内生镰刀菌Dzf17 多糖对盾叶薯蓣培养物薯蓣皂苷元积累的促进作用
2014-01-09李培琴曹宇恒刘洪卫傅小香周立刚
李培琴,曹宇恒,刘洪卫,傅小香,岳 阳,周立刚*
1西北农林科技大学林学院,杨凌 712100;2 中国农业大学农学与生物技术学院,北京 100193
Introduction
Plant endophytic fungi,which inhabit normal tissues of host plants without causing apparent symptoms of pathogenesis,are novel and rich sources of bioactive natural products[1].During the long period of coevolution,a friendly relationship has developed between each endophyte and its host plant[2].The host plant can supply plenteous nutriment and a safe habitation for the survival of its endophytes.On the other hand,the endophytes would produce a number of bioactive constituents for helping their host plants to resist external biotic and abiotic stresses,and benefiting for the host growth[3].
Dioscorea zingiberensis C.H.Wright (Dioscoreaceae)is a well-known traditional Chinese medicinal herb,indigenous to the south of China[4].Its rhizomes have a high content of diosgenin[5],which is an important precursor of various synthetic steroidal drugs[6].However,overexploitation of natural D.zingiberensis has led to a rapid decrease of this plant resource.Consequently,D.zingiberensis seedling and cell cultures have been regarded as alternative means for efficient and controllable production of diosgenin[7].In order to speed up their application,the enhancement of diosgenin yield in D.zingiberensis seedling and cell cultures is required.Many strategies (i.e.,medium optimization,elicitation by using polysaccharides and oligosaccharides,as well as two phase culture)have been developed to enhance the metabolite production of the either microorganism or plant cultures.Among them,elicitation was regarded as a convenient and effective approach[7-11].Saccharides (i.e.,polysaccharides and oligosaccharides)have been widely regarded as the preferable elicitors for overproduction of secondary metabolites in plant cell cultures[12-15].In our previous study,the autoclaved mycelia and fermented filtrate of the endophytic fungus Fusarium oxysporum Dzf17 were found to increase diosgenin production in D.zingiberensis cell cultures[16].In this work,three polysaccharides,namely exopolysaccharide (EPS),water-extracted mycelia polysaccharide(WPS)and sodium hydroxide-extracted mycelia polysaccharide (SPS)prepared from this fungus were studied for their enhancing effects on diosgenin production in D.zingiberensis seedling and cell cultures.
Materials and Methods
Instruments and chemicals
The freeze dryer (Labconco,Kansas City,MO,USA)was employed for freeze-drying the mycelia.The lyophilized mycelia were powdered in a disintegrator (HK-06A,Beijing,China).Diosgenin quantification was carried out on a high performance liquid chromatography(HPLC)system (Shimadzu,Japan),which consisted of two LC-20AT high-pressure solvent delivery pump units,a SPD-M20A photodiode array detector (PAD),a SIL-20AC autosampler,CTO-10AS column oven,and CBM-20Alite system controller.A reversed-phase Agilent TC-C18column (250 mm ×4.6 mm i.d.,5 μm)was used for the separation using a mobile phase of acetonitrile-water (90∶10,v/v)at a flow rate of 1 mL/min at 30 ℃.LCsolution multi-PDA workstation was employed to acquire and process chromatographic data.The injection volume was 20 μL.Changes in absorbance at 203 nm were recorded.The peak area was calibrated to diosgenin content with a chemical standard(Sigma).All the chemicals and reagents were of analytical grade.
Seedling and cell culture of D.zingiberensis
The seedlings of D.zingiberensis were subcultured on Murashige and Skoog (MS)medium supplemented with agar (8 g/L)and sucrose (30 g/L)at an interval of 30 days,which were placed in the growth chamber at 25 ℃under 12 h daily illumination of approximately 2,000 lux provided by cool fluorescent tubes[17].The calli were subcultured on MS medium supplemented with 6-benzyladenine (1.5 mg/L),naphthalene acetic acid (1.0 mg/L),agar (8 g/L)and sucrose (30 g/L)at an interval of 30 days in darkness[16].The pH was adjusted to 5.8 before autoclaving for 15 min at 121 ℃.All experiments were carried out in 125-mL Erlenmeyer flasks.The cell cultures were maintained on the above liquid medium on a rotary shaker at 120 rpm in darkness at 25 ℃.
