤(pH4.5)和亚热带集约化蔬菜地N2O主要排放途径(Zhang等, 2011a; Zhu 等, 2011),反硝化产生的N2O约占总N2O排放量的50%以上;而与之相应,在相似土壤含水率条件下(35%WFPS)温带牧羊草场(pH5.9)反硝化产生的N2O仅占总N2O排放量的10%以下(Rütting等, 2010)。但是在土壤含水率较高时(WFPS接近田间最大持水量),某德国温带酸性森林土壤(pH 3.8)原位试验结果表明反硝化是N2O产生的主要途径(Wolf和Brumme, 2002)。
好氧条件下反硝化是亚热带酸性森林土壤N2O的主要排放源,好氧条件下反硝化的发生是由于加入15N溶液后促进了由于微生物生长或土壤团聚体水分饱和所形成的厌氧微区的存在(Renault和Stengel, 1994)。好氧条件下也可能发生反硝化(Müller等, 2004; Xiong等, 2009),但其机理尚不明确。此外,化学反硝化也可能是酸性土壤N2O的排放源(Sørensen和Thorling, 1991),但是目前尚未将化学反硝化与生物反硝化区别开来。与温带森林土壤相比亚热带酸性森林土壤真菌生物量高(Shi等, 2002; Wu等, 2009),真菌具有同时进行反硝化和好氧呼吸的功能(Shoun等, 1992; Laughlin和Stevens, 2002),与需要在厌氧条件下才能进行的细菌反硝化相比真菌能在更宽范围的氧气浓度条件下进行反硝化(Firestone和Davidson, 1989; Granli和Bockman, 1994; Murray和Knowles, 2004)。热带亚热带生态系统土壤呈酸性可能不利于反硝化细菌的生长,进一步证实真菌参与的反硝化是N2O的重要排放源,真菌可能缺乏N2O还原酶(Shoun等, 1992),这就可以解释为什么亚热带森林土壤是全球最大的N2O排放源,尽管这些地区土壤生态系统自养硝化和反硝化作用较弱(Xu和Cai, 2007; Zhao等, 2007)。
在热带地区,N2O主要来源于反硝化,而NO主要来源于硝化作用(Veldkamp 等, 1998)。关于热带亚热带地区反硝化过程中NO排放的研究甚少。有研究表明热带亚热带土壤反硝化过程中产生的NO占总气态氮产物的比率比N2O占总气态氮产物的比率低(Zhang等, 2009)。
3.3 全球气候变化下的热带亚热带土壤反硝化环境效应
氮不足和氮过量分别是大多数温带和热带森林土壤生态系统的特征,它们对外源氮素施入的响应明显不同(Martinelli等, 1999)。热带森林土壤似乎已达到自然“氮饱和”状态,氮沉降的增加将可能导致较温带森林土壤更多的NO排放量(Li等, 2008),此外,热带森林土壤对人类活动产生的活性氮的保蓄能力没有北纬地区森林土壤高,湿润型热带亚热带森林额外的氮沉降将可能导致施入的氮素以NO损失的比例达到2%(Hall和Matson, 1999; Li等, 2008)。
值得注意的是,热带亚热带地区反硝化对全球变暖的贡献和环境效应需要进行综合考虑,因为其它温室气体(如甲烷CH4)的排放和还原,也会受到反硝化作用的影响。反硝化厌氧培养条件下,加入的NO3--N显著抑制了亚热带土壤CH4的产生和排放,这主要是由于NO3--N对产甲烷过程的竞争抑制作用或者反硝化产物对产甲烷菌的毒害作用造成的(Lindau, 1994; Kluber和Conrad, 1998; Lu等, 2005)。NO3--N对CH4排放的抑制效应可能较N2O的强,且NO3--N不仅能抑制CH4的产生和排放还能抑制Fe3+的还原(Xu等, 2008)。
CO2浓度升高(从463升高至780 ppm)可促进陆地生态系统反硝化和N2O的排放(van Groenigen等, 2011)。这可能主要是由于植物水分利用效率的提高,降低了由蒸腾作用导致的土壤水分损失(Wullschleger等, 2002);此外,CO2浓度升高可促进很多生态系统中土壤生物活性的增强(Zak等, 2000; Pendall等, 2004);这些生态响应有利于土壤厌氧微区的形成,从而促进了N2O主要产生途径之一的反硝化作用的增强。CO2浓度升高也会促进根系生物量的增加,矿质土壤的C源主要来自于根系分泌物,根系分泌物中易分解有效态C作为反硝化菌的能量物质和呼吸底物可促进反硝化的进行,因此根系生物量的增加将促进反硝化作用,进而促进N2O的排放(van Groenigen等, 2011)。
温室气体排放量的增加加剧了陆地生态系统的辐射强度,这些温室气体的排放将至少抵消之前由于大气CO2浓度升高、陆地生态系统C库增加所估计的全球气候变化消减潜力的16.6%(van Groenigen等, 2011),因此,可能过高估计了陆地生态系统减缓全球变暖的潜力。由此可知,评价反硝化的环境效应需要在全球气候变化的大背景条件下进行。
4 热带亚热带地区土壤防止反硝化N损失的策略
热带亚热带土壤普遍较弱的反硝化作用对于NO3--N的保蓄和淋失意义重大。依据Vitousek和Reiners提出的养分保持假说(Vitousek和Reiners, 1975),土壤N素含量较低的生态系统的关键特征之一是有机氮转化成矿质氮的速率通常较低,N循环和转化过程朝有利于防止N损失(如淋失或反硝化气态氮损失)的方向发展(Vitousek等, 1979; Huygen等, 2007)。为降低NO3--N损失风险热带亚热带土壤形成了一些保N策略和机制,其中可能的保N策略和机制综述如下。
4.1 降低硝化和矿化作用而增强固持作用
