南海软海绵Halichondria sp.化学成分研究
2014-01-08田永奇甘建红吴文惠
田永奇,甘建红,吴文惠*
上海海洋大学食品学院,上海 201306
海绵出现于寒武纪,是最原始、最简单的多细胞动物,分布极为丰富,自赤道到两极不同深度的海域都有海绵的存在。已知的海绵有1 万余种,其颜色、形状千姿百态,大小、质量相差非常大(直径1~15 m)。为了抵御外族的侵袭和恶劣的海洋环境,海绵群体在上亿年的积累中产生了很多化学防御物质。而这些化学防御物质由于具有新颖的结构和独特的生物活性,如今已经成为科学家研究的热点[1-2]。
软海绵属寻常海绵纲(Demospongiae),软海绵目(Halichondrida),软海绵科(Halichondridae),在亚热带地区的海绵中较为常见。Halichondria属软海绵多与其共生体如细菌等生活在一起,通过过滤海水摄入大量微生物有机体,产生各种各样的特异性代谢产物,如大环内酯、萜类、鞘类脂糖苷、甾醇、生物碱、内酰胺等。其中很多化合物都具有新颖的结构和显著的抗菌、抗肿瘤等生物活性[3]。1986年Hirata and Uemura[4]等从Halichondria okadai中分离得到Halichondrin B,以其为先导化合物研制而成的药物Eribulin 作为治疗转移性乳腺癌的新型药物,已经于2010年9月15 日在美国上市。1996年,Kuramoto[5]等从Halichondria.okadai 中分离到halichlorine,该化合物能够抑制血管黏附分子VCAM-1的诱导表达。具有该作用的化合物对于动脉硬化、冠心病、非心血管感染性疾病等有治疗作用。1997年Kobayashi[6]等从日本冲绳岛软海绵Halichondriasp.中发现化合物halishigamides A~D 其对鼠淋巴瘤L1210 细胞和人表皮样瘤KB 细胞均有细胞毒作用。Chill[7]等从Halichondriasp.中分得1 个新的结构新颖的四环双哌啶halichondramine,但未报到其生物活性。2007年张红军[8]等从Halichondria rugosa中分离得到一个酯甾醇化合物,具有有较强的体外抗HIV-1 蛋白酶和抗HIV-1 整合酶活性。2012年,Naonobu Tanaka[9]等从Halichondriasp.分离出2个结构非常新颖的倍半萜二聚体,它们对KB 肿瘤细胞株均具有中等的细胞毒性。
我国南海海绵资源极为丰富,为了寻找结构新颖且有药用活性的先导化合物,我们对2009年3月采集于海南西沙群岛的Halichondriasp.进行了化学成分研究。从二氯甲烷与甲醇(1∶1)提取物中分离得到了了7 个结构很新颖的化合物,经现代波谱技术分析,并结合文献对照,确定其结构分别为:4α-Isocyanogorgon-11-Ene(1),Homoverrucosanol (2),neoverru--cosanol(2),5α,6α-Epoxy-(22E)-ergosta-8,14,22-triene-3β,7α-diol (3),5α,8α-epidioxy-(22E,24R)-ergosta-6,22-dien-3β-ol (4),(22E,24R)-ergosta-7,22-diene-3β,5α,6α,9α-tetrol(6),3β,5α,9α,14β-tetrahydroxy-(22E)-ergosta-7,22-dien-6-one(7)(结构见图1).对首次从该属中分离得到的化合物进行了体外活性评价,发现化合物1与化合物3 对HeLa 显示出较弱的细胞毒性,其IC50值分别为33.7 μM 和43.8 μM。本文主要报道以上7 个化合物的分离纯化以及结构鉴定工作。
1 实验部分
1.1 仪器和材料
TLC:高效薄层层析板(HPTLC)为德国Merck公司产品和烟台江友硅胶开发有限公司产品。显色剂:10%硫酸香兰素溶液。EYELAN-1000 型旋转蒸发仪。HPLC:Waters 1525/2996,2998 HPLC;Agilent 1200;YMC-Pack(C8250 × 10 mm)。溶剂系统(石油醚:乙酸乙酯),(石油醚:丙酮),(正己烷:异丙醇),(二氯甲烷:甲醇)等。常用有机试剂均为国产的分析纯。
软海绵Halichondriasp.于2009年3月采集于海南西沙永兴岛,样品由中科院海洋研究所李锦和教授鉴定,现保存于第二军医大学长征医院海洋药物实验室,编号为SHT。
