下关生沱茶的成分分析以及对SD大鼠的盐酸/乙醇诱导胃损伤预防效果
2014-01-18王睿,赵欣*
王 睿,赵 欣*
(重庆第二师范学院生物与化学工程系,重庆 400067)
下关生沱茶的成分分析以及对SD大鼠的盐酸/乙醇诱导胃损伤预防效果
王 睿,赵 欣*
(重庆第二师范学院生物与化学工程系,重庆 400067)
为弄清下关生沱茶(Xiaguan raw Tuocha,XRT)的成分和其体内胃损伤预防效果,本实验利用LC-MS和细胞因子分析,同时还测定了胃液量和胃液的pH值。结果表明:XRT具有12种成分,这些成分使XRT具有胃损伤预防效果。高质量浓度的XRT相比低质量浓度的XRT,细胞因子IL-6 和TNF-α下降。1 000 mg/kg的XRT处理时小鼠显示出和正常小鼠接近的低胃液量(2.0 mL)和高的pH值(3.1)。由此可知,下关生沱茶包含很多功能性成分并且表现出强有力的胃损伤预防效果。
沱茶;胃损伤;细胞因子;大鼠
Xiaguan raw Tuocha (XRT), whose shape resembles a bird’s nest, is a compressed Pu-erh tea. Tuocha are convex to help the tea dry out after proccessing[1]. This tea is made from the large leaves of Camellia sinensis O. kuntze var. assamica Kitamura, and is a kind of Pu-erh tea[2]. Drinking Pu-erh tea for a long period of time can help maintain both mental and physical health. This tea is believed to help reduce high blood pressure and cholesterol levels and to play an important role in preventing heart disease and cancer[3]. Various phytochemicals, especially glycoside polyphenols can help improve health, including lowering the risk of cancer. The known bioactive components of Pu-erh tea are epicatechin and epigallocatechin[4].
Gastric injury is an injury to the stomach. It may be blunt or penetrating and may cause damage to the stomach organs. Ethanol promotes the rapid formation of injuries in the stomach, which occurs mainly due to inflammatory reaction[5]. Ethanol-induced gastric injury is characterized by epithelial cellular loss, mucosal edema, and subepithelial hemorrhage[6]. Cytokines, including interleukin IL-6and tumor necrosis factor TNF-α, are small proteins that are produced and released from a number of cells under physiological and pathological conditions[7]. IL-6 is increasingly recognized as an almost ubiquitous participant in numerous types of inflammatory processes[8].
TNF-α is a macrophage-derived cytokine with chemotactic potency, which has been implicated in the acute phase reaction under various inflammatory conditions[9]. In the current study, active compounds in Xiaguan raw Tuocha are identified by liquid chromatography-mass spectrometry (LC-MS) analysis, and their ability to prevent HCl/ethanol induced gastric injury is also examined.
1 Materials and Methods
1.1 Materials, reagents and instruments
Xiaguan raw Tuocha (XRT) (Xiaguan Tuocha Co. Ltd.) was purchased in Yunnan, China.
Dimethylsulfoxide (DMSO) was obtained from Amresco Co. (Soion, OH, USA); cytokines IL-6 and TNF-α were obtained from BioLegend Co. (San Diego, CA, USA).
Rotary evaporator (Eywla, N-1100, Tokyo, Japan); mass spectrometer and HPLC (high-performance liquid chromatography) System (Thermo Electron Co., Waltham, MA, USA); pH meter (ServenEasy pH, Mettler-Toledo, Schwerzenbach, Switzerland); microplate reader (iMark, Bio-Rad, Hercules, CA, USA ); digital camera (Canon D550, Tokyo, Japan).
1.2 Preparation of tea extract
The XRT was stored at -80 ℃and freeze-dried to produce a powder. A twenty-fold volume of methanol was added to the powdered sample and extracted twice by stirring overnight. The methanol extract was evaporated by a rotary evaporator, concentrated, and then dissolved in DMSO to adjust to the stock concentration (20 g/100 mL).
1.3 LC-MS analysis
The tea extracts were dissolved in DMSO to produce a final concentration of 10 mg/mL, and then diluted with 50% methanol to make a final concentration of 2 mg/mL. Five microliters of the diluted sample was analyzed by liquid chromatography followed by tandem mass spectrometry. LC-MS was performed using a Finnigan LCQ Advantage MAX ion trap mass spectrometer equipped with an electrospray ionization (ESI) source. Separation by HPLC was performed with a Finnigan Surveyor Modular HPLC System using an Xterra MS C18column ( 2.1 mm×150 mm, 5 øm). Mobile phase A was water, and mobile phase B was acetonitrile; both contained 0.1% formic acid. Gradient elution at a flow rate of 0.2 mL/min was carried out as follow: 0–5 min with 0–40% B (linear gradient), 5–20 min with 40%–80% B (linear gradient), 20–25 min with 80%–100% B (linear gradient), and 25–30 min with 100% B (isocratic). Full-scan mass spectra were obtained in the positive and negative ion modes at a range of m/z 100–1 000. To identify compound structures, data obtained were compared to that from the spectral library search[10].