大多数对于温带土壤有效的保N策略和机理同样对热带亚热带土壤有效。热带亚热带土壤降低以NO3--N形态的氮损失的必要前提是降低硝化速率,如淹水土壤施入NH4+-N形态的氮肥时,硝化过程减缓、NO3--N有效性限制了反硝化的进行(Aulakh等, 2000c)。此外,对于牧草地而言通过NO3--N渗透将其转移至水溶性C含量较低的25 cm土层以下也许是防止施入的15N反硝化损失的另一策略(Pu, 1996)。澳大利亚亚热带南洋杉林地枯落物降低了硝化作用而增强了固持作用,使得更多的N被固持而防止了NO3--N的大量淋失或反硝化损失,南洋杉林地土壤较相邻原始森林土壤矿质氮含量相对缺乏,这也许是南洋杉林地土壤保持矿质态N持续充足供应的机理之一(Pu等, 2002; Xu等, 2008; Pan等, 2009)。秸秆还田的整地措施和覆盖栽培的效果与林地枯落物的效果相似,通过降低净硝化速率从而降低NO3--N含量达到降低N淋失和反硝化损失风险的目的(Huang等, 2008)。
湿润型热带放牧草场较低的有机N矿化速率和硝化速率限制了反硝化的进行(Vitousek和Reiners, 1975; Buresh和Austin, 1988; Buresh和DeDatta, 1990; Scholes和Sanchez, 1990)。即使是在有机N矿化速率较高的中国亚热带阔叶林土壤(其矿化速率是针叶林的2倍以上),硝化速率同样极低,同时,这些阔叶林土壤产生的NO3--N中大约86%被微生物固持,有效防止了NO3--N淋失和反硝化损失(Zhang等, 2011b)。这种有机N矿化速率较高同时N固持效率也较高的机理同样也在澳大利亚亚热带枯落物覆盖的硬木树林地土壤上被证实,表明更多的N素被保存在土壤中,从而在较长时期内有利于树木的养分供应(Huang等, 2008; Stark和Hart, 1997)。
热带亚热带地区土壤较低的硝化速率本身就是减缓该高温高湿生态系统NO3--N淋失风险的策略(Vitousek等, 1979; Huygen等, 2007),同时较低的反硝化速率本身也是降低气态N损失的策略。即使当加入葡萄糖反硝化速率提高,亚热带土壤也具有降低N矿化来弥补反硝化N损失的保氮策略,并且这种保氮机理在有机C含量较低的土壤上更加突出(Jia等, 2010)。
4.2 NO3--N异化还原为铵和N的固持
微生物介导的产铵过程(如NO3--N异化还原为铵(DNRA)、微生物同化、NO3--N激发的有机N矿化)与反硝化竞争底物NO3--N,将N以NH4+形式保蓄下来,这对于降低反硝化N2O和NO排放以及降低NO3--N淋失导致的地表水和地下水富营养化风险具有重要的生态环境意义(Tobias等, 2001; Page等, 2003; Ma和Aelion, 2005)。
气候条件(如降雨量)可影响N的保蓄机理(Zhang等, 2011c),在潮湿的气候条件下由DNRA或NO3--N微生物固持过程所保蓄的N量显著高于温带或半干旱地区气候条件。Huygens等,发现智利南部假山毛榉原始森林生态系统中86%以上的新产生的NO3--N被立即消耗,其中99%以上是通过DNRA途径被消耗(Huygen等, 2007)。生态系统中DNRA途径的存在比我们想象的要更为普遍(Huygen等, 2007; Vitousek 和Sanford, 1986; Vitousek和Matson, 1988),特别是当土壤水分含量较高、厌氧微区较多时。不同生态系统中DNRA和NO3--N微生物固持作用的生态功能和角色以及DNRA和NO3--N微生物固持作用普遍发生的条件还有待开展更深入的研究,这些将为我们探索生态系统N保蓄机理提供更多的线索。
此外,DNRA也是N2O的排放途径,DNRA和反硝化对N2O排放的相对贡献至今尚不明确,因此应综合考虑并评价DNRA的生态环境效应。
4.3 NO3--N向可溶性有机N转化途径的增强
热带亚热带土壤长期厌氧培养条件下当土壤Eh降低时,加入的NO3--N由非生物固持作用快速转化为可溶性有机N(DON)(Zhang等, 2010),这一现象可用亚铁循环假说(Ferrous Wheel Hypothesis)加以解释,即厌氧微区的形成促进了有利于非生物NO3--N还原的氧化还原条件的形成,含铁(Fe)或含锰(Mn)矿物首先将NO3-还原成NO2-,大量存在的可溶性有机C(DOC)进一步和NO2-反应生成DON(Davidson等, 2003, 2008)。这一现象意味着当热带亚热带森林土壤枯落物层(含大量有机C)长期淹水条件下形成足够低的氧化还原势时DON会大量生成,这也许是热带亚热带土壤的另一NO3--N保蓄策略和机理,尽管DON的移动性较NO3--N低,这一途径也可能在温暖湿润的季节通过DON的渗透和径流而造成N损失环境污染威胁。
热带亚热带气候条件下,不同生态系统植被类型不同,与之协同进化形成的微生物区系和种群也不同,使土壤具有了不同的SOM特性,不同氮转化途径的精巧配合使热带亚热带土壤发挥了保氮和供氮功能,而土壤SOM在其中起到了关键的枢纽作用。
5 热带亚热带土壤反硝化研究展望
随着发展中国家和地区农业的不断发展,至2020年全球60%以上的N肥将施用在热带和亚热带地区,将进一步增加含氮化合物的迁移、转化、排放和沉降(Galloway等, 1994)。越来越多的证据表明热带亚热带土壤反硝化具有一些与温带土壤不同的特征,然而还需要进一步深入研究造成热带亚热带土壤和温带土壤反硝化特性不同的原因和机理,这将有助于加深我们对热带亚热带环境条件下土壤N循环的理解和认识。