Preparation of polysaccharides from F.oxysporum Dzf17
Endophytic F.oxysporum Dzf17 was cultured in the 1000-mL Erlenmeryer flasks for each one containing 300 mL of liquid medium consisting of glucose (50 g/L),peptone (13 g/L),NaCl (0.6 g/L),K2HPO4(0.6 g/L)and MgSO4·7H2O (0.2 g/L).All flasks maintained on a rotary shaker at 150 rpm and 25 ℃for 14 days.A total of 15 L of fermentation broth was centrifuged at 7,741 × g for 20 min.The supernatant and mycelia were collected separately.The supernatant was concentrated to a certain volume (0.5 L)and then kept in the freezer (4 ℃) for exopolysaccharide(EPS)preparation.Mycelia were washed twice with deionized water (500 mL (2),and then lyophilized.About 60 g of mycelia in dry weight was obtained for mycelial polysaccharide preparation.
EPS was prepared from the supernatant (15 L)mentioned above.The supernatant was concentrated under vacuum at 60 ℃by a rotary evaporator to a suitable volume (0.5 L)and mixed with three volumes of 95%ethanol,then kept at 4 ℃for 48 h.After that,the solution was centrifuged at 17,418 × g for 15 min,and the precipitate from ethanol dispersion was collected as crude EPS which was further subjected to deproteinization with Sevag reagent (chloroform-n-butanol=4∶1,v/v),decolorization with H2O2,and removal of small molecule impurities by dialysis.Polysaccharide mixture with molecular weight greater than 8,000-14,000 Da was kept in dialysis tube and then subjected to concentration.After lyophilization,the purified EPS (3.20 g)was obtained and stored in a desiccator at room temperature.The carbohydrate content of EPS was measured spectrophotometerically by anthrone-sulfuric acid method[18].
The lyophilized mycelia (60 g)were powdered,and then subjected to heat circumfluence extraction at 50℃by 95% ethanol-petroleum ether at 1∶1 (v/v)as the refluxing solvent to remove monosaccharide,disaccharide and lipid.The ratio of mycelia powder (g)to refluxing solvent (mL)was 1∶5 (w/v).Defatted mycelial powder was obtained by centrifugation (7,741× g,20 min)and drying in an oven at 40 ℃for 2 h,and then immersed in hot water at 90 ℃for 2 h with the ratio of water (mL)to the material (g)set at 30∶1 (v/w).After that,centrifugation was carried out at 7,741 × g for 20 min to separate the residue and the supernatant.The supernatant was condensed to a certain volume under vacuum at 60 ℃,and then mixed with three volumes of 95% ethanol,and then kept at 4℃for 48 h.The following procedure for polysaccharide preparation and purification was the same as the treatment of EPS.The gained polysaccharide (3.32 g)was named as water-extracted mycelial polysaccharide(WPS).The residue not containing WPS was further extracted with 10% sodium hydroxide (NaOH)solution at room temperature for 24 h.The remaining steps were the same described in the treatment of WPS.The obtained polysaccharide (3.59 g)was designated as sodium hydroxide-extracted mycelial polysaccharide(SPS).