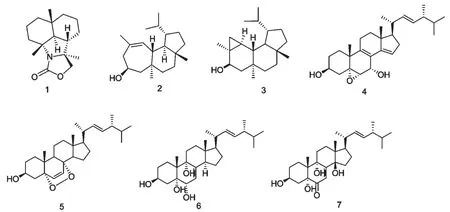
图1 化合物1~7 的结构式Fig.1 Structures of compounds 1-7
1.2 提取与分离
将浸泡于95%乙醇溶液中的海绵样品(湿重3 kg)取出,剪碎,放入3 L 的玻璃瓶中,加入甲醇和二氯甲烷各1000 mL 超声提取7 次。将原95%乙醇浸泡液与超声提取液合并旋蒸至无醇味。用乙酸乙酯提取出有机相。分别用正己烷,二氯甲烷,正丁醇萃取。得到正己烷层75.3 g,二氯甲烷层18 g,正丁醇层50 g。二氯甲烷层首先用凝胶柱(甲醇与二氯甲烷各500 mL)洗脱,分为D1~D3,D2(10 g)用反向硅胶柱梯度洗脱以甲醇(M)∶水(W)(3∶7,2∶3,1∶1,3∶2,7∶3,4∶1,9∶1)为流动相。得到14 个馏分(D2A~D2N).D2I 经硅胶柱分离,后采用高效液相色谱(YMC-Pack C8250 × 10 mm,2 mL/min)制备。以M∶W(3:1)为流动相得到化合物1(2.6 mg)。D2L 经硅胶柱分离以石油醚(P):乙酸乙酯(E)(20∶1,10∶1,5∶1,1∶1)乙酸乙酯为流动相,分离得到(D2L1~D2L8).D2L1 经硅胶柱洗脱(P∶E=30∶1)得到化合物2(221.3 mg),化合物3(11.2 mg)。D2M 经硅胶柱洗脱(P∶E=20∶1,10∶1,5∶1,1∶1),得到化合物4(10 mg),化合物5(112.3 mg),化合物6(6.3 mg),化合物7(16 mg)。
2 结果与讨论
2.1 结构鉴定
化合物1 无色油状物,ESI-MSm/z:263[M]+。分子式为C16H25NO2。1H NMR(600 Hz,CDCl3)δ:4.10(1H,d,J=6 Hz,H-11),3.97(1H,d,J=6 Hz,H-11),2.24(1H,m,H-3),1.87(1H,ddd,J=13.2,11.4,4.8 Hz,H-6),1.73(1H,m,H-7),1.67(1H,m,H-2),1.60(1H,m,H-8),1.53(1H,m,H-1),1.47(1H,d,J=13.2 Hz,H-5),1.47(1H,m,H-9),1.45(1H,m,H-3),1.42(1H,m,H-8),1.26(3H,s,H3-14),1.24(3H,s,H3-13),1.15(1H,m,H-7),1.06(1H,m,H-1),1.00(1H,m,H-9),0.98(3H,s,H3-15).13C NMR(150 MHz,CDCl3)δ:158.8(C-16),79.2(C-12),68.2(C -11),62.0(C-4),59.7(C-5),43.9(C-9),40.8(C-1),40.4(C-6),39.9(C-3),34.1(C-10),26.5(C-7),23.6(C-13),20.9(C-4),20.5(C-2),19.3(C-15),18.6(C-14)。其数据与文献[10]报道数据基本一致,故鉴定化合物1 为4α-Isocyanogorgon-11-Ene。
化合物2 白色固体,ESI-MSm/z:290 [M]+。分子式为C20H34O。1H NMR(600Hz,CDCl3)δ:5.29(1H,d,J=5 Hz,H-2),3.60(1H,ddt,J=2,3,11 Hz,H-5),2.54(1H,ddd,J=2,11,13 Hz,H-4),2.20(1H,dd,J=5,12 Hz,H-1),2.05(1H,m,H-15),2.03(1H,m,H-4),1.89(1H,ddd,J=2,3,13 Hz,H-6),1.76(3H,s,H3-18),1.71(1H,ddt,J=3,7,11 Hz,H-13),1.60(1H,m,H-12),1.54(1H,dt,J=4,14 Hz,H-8),1.47(1H,dd,J=11,13 Hz,H-6),1.40(1H,m,H-9),1.38(1H,m,H-12),1.37(1H,m,H-11),1.32(1H,dt,J=3,14 Hz,H-9),1.23(1H,dt,J=3,14 Hz,H-8),1.16(1H,t,J=11,H-14),1.03(1H,q,J=11 Hz H-11),0.86(3H,d,J=7 Hz,H3-17),0.86(3H,s,H3-19),0.83(3H,d,J=7 Hz,H-16),0.78 (3H,s,H3-20)。