全球气候变化已造成陆地生态系统许多物理、化学和生物学方面的变化(如大气CO2浓度升高、气温升高、降水模式的改变、大气N沉降、土地利用方式改变、森林火灾/生物质燃烧等),全球气候变化可能会导致N生物地区化学循环的改变,反之进一步调节生态系统对环境变化的响应。大多数关于环境变化引起N循环改变的生态后果的研究都集中在温带地区,这些地区生态系统中的生化过程通常受到土壤N含量过低的限制。但是,热带亚热带生态系统通过调节生物地球化学循环、进而调节其对环境变化的响应机制尚不清楚,特别是全球气候变化和N素以及其它养分元素生物地球化学循环之间的关系仍是研究空白。因此,我们针对全球气候变化和反硝化之间的可能的耦合过程和机理提出了一些科学假设,两者之间的耦合过程和机理通过C和其它营养元素的生物地球化学循环交织在一起。C生物地球化学循环是陆地生态系统和气候系统之间的关键交织点,而N的生物地球化学循环进一步受C以及其它养分元素有效性的促进或抑制。值得指出的是,全球水循环也是全球气候变暖背景下驱动C和其它养分元素循环以及反硝化的重要生态过程,因此需要在研究上述科学问题时加以充分考虑。
此外,研究环境变化对N循环的影响以及评价N循环在调节生态系统对环境变化的响应机制中的作用时,应将N循环放在C、N、P生物地球化学循环之间交互作用的背景下,利用土壤化学、微生物生态学、植物生理学、分子生物学等多学科交叉的研究方法,这些研究将为更好地认识反硝化的相对重要性及其与其它元素循环的关系提供理论依据。
AHN Y H. 2006. Sustainable nitrogen elimination biotechnologies: A review [J]. Process Biochemistry, 41: 1709-1721.
ATTARD E, RECOUS S, CHABBI A, et al. 2011. Soil environmental conditions rather than denitrifier abundance and diversity drive potential denitrification after changes in land uses [J]. Global Change Biology, 17: 1975-1989.
AULAKH M S, DORAN J W, MOSIER A R. 1992. Soil denitrification -significance, measurement, and effects of management[C]//Stewart B A. Advances in Soil Science, Vol 18. New York: Springer-Verlag.
AULAKH M S, KHERA T S, DORAN J W, et al. 2001. Managing crop residue with green manure, urea, and tillage in a rice-wheat rotation [J]. Soil Science Society of America Journal, 65: 820-827.
AULAKH M S, KHERA T S, DORAN J W. 2000c. Mineralization and denitrification in upland, nearly saturated and flooded subtropical soil I. Effect of nitrate and ammoniacal nitrogen [J]. Biology and Fertility of Soils, 31: 162-167.
AULAKH M S, KHERA T S, DORAN J W. 2000b. Mineralization and denitrification in upland, nearly saturated and flooded subtropical soil II. Effect of organic manures varying in N content and C:N ratio [J]. Biology and Fertility of Soils, 31: 168-174.
AULAKH M S, KHERA T S, SINGH K, et al. 2000a. Yields and nitrogen dynamics in a rice-wheat system using green manure and inorganic fertilizer [J]. Soil Science Society of America Journal, 64: 1867-1876.
H社区还与所属街道、周边商铺、企业签署消防安全责任状,将消防安全纳入安全生产目标管理考核范畴,根据考核结果对先进社区给予通报表彰,对不合格社区给予通报批评。 对于安全生产这块,H社区实行的是党政同责,一岗双责制。 H社区的书记说道:
BACON P E, FRENEY J R. 1989. Nitrogen loss from different tillage systems and the effect on cereal grain yield [J]. Fertilizer Research 20: 59-66.