Application of polysaccharides in seedling and cell culture of D.zingiberensis
EPS,WPS and SPS (100 mg for each polysaccharide)were separately dissolved in 10 mL of sterile distilled water as the stock solutions,and then filtered through a 0.45 μm sterile filter membrane.For elicitation in seedling cultures,each 125-mL flask was filled with 50 mL MS solid medium.After the MS medium was autoclaved,gradient concentrations of sterile polysaccharides solutions were immediately added to MS medium,and shaken vigorously.The final concentrations of each polysaccharide in medium were 10,20,40,80 and 160 mg/L,separately.When the MS medium was solidified,1.0 g fresh seedling was inoculated in each flask.All the seedling cultures were harvested on day 32.For elicitation in suspension cell cultures,25-day-old cell cultures were respectively treated by the three polysaccharides elicitors at 10,20,40,80 and 160 mg/L.Each 125-mL flask was filled with 30 mL liquid medium with the inoculated weight as 0.3 g cell cultures.The suspension cell cultures were harvested on day 30.The same volume of sterilized water was used as the control in the seedling and cell culture.
Biomass determination and diosgenin quantification
D.zingiberensis seedling cultures were harvested from the Erlenmeyer flasks and washed with distilled water to remove residual medium.The suspension cell cultures were harvested by filtration under vacuum.Both the seedling and cell cultures were lyophilized to a constant weight.
Diosgenin extraction and determination were carried out as described in our previous study[17].Dried and powdered seedlings or cell cultures (200 mg)was weighed and transferred into a tube with 40 mL of 95% ethanol,and then subjected to ultrasonic extraction for 1 h.After that,40 mL of 1 mol/L sulfuric acid was added to each tube,and hydrolyzed at 121 ℃for 2 h.The hydrolyte was extracted for three times with petroleum ether.The combined petroleum ether solution was washed twice with 1 mol/L of NaOH solution,and then twice with distilled water.After dehydration with anhydrous sodium sulfate,the petroleum ether solution was concentrated to dryness under vacuum on a rotary evaporator.The extract was dissolved in acetonitrile,and then filtered through a 0.22 μm filter before analysis.Diosgenin quantification was carried out by HPLC.Diosgenin content in the culture medium was negligible and not determined.
Statistical analysis
All treatments were carried out in triplicate,and the results were represented by their mean values and the standard deviations (SD).The data were analyzed to detect significant differences by PROC ANOVA of SAS version 8.2.
Results and Discussion
Enhancement of polysaccharides on diosgenin production in seedling culture of D.zingiberensis
The effects of EPS,WPS and SPS from F.oxysporum Dzf17 on growth and diosgenin accumulation of D.zingiberensis seedling cultures were shown in Fig.1.In general,the effects of polysaccharides on growth of the seedlings were not obvious.As presented in Fig.1A,the maximum seedling dry weight (14.32 g/L)was observed when the seedlings were treated with 20 mg/L of WPS,which was 1.18-fold as compared with control(12.09 g/L).For EPS,the maximum seedling dry weight (13.54 g/L)was observed when the seedlings were treated at 20 mg/L,which was 1.12-fold as that of control and identical to that of SPS.As polysaccharide concentration was increased from 40 mg/L to 160 mg/L,the augmentation of dry weight displayed decreasing trend,even disappeared and then changed into suppressing effects.
Fig.1B presented the effects of polysaccharides on diosgenin content,from which the evident enhancement on diosgenin content was caused by WPS.When the seedlings were treated with 20 mg/L of WPS,the highest diosgenin content (0.70 mg/g)was obtained,which was 2.63-fold as compared with control (0.27 mg/g).The slight enhancement of diosgenin content was also observed in the seedlings treated with WPS or EPS at 40 mg/L,which were respectively 1.95 and 1.30-fold as that of control.However,diosgenin content was decreased when the seedlings were treated by the high concentration polysaccharide (i.e.,160 mg/L).SPS exhibited obviously inhibitory effects on diosgenin content at 40,80,and 160 mg/L.