13C NMR (150 MHz,CDCl3)δ:131.7(C-2),131.3(C-3),65.6(C-5),58.9(C-6),47.8(C-14),46.8(C-13),43.8(C-1),42.6(C-4),42.6(C-10),39(C-8),38.8(C-11),38(C-7),35.1(C-9),28.0(C-15),25.8(C-18),22.9(C-17),21.4(C-12),20.0(C-19),18.1(C-20),14.9(C-16)。其数据与文献[11]报道数据基本一致,故鉴定化合物2 为homoverrucosanol。
化合物3 白色固体,ESI-MSm/z:290[M]+分子式为C20H34O。1H NMR(600 Hz,CDCl3)δ:4.03(1H,dd,J=7,11 Hz,H-5),2.15(1H,m,H-15),1.90(1H,m,H-13),1.69(1H,dd,J=7,13 Hz,H-6),1.60(1H,m,H-12),1.42(1H,m,H-9),1.40(1H,m,H-12),1.37(1H,m,H-11),1.35(1H,m,H-9),1.29(1H,dt,J=4,13 Hz,H-8),1.19(1H,s,H-18),1.16(1H,t,J=12 Hz,H-14),1.10(1H,ddd,J=2,4,13 Hz,H-8),1.05(1H,m,H-11),1.02(1H,dd,J=4,12 Hz,H-1),0.91(1H,d,J=7 Hz,H-17),0.85(1H,ddd,J=4,5,8 Hz,H-2),0.83(1H,s,H-19),0.82(1H,d,J=7 Hz,H-16),0.74((1H,s,H-20),0.68(1H,dd,J=11,13 Hz,H-6),0.56(1H,dd,J=5,8 Hz,H-3),0.28(1H,t,J=5 Hz,H-3)。13C NMR(150 MHz,CDCl3)δ:71.2(C-5),47.6(C-1),47.2(C-6),46.9(C-14),45.2(C-13),44(C-10),39.2(C-11),37.1(C-7),35.3(C-8),34.6(C-9),28.7(C-15),25.8(C-18),25.6(C-2),22.7(C-17),22.0(C-4),21.6(C-12),19.6(C-3),18.6(C-20),17.2(C-19),14.9(C-16)。其数据与文献[11]报道数据基本一致,故鉴定化合物3 为neoverrucosanol。
化合物4 无色针晶,ESI-MSm/z:426 [M]+。分子式C28H42O3。1H NMR(600 Hz,CDCl3)δ:6.5(1H,dd,J=1.8,3.3 Hz,H-15),5.33(1H,dd,J=7.3,15.4 Hz,H-23),5.28(1H,dd,J=8.1,15.4 Hz,H-22),4.34(1H,dd,J=2.6,11.2 Hz,H-7),3.02(1H,d,J=2.6 Hz,H-6),3.82(1H,m,H-3),2.45(1H,ddd,J=3.3,7.3,16.9 Hz,H-16),2.18(1H,dd,J=11.4,16.9 Hz,H-16),2.31(1H,m,H-20),1.87(1H,dd,J=11.4,12.8 Hz,H-4),1.63(1H,m,H-17),1.13(3H,d,J=6.6 Hz,H3-21),1.00(3H,d,J=6.6 Hz,H3-28),0.92(3H,d,J=7.0 Hz,H3-27),0.91(3H,d,J=7.0 Hz,H3-26),0.84(3H,s,H3-18),0.83(3H,s,H3-19)。13C NMR(150 MHz,CDCl3)δ:146.9(C-14),139.1(C-9),136.0 (C-22),132.5 (C-23),123.9 (C-15),123.0(C-8),68.3(C-3),67.7(C-7),65.2(C-5),62.5(C-6),56.6(C-17),45.6(C-13),43.3(C-24),39.5(C-4),39.3(C-20),38.9(C-10),37.6(C-16),35.7(C-12),33.4(C-25),31.3(C-1),30.8(C-2),23.5(C-11),23.1(C-19),21.3(C-21),20.2(C-27),19.9(C-26),18(C-28),15.6(C-18).其数据与文献[12]报道数据基本一致,故鉴定化合物4 为5α,6α-Epoxy-(22E)-ergosta -8,14,22-triene-3β,7α-diol。