1.4 Gastric injury experiment
Male Sprague-Dawley (SD) rats (n=36, 7 week old) were purchased from Experimental Animal Center of Chongqing Medical University (Chongqing, China). The rats were maintained in a temperature-controlled facility (temperature, (25±2) ℃; relative humidity, (50±5)%) with a 12 h light/dark cycle and free access to a standard rat chow diet and water. The experimental design was as follows: the normal and control groups received 14-day repeated oral administration of distilled water and a single dose of olive oil (2 mL/kg); the sample groups received 14-day repeated oral administration of 250, 500 or 1 000 mg/kg tea extract. Then, the control and sample group rats were administered 1 mL HCl/ethanol (60% in 150 mmol/L HCl) by oral gavage and were then sacrificed 1 h later under deep ether anesthesia. The stomachs were removed, inflated by injecting 10 mL 1% formalin for 10 min to fix the tissue walls and opened along the greater curvature. The area (mm2) of hemorrhagic lesions developed in the stomach was measured using a digital camera with a square grid and the images were analyzed by ImageJ software[11].
Inhibitory rate/%= (Gastric injury dimensions of control group rats-Gastric injury dimensions of sample treated group rats) / Gastric injury dimensions of control group rats×100
1.5 Analysis of cytokines in serum by enzyme-linked immunosorbent assay (ELISA)
For the serum cytokine assay, blood from the inferior vena cava was collected in a tube and centrifuged (1 370×g for 10 min at 4 ℃). The serum was aspirated and assayed as follows: concentrations of the cytokines IL-6 and TNF-α in serum were measured by ELISA according to the kit manufacturer’s instructions. Briefly, after the biotinylated antibody reagent was added to 96-well plates, supernatants of homogenized serum were incubated at 37 ℃ in CO2for 2 h. After washing with phosphate-buffered saline (PBS),horseradish peroxidase (HRP)-conjugated streptavidin peroxidase solution was added and the plate was incubated for 30 min at room temperature. The absorbance was then measured at 450 nm using a microplate reader[12].
1.6 Statistical analysis
Data from the experiments for each specimen was subjected to an analysis of variance (ANOVA). Results are presented as the±s. Differences between mean values for the various groups were assessed by a one-way ANOVA and Duncan’s multiple range tests. Differences were considered significant when P<0.05. The SAS v9.1 statistical software package was used to perform these analyses.
2 Results and Analysis
2.1 Analysis of Xiaguan raw Tuocha composition
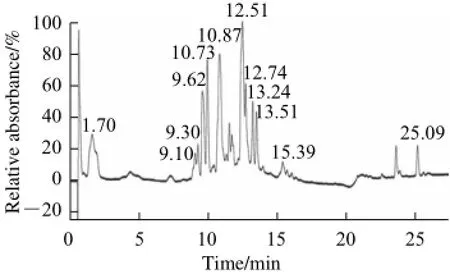
Fig.1 HPLC-PDA chromatogram (200–600 nm) of Xiaguan raw Tuocha
An initial LC-MS analysis of the methanol extract of XRT showed that there were 12 known peaks. These peaks (Fig. 1) were for gallic acid (1.70 min, m/z 169), epigallocatechin (9.10 min, m/z 305), catechin (9.30 min, m/z 289), caffeine (9.62 min, m/z 195), epicatechin (10.73 min, m/z 289), epigallocatechin gallate (10.87 min, m/z457), epicatechin gallate (12.51 min, m/z 441), quercetin-3-galactoside (12.74 min, m/z 463), kaempferol-3-rutinoside (13.24 min, m/z 593), kaempferol-3-glucoside (13.51 min, m/z 447), quercetin (15.39 min, m/z 301), and lutein (25.09 min, m/z 568). Tea has been reported to exert antiinflammatory effects, which is associated with its many functional compounds, such as polypenols, catechines, amino acids and vitamins[13-15]. Gallic acid was found to possess anti-inflammatory activity towards acute food pad swelling in animal experiments[16]. The antioxidant properties, immunomodulator proteins and inhibition of mitochondrial apoptosis may contribute to the gastroprotective activity of gallic acid and its novel derivatives[17]. Naturally occurring flavonoids such as kaempferol and quercetin also showed strong anti-inflammatory activity[18]. Kaempferol could decrease the damage of gastric ulcer[19]and quercetin could also decrease the gastric mucosal injury[20]. There was a study found that lutein has anti-inflammatory effects by inhibiting the NF-κB dependent signaling pathway and the subsequent production of proinflammatory mediators[21], and its protective effect of ethanol induced gastric ulcer was report[22].