The effects of three polysaccharides on diosgenin yield in seedlings were graphed in Fig.1C,which displayed the almost identical variance trend as that of diosgenin content.The highest diosgenin yield (10.04 mg/L)in the seedlings was caused by 20 mg/L of WPS,which was 3.11-fold as that of control.EPS at 10,20 and 40 mg/L exhibited slight enhancement on diosgenin yield of seedlings.At 40,80,and 160 mg/L,SPS exhibited obviously inhibitory effects on diosgenin yield which indicated that higher SPS concentrations (i.e.,more then 40 mg/L)could inhibit diosgenin biosynthesis.
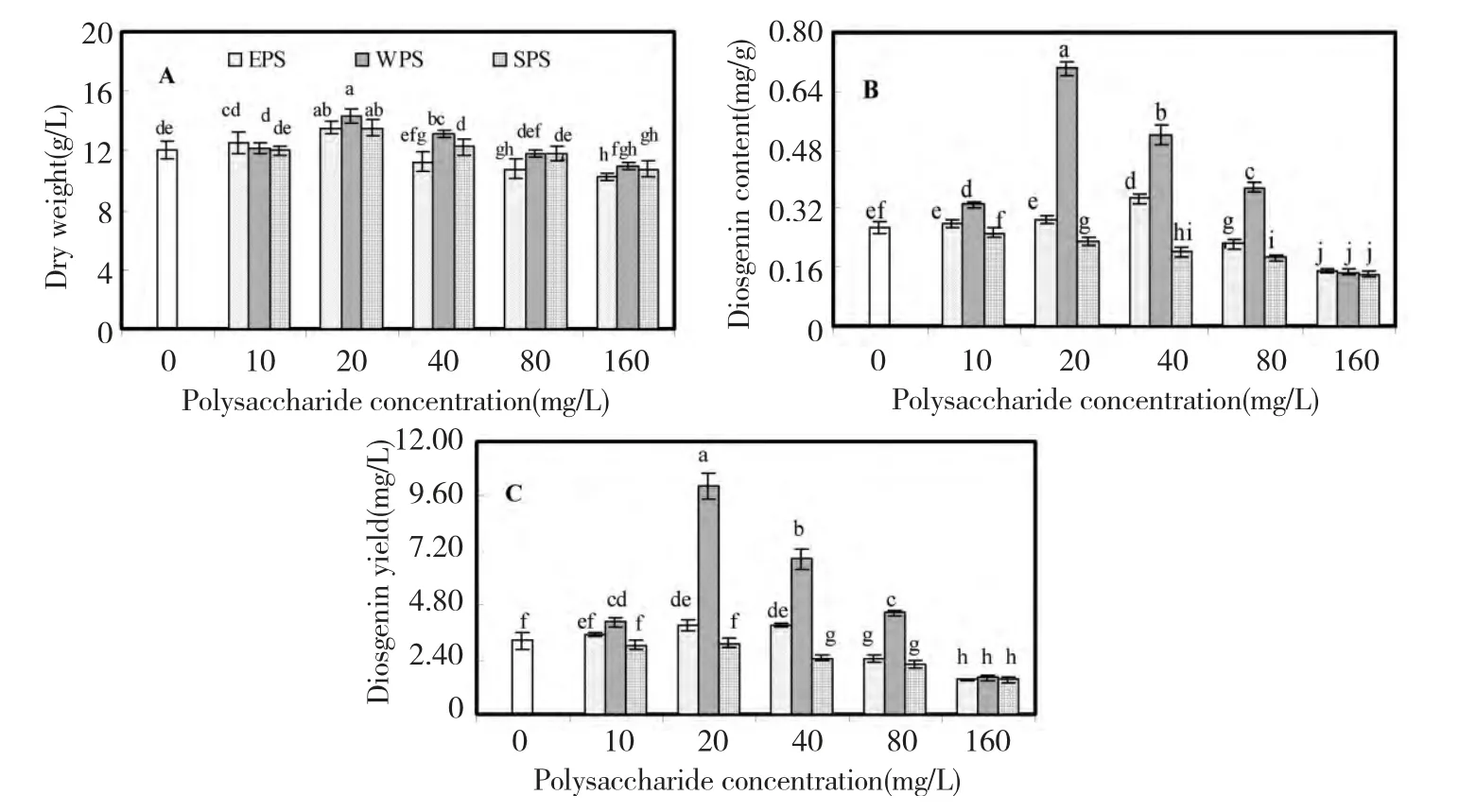
Fig.1 Effects of EPS,WPS and SPS on biomass (A),diosgenin content (B)and diosgenin yield (C)in seedling culture of D.zingiberensis.Each value was expressed as mean ± standard deviation (n=3).Different letters indicated significant differences among the treatments at P=0.05 level.