化合物5 无色针晶,ESI-MSm/z:428[M]+分子式C28H44O3。1H NMR(600 Hz,CDCl3)δ:6.49(1H,d,J=8.5 Hz,H-7),6.23(1H,d,J=8.5 Hz,H-6),5.20(1H,dd,J=7.6,15.2 Hz,H-23),5.12(1H,dd,J=7.6,15.2 Hz,H-22),3.94(1H,m,H-3),2.08~1.49(20H,m),1.06(3H,s,H-19),0.97(3H,d,J=6.4 Hz,H-21),0.89(3H,d,J=5.2 Hz,H-28),0.86(3H,s,H-18),0.83(3H,d,J=4.8 Hz,H-26),0.82(3H,d,J=4.8 Hz,H-27)。13C NMR(CDCl3,150 MHz)δ:135.5(C-6),135.2(C-22),132.4(C-23),130.8(C-7),82.1(C-5),79.4(C-8),66.5(C-3),56.3(C-17),51.7(C-14),51.2(C-9),44.6(C-13),42.8(C-24),39.7(C-20),39.4(C-12),37.0(C-4),37.0(C-10),34.6(C-1),33.1(C-25),30.2(C-2),28.6(C-16),23.4(C-11),20.9(C-21),20.6(C-15),19.6(C-27),19.1(C-26),18.2(C-19),17.6(C-28),12.9(C-18)。其数据与文献[13,14]报道数据基本一致,故鉴定化合物5 为5α,8α-epidioxy-(22E,24R)-ergosta-6,22-dien-3β-ol。
化合物6 无定型粉末,ESI-MSm/z:446[M]+。分子式C28H46O4。1H NMR(600 Hz,CDCl3)δ:5.22(1H,dd,J=7.7,15.4 Hz,H-23),5.17(1H,dd,J=7.7,15.4 Hz,H-22),5.06(1H,dd,J=1.8,1.8 Hz,H-7),4.03(1H,m,H-3),3.96(1H,brs,H-6),2.25(1H,ddd,J=4.0,13.6,13.6 Hz,H-1),1.95(1H,d,J=12.1 Hz,H-2),2.48(1H,m,H-14),2.02(1H,m,H-20),1.05(3H,s,H3-19),1.02(1H,d,J=6.6 Hz,H-21),0.92(3H,d,J=7.0 Hz,H3-28),0.84(3H,d,J=6.6 Hz,H3-27),0.82(3H,d,J=7.0 Hz,H3-26),0.58(3H,s,H3-18)。13C NMR(CDCl3,150 MHz)δ:142.6(C-8),135.4(C-22),132.7(C-23),120.3(C-7),77.1(C-5),74.5(C-9),70.3(C-6),67.3(C-3),55.8(C-17),50.5(C-14),43.8(C-13),42.8(C-24),41.0(C-10),40.4(C-20),40.2(C-4),35.1(C-12),33.1(C-25),30.3(C-2),28.1(C-16),28.0(C-11),26.5(C-1),22.8(C-15),21.1(C-21),20.3(C-19),20.0(C-27),19.6(C-26),17.6(C-28),11.7(C-18)。其数据与文献[15]报道数据基本一致,故鉴定化合物6 为(22E,24R)-ergosta-7,22-diene-3β,5α,6α,9α-tetrol(6)。