BENNEMA J, JONGERIUS A, LEMOS R C. 1970. Micromorphology of some oxic and argillic horizons in south Brazil in relation to weathering sequences [J]. Geoderma, 4: 333-355.
BOWDEN W B. 1986. Gaseous nitrogen emissions from undisturbed terrestrial ecosystems: an assessment of their impacts on local and global nitrogen budgets [J]. Biogeochemistry, 2: 249-279.
BOYER E W, ALEXANDER R B, PARTON W J, et al. 2006. Modeling denitrification in terrestrial and aquatic ecosystems at regional scales [J]. Ecological Applications, 16: 2123-2142.
BURESH R J, AUSTIN E R. 1988. Direct measurement of dinitrogen and nitrous oxide flux in flooded rice fields [J]. Soil Science Society of America Journal, 52: 681-688.
BURESH R J, DEDATTA S K. 1990. Denitrification losses from puddled rice soils in the tropics [J]. Biology and Fertility of Soils, 9: 1-13.
Chapuis-Lardy L, Wrage N, Metay A, et al. 2007. Soils, a sink for N2O? A review [J]. Global Change Biology, 13: 1-17.
DAVIDSON E A, CHOROVER J, DAIL D B. 2003. A mechanism ofabiotic immobilization of nitrate in forest ecosystems: The ferrous wheel hypothesis [J]. Global Change Biology, 9: 228-236.
DAVIDSON E A, CHOROVER J, DAIL D B. 2008. Iron interference in the quantification of nitrate in soil extracts and its effect on hypothesized abiotic immobilization of nitrate [J]. Biogeochemistry, 90: 65-73.
DAVIDSON E A, KINGERLEE W. 1997. A global inventory of nitric oxide emissions from soils [J]. Nutrient Cycling in Agroecosystems, 48: 37-50.
DRURY C F, OLOYA T O, MCKENNEY D J, et al. 1998. Long-term effects of fertilization and rotation on denitrification and soil carbon [J]. Soil Science Society of America Journal, 62: 1572-1579.
FELLOWS C S, HUNTER H M, ECCLESTON C E A, et al. 2011. Denitrification potential of intermittently saturated floodplain soils from a subtropical perennial stream and an ephemeral tributary [J]. Soil Biology and Biochemistry, 43: 324-332.
FIRESTONE M, DAVIDSON E. 1989. Microbiological basis of NO and N2O production and consumption in soil[C]// Andreae M, SCHIMEL D. Exchange of Trace Gases between Terrestrial Ecosystems and the Atmosphere. Chichester, England: Wiley: 7-21.
GALLOWAY J N, DENTENER D G, CAPONE E W, et al. 2004. Nitrogen cycles: past, present and future [J]. Biogeochemistry, 70: 153-226.
GALLOWAY J N, LEVYLL H, KASIBHATLA P S. 1994. Year 2020: Consequences of population growth and development on the deposition of oxidized nitrogen [J]. Ambio, 23: 120-123.
GALLOWAY J N, TOWNSEND A R, ERISMAN J W, et al. 2008. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions [J]. Science, 320: 889-892.
GOODROAD L L, KEENEY D R. 1984. Nitrous oxide emissions from soils during thawing [J]. Canadian Journal of Soil Science, 64: 187-194.
GRANLI T, BOCKMAN O C. 1994. Processes that from N2O in soils, nitrogen oxide from agriculture [J]. Norway Journal of Agricultural Science, 12: 18-22.
GRIFFITHS R P, CALDWELL B A, SOLLINS P. 1993. Effects of vegetation regime on denitrification potential in two tropical volcanic soils [J]. Biology and Fertility of Soils, 16: 157-162.
GROFFMAN P M, TIEDJE J M. 1989. Denitrification in north temperate forest soils: Relationships between denitrification and environmental factors at the landscape scale [J]. Soil Biology and Biochemistry, 21: 621-626.
GROFFMAN P M. 1995. A conceptual assessment of the importance of denitrification as a source of soil nitrogen loss in tropical agro-ecosystems [J]. Fertilizer Research, 42: 139-148.
HALL S J, MATSON P A. 1999. Nitrogen oxide emissions after nitrogen additions in tropical forests [J]. Nature, 400: 152-155.
HEINEN M. 2006. Simplified denitrification models. Overview and properties [J]. Geoderma, 133: 444-463.
HOFSTRA N, BOUWMAN A F. 2005. Denitrification in agricultural soils: summarizing published data and estimating global annual rates [J]. Nutrient Cycling in Agroecosystems, 72: 267-278.
HOULTON B Z, SIGMAN D M, HEDIN O. 2006. Isotopic evidence for large gaseous nitrogen losses from tropical rainforests [J]. Proceedings of the National Academy of Sciences of the USA, 103: 8745-8750.
HUANG Z, XU Z, BLUMFIELD T J, et al. 2008. Soil nitrogen mineralization and fate of (15NH4)2SO4in field-incubated soil in a hardwood plantation of subtropical Australia: the effect of mulching [J]. Journal of Soils and Sediments, 8: 389-397.
HUYGENS D, RÜTTING T, BOECKX P, et al. 2007. Soil nitrogen conservation mechanisms in a pristine south Chilean Nothofagus forest ecosystem [J]. Soil Biology and Biochemistry, 39: 2448-2458.