Enhancement of polysaccharides on diosgenin production in cell culture of D.zingiberensis
Fig.2 showed the effects of three polysaccharides from F.oxysporum Dzf17 on growth and diosgenin accumulation in D.zingiberensis cell cultures.The growth was expressed as dry weight per liter medium (g/L),which was presented in Fig.2A.As compared with control(4.02 g/L),dry weight of cell cultures was not influenced obviously when treated with the polysaccharides.The highest dry weight (5.39 g/L)was induced by 20 mg/L of WPS,which was 1.34-fold in comparison to that of control but no significant difference to that(5.20 g/L)treated with 40 mg/L of EPS.However,as the concentration of polysaccharide EPS or WPS was increased to 160 mg/L,cell growth was inhibited.
Fig.2 B displayed the effects of polysaccharides on diosgenin content of cell cultures,from which no significant inhibitory effects on diosgenin content were observed.When cell cultures were treated with 20 mg/L of EPS,diosgenin content was increased to the highest with the value of 0.39 mg/g,which was 2.50-fold as that of control (0.15 mg/g).When WPS was treated at 20 mg/L,the most obvious enhancement of diosgenin content (0.45 mg/g)was observed,which was 2.90-fold of control.However,SPS exhibited weaker stimulating effects on dosgenin content than EPS or WPS.The highest diosgenin content caused by SPS was just 0.30 mg/g,which was only 1.94-fold as that of control.
Diosgenin yield (mg/L)is the synthesized result of dry weight (g/L)and diosgenin content (mg/g).From Fig.2C,diosgenin yield showed a diosgenin content-dependent manner,for larger varied range of diosgenin content caused by polysaccharides elicitors.When WPS was treated at 20 mg/L,the maximum of diosgenin yield (2.40 mg/L)was observed,which was 3.87-fold as that of control (0.62 mg/L).When EPS was treated at 20 mg/L,the highest diosgenin yield (1.75 mg/L)was observed,which was 2.82-fold as that of control.When SPS was treated at 80 mg/L,diosgenin yield was increased to the maximum (1.26 mg/L).