2.2 Cytokine levels in serum
The IL-6 level of normal rats was (54.3±3.7) pg/mL; however, that of the control rats was significantly increased to (293.2±11.3) pg/mL following the induction of gastric injury. The levels of IL-6 in rats fed with 1 000 mg/kg XRT were lower than 250 mg/kg and 500 mg/kg XRT-treated rats (Table 1). The TNF-α levels in normal, control and 250, 500, 1 000 mg/kg XRT-treated rats showed the similar trend as IL-6 levels. The serum IL-6 and TNF-α levels in the rats of the XRT-treated groups were significantly lower than those in the control group. It is reported that the serum levels of cytokines, including IL-6 and TNF-α, in patients with inflammatory diseases are higher than those in healthy individuals[23]. Thus, lower levels of IL-6 and TNF-α as indicative of anti-inflammatory effects and XRT demonstrate a good protective effect of XRT against gastric damage. Hepatocytes bear a variety of cytokine receptors[24]. IL-6 is secreted by T cells and macrophages to stimulate the immune response, particularly in the process of tissue damage leading to inflammation[25]. TNF-α is a cytokine involved in systemic inflammation and is a member of a group of cytokines that stimulate the acute phase reaction[26]. The inflammatory cytokines IL-6 and TNF-α play pathogenic roles in diseases of the stomach[27]. Although systemic IL-6 levels are elevated following traumatic hemorrhage, impaired hepatocellular function and gastric injury[28]. TNF-α is also a key mediator in a number of experimental models of stomach injury[29].

Table 1 Effect of Xiaguan raw Tuocha on the serum IL-6 and TNF-α levels in rats with HCl/ethanol-induced gastric injury
2.3 Gastric secretion volume and pH of gastric juice
Materials that induce gastric injury cause increased gastric secretion volume and acid output with significantly decreased gastric pH, and low stomach acidity is an importantmarker of gastric injury[30]. The gastric secretion volume of the normal rats showed the lowest quantity, and the volume of the control rats increased, higher than XRT (Table 2). 1 000 mg/kg XRT was associated with the lowest gastric secretion volume and highest pH compared to the other concentrations of XRT. This could explain in part why the ability of high concentration of XRT to protect against gastric injury was greater than that of the lower concentration of XRT.
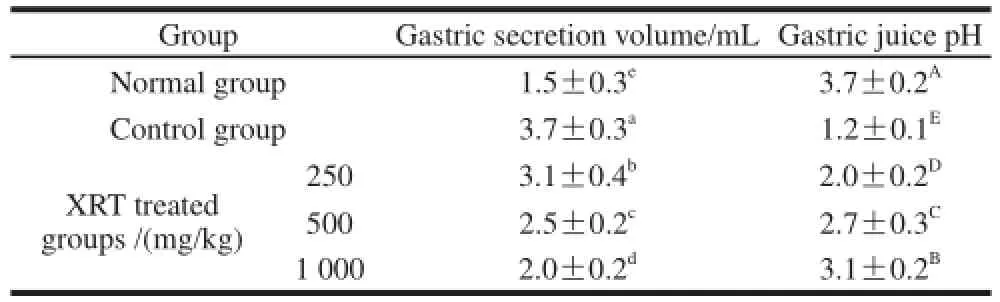
Table 2 Gastric secretion volume and gastric juice pH in rats treated with Xiaguan raw Tuocha after the induction of gastritis with HCl/ethhaannooll
2.4 Gastric injury levels
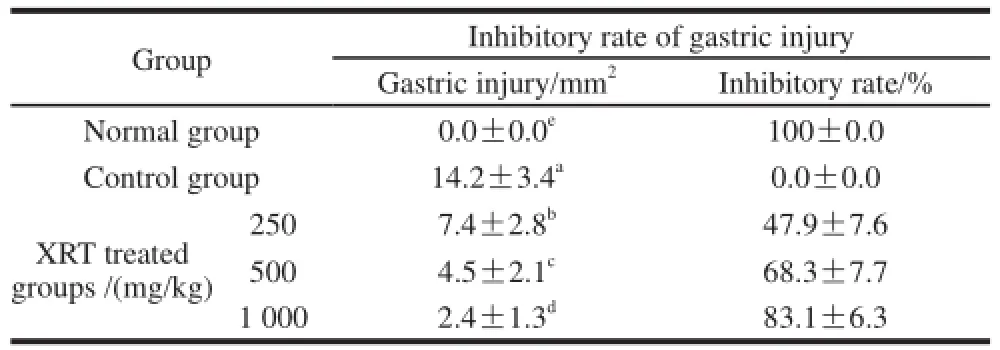
Table 3 Prevention of HCl/ethanol-induced gastric injury by treatment with Xiaguan raw Tuocha
The administration of XRT to rats prior to the induction of gastritis led to reduced gastric injury. The rats of the control group demonstrated a gastric injury area of (14.2±3.4) mm2. Treatment with 250 mg/kg and 500 mg/kg XRT resulted in the high gastric injury inhibition rates, while 1 000 mg/kg XRT demonstrated the best gastritis preventive effect (Table 3 and Fig.2). The results suggest that XRT has a strong preventive effect on gastric injury.