Islam N. 1992. Denitrification under Different Tillage and Cropping Systems of Eastern Australia [D]. Queensland, Australia: Griffith University.
JIA J, LI Z, LIU M, et al. 2010. Effects of glucose addition on N transformations in paddy soils with a gradient of organic C content in subtropical China [J]. Agricultural Sciences in China, 9: 1309-1316.
KELLER M, GOREAU T J, WOFSY S C, et al. 1983. Production of nitrous oxide and consumption of methane by forest soils [J]. Geophysical Research Letters, 10: 1156-1159.
KELLER M, KAPLAN W A, WOFSY S C. 1986. Emissions of N2O, CH4and CO2from tropical forest soils [J]. Journal of Geophysical Research, 91: 11791-11802.
KLUBER H D, CONRAD R. 1998. Inhibitory effects of nitrate, nitrite, NO and N2O on methanogenesis by Methanosarcina barkeri and Methanobacterium bryantii [J]. FEMS Microbiology Ecology, 25: 331-339.
KUMARASWAMY R, SJOLLEMA K, KUENEN G, et al. 2006. Nitrate-dependent [Fe (II) EDTA]2−oxidation by Paracoccus ferrooxidans sp. nov., isolated from a denitrifying bioreactor [J]. Systematic and Applied Microbiology, 29: 276-286.
LANDAU C W. 1994. Methane emissions from Louisiana rice fields amended with nitrogen fertilizers [J]. Soil Biology and Biochemistry, 26: 353-359.
LASHOF D A, AHUJA D R. 1990. Relative contribution of greenhouse emissions to global warming [J]. Nature, 344: 529-531.
LAUGHLIN R J, STEVENS R J. 2002. Evidence for fungal dominance of denitrification and codenitrification in grassland soil [J]. Soil Science Society of America Journal, 66: 1540-1548.
LI D, WANG X, SHENG G, et al. 2008. Soil nitric oxide emissions after nitrogen and phosphorus additions in two subtropical humid forests [J]. Journal of Geophysical Research, 113 D16301. doi: 10.1029/2007JD009375.
LI Y, YU S, STRONG J, et al. 2012. Are the biogeochemical cycles of carbon, nitrogen, sulfur, and phosphorus driven by the “FeIII–FeII redox wheel” in dynamic redox environments [J]? Journal of Soils and Sediments, 12: 683-693.
LU Y, WASSMANN R, NEUE H U, et al. 2005. Dissolved organic carbon and methane emissions from a rice paddy fertilized with ammonium and nitrate [J]. Journal of Environmental Quality, 29: 1733-1740.
MA H B, AELION C M. 2005. Ammonium production during microbial nitrate removal in soil microcosms from a developing marsh estuary [J]. Soil Biology and Biochemistry, 37: 1869-1878.
MAAG M, VINTHER F P. 1999. Effect of temperature and water on gaseous emissions from soils treated with animal slurry [J]. Soil Science Society of America Journal, 63: 858-865.
MAHMOOD T, ALI R, ASLAM Z. 2001. Fate of the (15)N-labelled urea applied to irrigated wheat grown after cotton harvest under semiarid subtropical conditions [J]. Pakistan Journal of Botany, 33: 389-392.
MAHMOOD T, ALI R, MALIK K A, et al. 2005. Seasonal pattern of denitrification under an irrigated wheat-maize cropping system fertilized with urea and farmyard manure in different combinations [J]. Biology and Fertility of Soils, 42: 1-9.
MAHMOOD T, ALI R, SAJJAD M I, et al. 2000. Denitrification and total fertilizer-N losses from an irrigated cotton field [J]. Biology and Fertility of Soils, 31: 270-278.
MANCONI I, MAAS P, LENS P N L. 2006. Effect of sulfur compounds on biological reduction of nitric oxide in aqueous Fe(II)EDTA2-solutions [J]. Nitric Oxide–Biology and Chemistry, 15: 40-49.
MARTINELLI L A, PICCOLO M C, TOWNSEND A R, et al. 1999. Nitrogen stable isotopic composition of leaves and soil: tropical versus temperate forests [J]. Biogeochemistry, 46: 45-65.
MATHIEU O, LÉVÊQUE J, HÉNAULT C, et al. 2006. Emissions and spatial variability of N2O, N2and nitrous oxide mole fraction at the field scale, revealed with15N isotopic techniques [J]. Soil Biology and Biochemistry, 38: 941-951.
MATSON P A, VITOUSEK P M. 1990. Ecosystem approach for the development of a global nitrous oxide budget [J]. Bioscience, 40: 667-672.
MCLAIN J E T, MARTENS D A. 2006. N2O production by heterotrophic N transformations in a semiarid soil [J]. Applied Soil Ecology, 32: 253-263.
MÜLLER C, STEVENS R J, LAUGHLIN R J, et al. 2004. Microbial processes and the site of N2O production in a temperate grassland soil [J]. Soil Biology and Biochemistry, 36: 453-461.