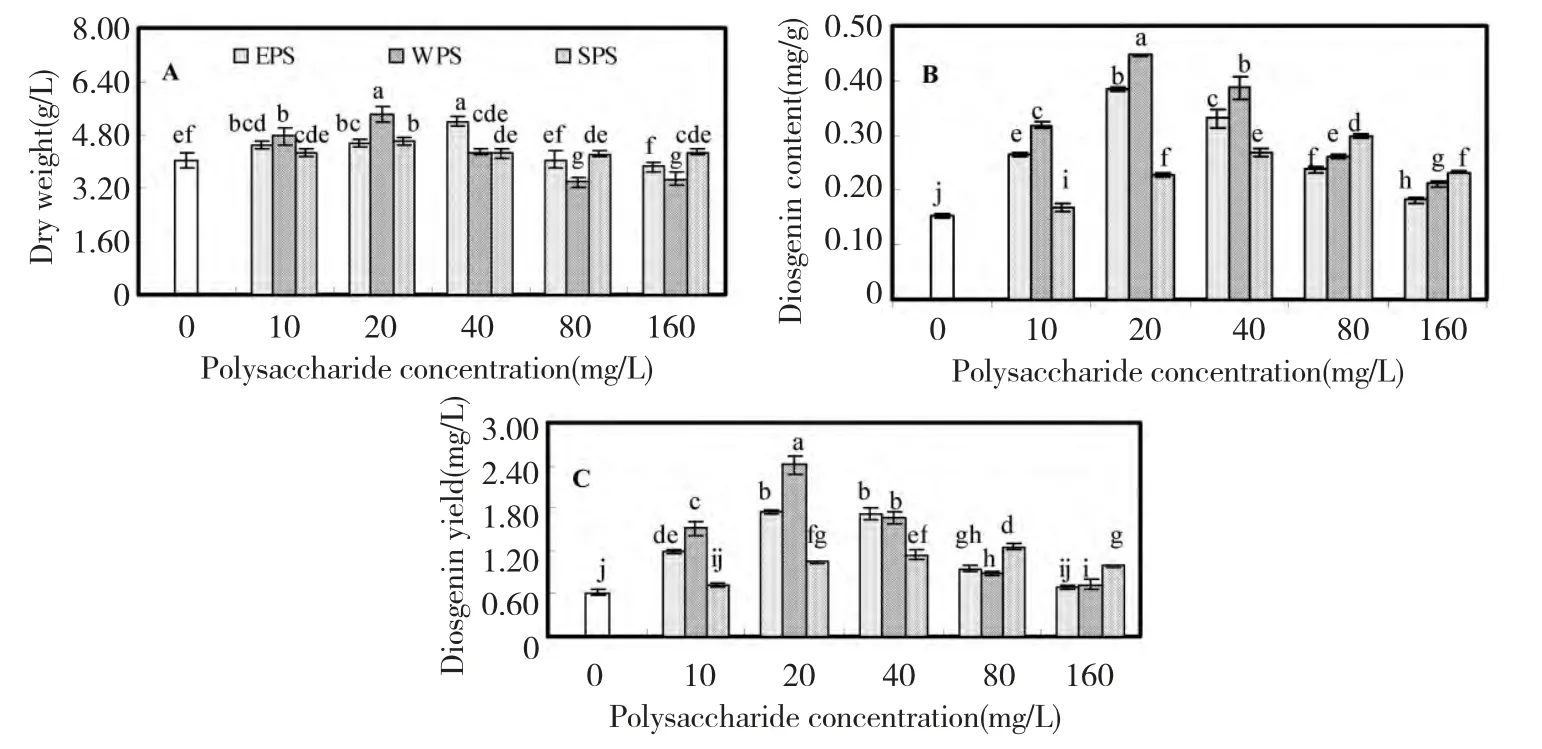
Fig.2 Effects of EPS,WPS and SPS on biomass (A),diosgenin content (B)and diosgenin yield (C)in cell suspension culture of D.zingiberensis.Each value was expressed as mean ± standard deviation (n=3).Different letters indicated significant differences among the treatments at P=0.05 level.
Conclusion
The results from the present study showed that the polysaccharides (EPS,WPS and SPS)from the endophytic fungus F.oxysporum Dzf17 notably stimulated diosgenin production in the seedling and cell cultures of D.zingiberensis.Among them,WPS was found to be the most effective polysaccharide.It indicated that there should be a close relationship between the endophytic F.oxysporum Dzf17 and its host D.zingiberensis which needs further investigation.There is a need to characterize the chemical structures (i.e.,homogeneity,monosaccharide composition,and linkage of the monosaccharide residues)of the polysaccharides from F.oxysporum Dzf17,as well as to investigate their structure–activity relationships and action mechanisms.The oligosaccharides prepared by partial hydrolysis from other polysaccharides have been reported to show enhancement effects on production of secondary metabolites[14,15].This indicates that the added polysaccharide in medium was possibly catabolized into the oligosaccharide fragments which could be the active components to affect growth and diosgenin production in seedling and cell cultures of D.zingiberensis.The present work could provide more information for further utilization of endophytic fungus F.oxysporum Dzf17.Addition of the polysaccharides from the endophytic fungus F.oxysporum Dzf17 could be an effective strategy for large-scale production of diosgenin in seedling and cell cultures of D.zingiberensis in the future.