Fig.2 Stomach of rats treated with Xiaguan raw Tuocha after the induction of gastritis with HCl/ethanol
3 Conclusions
The current study demonstrated that Xiaguan raw Tuocha was an effective agent for the prevention of HCl/ ethanol-induced gastric injury in SD rats. This effect was attributed to the bioactive compounds in Xiaguan raw Tuocha, such as gallic acid, epigallocatechin, catechin, caffeine, epicatechin, epigallocatechin gallate, epicatechin gallate, quercetin-3-galactoside, kaempferol-3-rutinoside, kaempferol-3-glucoside, quercetin, and lutein. Our results indicated that the protective effects of Xiaguan raw Tuocha may work through the decreased levels of proinflammatory cytokines, including IL-6 and TNF-α. Analysis of the stomachs of various rat treatment groups indicated that Xiaguan raw Tuocha represents a potentially useful agent for the treatment or prevention of chemical-induced gastric injury in vivo.
ces:
[1] XU Lu, DENG Dehua, CAI Chenbo. Predicting the age and type of tuocha tea by fourier transform infrared spectroscopy and chemometric data analysis[J]. Journal of Agricultural and Food Chemistry, 2011, 59(19): 10461-10469.
[2] PENG Cuizhen, LIU Chuan, LI Wanyi. Study on inoculated fermentation of Yunnan Puerh tea[J]. Journal of Yunnan University, 2008, 30: 351-355.
[3] DUH P D, YEN G C, YEN W J, et al. Effects of Pu-erh tea on oxidative damage and nitric oxide scavenging[J]. Journal of Agricultural and Food Chemistry, 2004, 52(26): 8169-8176.
[4] ZHOU Zhihong, YANG Chongren. Chemical constituents of crude green tea, the material of Pu-er Tea in Yunnan[J]. Acta Botanica Yunnanica, 2000, 22: 343-350.
[5] SZABO S, TRIER J S, BROWN A, et al. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat[J]. Gastroenterology, 1985, 88(1): 228-236.
[6] MEDEIROS J V, GADELHA G G, LIMA S J, et al. Role of the NO/cGMP/KATP pathway in the protective effects of sildenafil against ethanol-induced gastric damage in rats[J]. British Journal of Pharmacology, 2008, 153(4): 721-727.
[7] RAMADORI G, ARMBRUST T. Cytokines in the liver[J]. European Journal of Gastroenterology and Hepatology, 2001, 13(7): 777-784.
[8] MCCURRY K R, CAMPBELL D A, Jr, SCALES W E, et al. Tumor necrosis factor, interleukin 6, and the acute phase response followinghepatic ischemia/reperfusion[J]. Journal of Surgical Research, 1993, 55(1): 49-54.
[9] MING W J, BERSANI L, MANTOVANI A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes[J]. The Journal of Immunology, 1987, 138(5): 1469-1472.
[10] LEE J S, KIM D H, LIU K H, et al. Identification of flavonoids using liquid chromatography with electrospray ionization and ion trap tandem mass spectrometry with an MS/MS library[J]. Rapid Communications in Mass Spectrometry, 2005, 19(23): 3539-3548.
[11] WANG Qiang, ZHAO Xin, QIAN Yu, et al. in vitro antioxidative activity of yellow tea and its in vivo preventive effect on gastric injury[J]. Experimental and Therapeutic Medicine, 2013, 6(2): 423-426.
[12] PARK H S, PARK J Y, YU R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6[J]. Diabetes Research and Clinical Practice, 2005, 69(1): 29-35.