MURRAY R E, KNOWLES R. 2004. Trace amounts of O2affect NO andN2O production during denitrifying enzyme activity (DEA) assays [J]. Soil Biology and Biochemistry, 36: 513-517.
MYROLD D D. 1988. Denitrification in ryegrass and winter wheat cropping systems of western Oregon [J]. Soil Science Society of America Journal, 52: 412-415.
NIEDER R, SCHOLLMAYER G, RICHTER J. 1989. Denitrification in the rooting zone of cropped soils with regard to methodology and climate: A review [J]. Biology and Fertility of Soils, 8: 219-226.
PAGE K L, DALAL R C, MENZIES N M. 2003. Nitrate ammonification and its relationship to the accumulation of ammonium in a Vertisol subsoil [J]. Australian Journal of Soil Research, 41: 687-697.
PAN K, XU Z, BLUMFIELD T J, et al. 2009. Application of (15NH4)2SO4to study N dynamics in hoop pine plantation and adjacent native forest of subtropical Australia: the effects of injection depth and litter addition [J]. Journal of Soils and Sediments, 9: 515-525.
PARKIN T B. 1990. Characterizing the variability of soil denitrification [C]//Revsbech N P, Sorensen J. Denitrification in Soil and Sediment. New York: Plenum Press: 213-228.
PARSONS W F J, MITRE M E, KELLER M, et al. 1993. Nitrate limitation of N2O production and denitrification from tropical pasture and rain forest soils [J]. Biogeochemistry, 22: 179-193.
PAYNE W J. 1981. Denitrification[M]. New York: John Wiley&Sons.
PENDALL E, BRIDGHAM S, HANSON P J, et al. 2004. Below-ground process responses to elevated CO2and temperature: a discussion of observations, measurement methods, and models [J]. New Phytologist, 162: 311-322.
PETT-RIDGE J, SILVER W L, FIRESTONE M K. 2006. Redox fluctuations frame microbial community impacts on N-cycling rates in a humid tropical forest soil [J]. Biogeochemistry, 81: 95-110.
PHILIPS S, RABAEY K, VERSTRAETE W. 2003. Impact of iron salts on activated sludge and interaction with nitrite or nitrate [J]. Bioresource Technology, 88: 229-239.
PU G X, SAFFIGNA P G, XU Z H. 2001. Denitrification, leaching and immobilization of15N-labelled nitrate in winter under windrowed harvesting residues in hoop pine plantations of 1–3 years old in subtropical Australia [J]. Forest Ecology and Management, 152: 183-194.
PU G X, SAFFIGNA P G, XU Z H. 2005. Transformations of nitrate N-15 under different forest harvest residue regimes in a hoop pine plantation in Australia [J]. Journal of Tropical Forest Science, 17: 372-385.
PU G X, XU Z H, SAFFIGNA P G. 2002. Fate of15N-labelled nitrate in a wet summer under different residue management regimes in young hoop pine plantations [J]. Forest Ecology and Management, 170: 285-298.
PU G X. 1996. Denitrification in Cereal and Sugarcane Soils of Australia [D]. Queensland, Australia: Griffith University.
QAFOKU N P, VAN RANST E, NOBLE A, et al. 2004. Variable charge soils: their mineralogy, chemistry and management [J]. Advances in Agronomy, 84: 159-215.
QIAN C, CAI Z C. 2007. Leaching of nitrogen from subtropical soils as affected by nitrification potential and base cations [J]. Plant and Soil, 300: 197-205.
RENAULT P, STENGEL P. 1994. Modeling oxygen diffusion in aggregated soils. I. Anaerobiosis inside the aggregates [J]. Soil Science Society of America Journal, 58: 1017-1023.
ROBERTSON G P, TIEDJE J M. 1988. Deforestation alters denitrification in a lowland tropical rain forest [J]. Nature, 336: 756-759.
RÜTTING T, CLOUGH T J, MÜLLER C, et al. 2010. Ten years of elevated atmospheric carbon dioxide alters soil nitrogen transformations in a sheep-grazed pasture [J]. Global Change Biology, 16: 2530-2542.
SANCHEZ P A, PALM C A, SZOTT L T, et al. 1989. Organic input management in tropical agroecosystems[C]// Coleman D C, OADES J M, UEHARA G. Dynamics of Soil Organic Matter in Tropical Ecosystems, NifTAL Project. Honolulu, HI: University of Hawaii Press: 125-152.
SCHMIDT J, SEILER W, CONRAD R. 1988. Emission of nitrous oxide from temperate forest soils into the atmosphere [J]. Journal of Atmospheric Chemistry, 6: 95-115.
SCHOLES M C, SANCHEZ P A. 1990. Low soil nitrogen mineralization rates in a humid tropical pasture [J]. Tropical Ecology, 31: 12-15.
SHOUN H, KIM D, UCHIYAMA H, et al. 1992. Denitrification by fungi [J]. FEMS Microbiology Letters, 94: 277-282.
ŠIMEK M, COOPER J E, PICEK T, et al. 2000. Denitrification in arable soils in relation to their physico-chemical properties and fertilization practice [J]. Soil Biology and Biochemistry, 32: 101-110.
ŠIMEK M, COOPER J E. 2002. The influence of soil pH on denitrification: progress towards the understanding of this interaction over the last 50 years [J]. European Journal of Soil Science, 53: 345-354.