1 Backman PA,Sikora RA.Endophytes:an emerging tool for biological control.Biol Control,2008,46:1-3.
2 Zhao J,Shan T,Mou Y,et al.Plant-derived bioactive compounds produced by endophytic fungi.Mini-Rev Med Chem,2011,11:159-168.
3 Silvia F,Sturdikov M,Muckova M.Bioactive secondary metabolites produced by microorganisms associated with plants.Biologia,2007,62:251-257.
4 Li H,Ni J.Treatment of wastewater from Dioscorea zingiberensis tubers used for producing steroid hormones in a microbial fuel cell.Bioresource Technol,2011,102:2731-2735.
5 Zhu YL,Huang W,Ni JR,et al.Production of diosgenin from Dioscorea zingiberensis tubers through enzymatic saccharification and microbial transformation.Appl Microbiol Biotechnol,2010,85:1409-1416.
6 Sautour M,Mitaine-Offer AC,Lacaille-Dubois MA.The Dioscorea genus:a review of bioactive steroid saponins.J Nat Med,2007,61:91-101.
7 Zhou L,Wu J.Development and application of medicinal plant tissue cultures for production of drugs and herbal medicinals in China.Nat Prod Rep,2006,23:789-810.
8 Zhou L,Cao X,Zhang R,et al.Stimulation of saponin pro-duction in Panax ginseng hairy roots by two oligosaccharides from Paris polyphylla var.yunnanensis.Biotechnol Lett,2007,29:631-634.
9 Zhao J,Zhou L,Wu J.Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures.Appl Microbiol Biotechnol,2010,87:137-144.
10 Zhao J,Zheng B,Li Y,et al.Enhancement of diepoxin ζ production by yeast extract and its fractions in liquid culture of Berkleasmium-like endophytic fungus Dzf12 from Dioscorea zingiberensis.Molecules,2011,16:847-856.
11 Li Y,Li P,Mou Y,et al.Enhancement of diepoxin ζ production in liquid culture of endophytic fungus Berkleasmium sp.Dzf12 by polysaccharides from its host plant Dioscorea zingiberensis.World J Microbiol Biotechnol,2012,28:1407-1413.
12 Dornenburg H,Knorr D.Effectiveness of plant-derived and microbial polysaccharides as elicitors for anthraquinone synthesis in Morinda citrifolia cultures.J Agric Food Chem,1994,42:1048-1052.
13 Zhou L,Zheng G,Wang S,et al.Effects of polysaccharides on cultured cells of Onosma paniculatum.Nat Prod Res Dev,1991,3:34-37.
14 Zhou L,Yang C,Li J,et al.Heptasaccharide and octasaccharide isolated from Paris polyphylla var.yunnanensis and their plant growth-regulatory activity.Plant Sci,2003,165:571-575.
15 Zhang R,Li P,Xu L,et al.Enhancement of diosgenin production in Dioscorea zingiberensis cell culture by oligosaccharide elicitor from its endophytic fungus Fusarium oxysporum Dzf17.Nat Prod Commun,2009,4:1459-1462.
16 Zhang R,Li P,Zhao J,et al.Endophytic fungi from Dioscorea zingiberensis and their effects on the growth and diosgenin production of the host plant cultures.Nat Prod Res Dev(天然产物研究与开发),2010,22:11-15.
17 Yin C,Li P,Li H,et al.Enhancement of diosgenin production in Dioscorea zingiberensis seedling and cell cultures by beauvericin from the endophytic fungus Fusarium redolens Dzf2.J Med Plants Res,2011,5:6550-6554.
18 Wang Z,Luo D,Ena C.Optimization of polysaccharides extraction from Gynostemma pentaphyllum Makino using uniform design.Carbohyd Polym,2007,69:311-317.