[13] TIPOE G L, LEUNG T M, HUNG M W, et al. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection[J]. Cardiovasc Hematol Disord Drug Targets, 2007, 7(2): 135-144.
[14] MANDEL S, AMIT T, REZNICHENKO L, et al. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders[J]. Molecular Nutrition and Food Research, 2006, 50(2): 229-234.
[15] TROUILLAS P, CALLISTE C A, ALLAIS D P, et al. Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas[J]. Food Chemistry, 2003, 80(3): 399-407.
[16] KROES B H, van den BERG A J, QUARLES van UFFORD H C, et al. Anti-inflammatory activity of gallic acid[J]. Planta Medica, 1992, 58(6): 499-504.
[17] ABDELWAHAB S I. Protective mechanism of gallic acid and its novel derivative against ethanol-induced gastric ulcerogenesis: involvement of immunomodulation markers, Hsp70 and Bcl-2-associated X protein[J]. International Immunopharmacology, 2013, 16(2): 296-305.
[18] HÄMÄLÄINEN M, NIEMINEN R, VUORELA P, et al. Anti-Inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages[J]. Mediators of Inflammation, 2007, article ID: 45673.
[19] GOEL R K, PANDEY V B, DWIVEDI S P, et al. Antiinflammatory and antiulcer effects of kaempferol, a flavone, isolated from Rhamnus procumbens[J]. International Immunopharmacology, 1988, 26(2): 121-124.
[20] MOJZIS J, HVISCOV˘ K, GERMANOVA D, et al. Protective effect of quercetin on ischemia/reperfusion-induced gastric mucosal injury in rats[J]. Physiological Research, 2001, 50(5): 501-506.
[21] JIN X H, OHGAMI K, SHIRATORI K, et al. Inhibitory effects of lutein on endotoxin-induced uveitis in Lewis rats[J]. Investigative Ophthalmology and Visual Science, 2006, 47(6): 2562-2568.
[22] SINDHU E R, KUTTAN R. Carotenoid lutein protects rats from gastric ulcer induced by ethanol[J]. Journal of Basic and Clinical Physiology and Pharmacology, 2012, 23(1): 33-37.
[23] GRATACÓS J, COLLADO A, FILELLA X, et al. Serum cytokines (IL-6, TNF-α, IL-1β and IFN-γ) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity[J]. Rheumatology, 1994, 33(10): 927-931.
[24] FERGUSON-SMITH A C, CHEN Y F, NEWMAN M S, et al. Regional localization of the interferon-beta 2/B-cell stimulatory factor 2/hepatocyte stimulating factor gene to human chromosome 7p15-p21[J]. Genomics, 1988, 2(3): 203-208.
[25] van der POLL T, KEOGH C V, GUIRAO X, et al. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia[J]. The Journal of Infectious Diseases, 1997, 176(2): 439-444.
[26] GOSSELIN D, RIVEST S. Role of IL-1 and TNF in the brain: twenty years of progress on a Dr. Jekyll/Mr. Hyde duality of the innate immune system[J]. Brain, Behavior and Immunity, 2007, 21(3): 281-289.
[27] ABDOLLAHI H, SHAMS S, ZAHEDI M J, et al. IL-10, TNF-α and IFN-γ levels in serum and stomach mucosa of Helicobacter pylori-infected patients[J]. Iranian Journal of Allergy, Asthma and Immunology, 2011, 10(4): 267-271.
[28] GISLASON H, R±KKE O, SVANES K. Release of cytokines associated with gastric mucosal injury[J]. European Surgical Research, 1996, 28(4): 278-286.
[29] KUZUHARA T, SUGANUMA M, OKA K, et al. DNA-binding activity of TNF-inducing protein from Helicobacter pylori[J]. Biochemical and Biophysical Research Communications, 2007, 362(4): 805-810.
[30] VAANANEN P M, MEDDINGS J B, WALLACE J L. Role of oxygen-derived free radicals in indomethacin-induced gastric injury[J]. American Journal of Physiology, 1991, 261(3): 470-475.
TS272.5
A
1002-6630(2014)13-0271-05
10.7506/spkx1002-6630-201413054
2013-07-27
重庆市高校创新团队建设计划项目(KJTD201325);重庆第二师范学院引进高层次人才科研启动费基金项目
王睿(1982—),男,讲师,硕士,研究方向为食品化学与营养学。E-mail:jackywangrui@163.com
*通信作者:赵欣(1981—),男,教授,博士,研究方向为食品化学与营养学。E-mail:foods@live.cn