SØRENSEN J, THORLING L. 1991. Stimulation by lepidocrocite (7-FeOOH) of Fe (II)- dependent nitrite reduction [J]. Geochimica et Cosmochimica Acta, 55: 1289-1294.
STARK J M, HART S C. 1997. High rates of nitrification and nitrate turnover in undisturbed coniferous forests [J]. Nature, 385: 61-64.
STEHFEST E, BOUWMAN L. 2006. N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions [J]. Nutrient Cycling in Agroecosystems, 74: 207-228.
TIEDJE J M. 1988. Ecology of denitrification and dissimilatory nitrate reduction to ammonia[C]//Zehnder A J B. Biology of Anaerobic Microorganisms. New York: John Wiley & Sons: 179-244.
TOBIAS C R, ANDERSON I C, CANUEL E A. 2001. Nitrogen cycling through a fringing marsh-aquifer ecotone [J]. Marine Ecology Progress Series, 210: 25-39.
VAN GROENIGEN K, OSENBERG C W, HUNGATE B A. 2011. Increased soil emissions of potent greenhouse gases under increased atmospheric CO2[J]. Nature, 475: 215-216.
VELDKAMP E, KELLER M, NUÑEZ M. 1998. Effects of pasture management on N2O and NO emissions from soils in the humid tropics of Costa Rica [J]. Global Biogeochemistry Cycling, 12: 71-79. VITOUSEK P, SANFORD R. 1986. Nutrient cycling in moist tropical forest [J]. Annual Review of Ecology and Systematics, 17: 137-167.
VITOUSEK P M, GOSZ J R, GRIER C C, et al. 1979. Nitrate losses from disturbed ecosystems [J]. Science, 204: 469-474.
VITOUSEK P M, MATSON P A M. 1988. Nitrogen transformations in a range of tropical forest soils [J]. Soil Biology and Biochemistry, 20: 361-367.
VITOUSEK P M, REINERS W A. 1975. Ecosystem succession and nutrient retention: a hypothesis [J]. Bioscience, 25: 376-381.
WANG L, CAI Z. 2008. Nitrous oxide production at different soil moisture contents in an arable soil in China [J]. Soil Science and Plant Nutrition, 54: 786-793.
WEIER K L, MACRAE I C, MYERS R J K. 1993. Denitrification in a clay soil under pasture and annual crop: estimation of potential losses using intact soil cores [J]. Soil Biology and Biochemistry, 25: 991-997.
WEIER K L, MACRAE I C, MYERS R J K. 1991. Seasonal variation in denitrification in a clay soil under a cultivated crop and a permanent pasture [J]. Soil Biology and Biochemistry, 23: 629-635.
WEIER K L. 1994. Nitrogen use and losses in agriculture in subtropical Australia [J]. Fertilizer Research, 39: 245-257.
WIJLER J, DELWICHE C C. 1954. Investigations on the denitrifying process in soil [J]. Plant and Soil, 5: 155-169.
WOLF I, BRUMME R. 2002. Contribution of nitrification and denitrification sources for seasonal N2O emissions in an acid German forest soil [J]. Soil Biology and Biochemistry, 34: 741-744.
WOLF I, BRUMME R. 2003. Dinitrogen and nitrous oxide formation in beech forest floor and mineral soils [J]. Soil Science Society of America Journal, 67: 1862-1868.
WU Y, MA B, ZHOU L, et al. 2009. Changes in the soil microbial community structure with latitude in eastern China, based on phospholipid fatty acid analysis [J]. Applied Soil Ecology, 43:234-240.
WULLSCHLEGER S D, TSCHAPLINSKI T J, NORBY R J. 2002. Plant water relations at elevated CO2–implications for water-limited environments [J]. Plant Cell and Environment, 25: 319-331.
XIONG Z Q, KHALIL M A K, XING G, et al. 2009. Isotopic signatures andconcentration profiles of nitrous oxide in a rice-based ecosystem during the drained crop-growing season [J]. Journal of Geophysical Research, 114, G02012. doi:10.1029/2008JG000827.
XU Y B, CAI Z C, LEI B K. 2008. Effect of NO3--N on CH4emission during denitrification in subtropical soils [J]. Journal of Environmental Sciences-China, 29: 3513-3519.
XU Y B, CAI Z C, XU Z H. 2012. Production and consumption of N2O during denitrification in subtropical soils of China [J]. Journal of Soils and Sediments, 12: 1339-1349.
XU Y B, CAI Z C. 2007. Denitrification characteristics of subtropical soils in China affected by soil parent material and land use [J]. European Journal of Soil Science, 58: 1293-1303.
XU Z H, WARD S, CHEN C R, et al. 2008. Soil carbon and nutrient pools, microbial properties and gross nitrogen transformations in adjacent natural forest and hoop pine plantations of subtropical Australia [J]. Journal of Soils and Sediments, 8: 99-105.
ZAK D R, PREGITZER K S, KING J S, et al. 2000. Elevated atmospheric CO2, fine roots and the response of soil microorganisms: a review and hypothesis [J]. New Phytologist, 147: 201-222.
ZECH W, SENESI N, GUGGENBERGER G, et al. 1997. Factors controlling humification and mineralization of soil organic matter in the tropics [J]. Geoderma, 79: 117-161.
ZHANG J, CAI Z, CHENG Y, et al. 2009. Denitrification and total nitrogen gas production from forest soils of Eastern China [J]. Soil Biology and Biochemistry, 41:2551-2557.
ZHANG J, CAI Z, CHENG Y, et al. 2010. Nitrate immobilization in anaerobic forest soils along a North–South transect in east China [J]. Soil Science Society of America Journal, 74: 1193-1200.
ZHANG J, CAI Z, ZHU T. 2011a. N2O production pathways in the subtropical acid forest soils in China [J]. Environmental Research, 111: 643-649.
ZHANG J, MÜLLER C, ZHU T, et al. 2011b. Heterotrophic nitrification is the predominant NO3−production mechanism in coniferous but not broad-leaf acid forest soil in subtropical China [J]. Biology and Fertility of Soils, 47: 533-542.
ZHANG J, ZHU T, CAI Z, et al. 2011c. Nitrogen cycling in forest soils across climate gradients in Eastern China [J]. Plant and Soil, 342: 419-432.
ZHAO W, CAI Z C, XU Z H. 2007. Does ammonium-based N addition influence nitrification and acidification in humid subtropical soils of China[J]? Plant and Soil, 297: 213-221.
ZHU T, ZHANG J, CAI Z. 2011. The contribution of nitrogen transformation processes to total N2O emissions from soils used for intensive vegetable cultivation [J]. Plant and Soil, 343: 313-327.
丁昌璞. 2008. 中国自然土壤、旱作土壤、水稻土的氧化还原状况和特点[J].土壤学报, 45: 66-75.
史央, 戴传超, 陆玲. 2002. 中国科学院红壤生态站不同土壤中的微生物类群调查[J]. 南京师范大学学报, 25: 32-36.
徐仁扣, 李九玉, 姜军. 2014. 可变电荷土壤中特殊化学现象及其微观机制的研究进展[J].土壤学报, 51(2): 1-9.
Progresses in Research on Denitrification in Tropical and Subtropical Soils of Terrestrial Ecosystems
XU Yongbo1, XU Zhihong2, CAI Zucong3
1. College of Tobacco Science, Yunnan Agricultural University, Kunming 650201, China; 2. Environmental Futures Centre and School of Biomolecular and Physical Sciences, Griffith University, QLD 4111, Australia; 3. School of Geography Sciences, Nanjing Normal University, Nanjing 210097, China
Denitrification has been extensively studied in soils from temperate zones in industrialized countries. However, few studies quantifying denitrification rates in soils from tropical and subtropical zones have been reported. Denitrification mechanisms in tropical/subtropical soils may be different from other soils, due to their unique soil characteristics. The identification of denitrification in the area is crucial to understand the role of denitrification in the global nitrogen (N) cycle in terrestrial ecosystems, and in the interaction between global environmental changes and ecosystem responses. We review the existing literature on microbially-mediated denitrification in tropical/subtropical soils, attempting to provide a better understanding about and new research directions for denitrification in these regions. Tropical and subtropical soils might be characterized by generally lower denitrification capacity than temperate soils, with greater variability due to land use and management practices varying temporally and spatially. Factors that influence soil water content and the nature and rate of carbon (C) and N turnover are the landscape and field scale controls of denitrification. High redox potential in the field, which is mainly attributed to soil oxide enrichment, may be at least one critical edaphic variable responsible for slow denitrification rates in the humid tropical and subtropical soils. However, soil pH is not responsible for these slow denitrification rates. Organic C mineralization is more important than total N content and C/N in determining denitrification capacity in humid subtropical soils. There is increasing evidence that the ecological consequence of denitrification in tropical and subtropical soils may be different from that of temperate zones. Contribution of denitrification in tropical and subtropical regions to the global climate warming should be considered comprehensively since it could affect other greenhouse gases, such as methane (CH4) and carbon dioxide (CO2), and N deposition. Tropical/subtropical soils have developed several N conservation strategies to prevent N losses via denitrification from the ecosystems. However, the mechanisms involved in the biogeochemical regulation of tropical and subtropical ecosystem responses to environmental changes are largely unknown. These works are important for accurately modeling denitrification and all other simultaneously operating N transformations.
denitrification; environmental implication; nitrate leaching; nitrogen retention strategies; nitrous oxide
S15
A
1674-5906(2014)09-1557-10
续勇波,XU Zhihong,蔡祖聪. 热带亚热带土壤氮素反硝化研究进展[J]. 生态环境学报, 2014, 23(9): 1557-1566.
XU Yongbo, XU Zhihong, CAI Zucong. Progresses in Research on Denitrification in Tropical and Subtropical Soils of Terrestrial Ecosystems [J]. Ecology and Environmental Sciences, 2014, 23(9): 1557-1566.
国家自然科学基金项目(31101605;31260503);云南省自然科学基金项目(2010ZC083)
续勇波(1974年生),女,副教授,博士,主要从事养分资源利用与环境效应研究。*通信作者
2014-06-16