化合物7 白色固体,ESI-MSm/z:460[M]+。分子式C28H44O5。1H NMR(600 MHz,CDCl3)δ:6.50(1H,s,H-7),5.45(1H,dd,J=8.8,15.4 Hz,H-22),5.34(1H,dd,J=8.8,15.4 Hz,H-23),4.06(1H,m,H-3),2.80(1H,m,H-15),2.38(1H,m,H-20),2.33(1H,ddd,J=4.0,13.9,13.9 Hz,H-1),1.00(3H,s,H3-18),1.00(3H,d,J=6.6 Hz,H3-21),0.99(3H,s,H3-19),0.95(3H,d,J=6.6 Hz,H3-28),0.85(3H,d,J=6.6 Hz,H3-27),0.83(3H,d,J=6.6 Hz,H3-26)。13C NMR(CDCl3,150MHz)δ:199.4(C-6),166.9(C-8),135.3(C-22),132.9(C-23),122.8(C-7),84.6(C-14),79.3(C-5),75.9(C-9),66.7(C-3),56.1(C-17),49.8(C-13),43.3(C-24),43.3(C-10),41.9(C-15),39.5(C-20),38.1(C-4),37.4(C-12),33.4(C-25),31.7(C-2),28.6(C-16),28.0(C-11),26.0(C-1),22.9(C-19),20.4(C-21),20.2(C-27),19.9(C-26),17.9(C-28),17.5(C-18)。其数据与文献[16]报道数据基本一致,故鉴定化合物7 为3β,5α,9α,14β-tetrahydroxy-(22E)-ergosta-7,22-dien-6-one。
2.2 讨论
化合物1,2,3 属于结构新颖的萜类化合物。化合物2,3 最初从植物中分离出来,这种verrucosane类的二萜在海绵中非常罕见。本文是第二次报道verrucosane 型二萜从海绵中分离出来,这为研究海洋生物与陆地生物生态学之间的关系提供了材料。化合物4,5,6 都为高度氧化的麦角甾醇衍生物,结构较为新颖。在细胞毒活性筛选中,对首次从Halichondriasp.中分离出来7 个化合物进行了活性评价,化合物1,3 对HeLa 显示出了较弱的细胞毒活性,IC50值分别为33.7 和43.8 μM。
1 Blunt JW,Copp BR,Hu WP,et al.Marine natural products.Nat Prod Rep,2009,26:170-244.
2 Hu J(胡静),Yang B(杨斌),Sun JF(孙见凡),et al.Chemical Constituents of Marine SpongeHalichondriasp.from the South China sea.Nat Prod Res Dev(天然产物研究与开发),2012,24:614-617.
3 Sun JB(孙晶波),Lin HW(林厚文),Li SL(李水林),et al.Secondary metabolites and their bioactivities from marine spongeHalichondria.Chin Tradit Herb Drugs(中草药).2005,36:609-613.
4 Hirata Y,Uemura D.Halichondrins-antitumor polyether macrolides from a marine sponge.Pure Appl Chem,1986,58:701-710.
5 Kuramoto M,Tong C,Yamada K,et al.Halichlorine aninhibitor of VCAM-induction from the marine spongeHalichondria okadaiKadota.Tetrahedron Lett,1996,37:3867-3870.
6 Kobayashi J,Tsuda M,Fuse H,et al.Halishigamides A-D,new cytotoxic oxazole-containing metabolites from Okinaw an spongeHalinchondriasp..J Nat Prod,1997,60:150-154.
7 Chill L,Yosief T,Kashman Y,et al.Halichondramine,a new tetracyclic bipiperidine alkaloid from the marine spongeHalichondriasp..J Nat Prod,2002,65:1738-1741.
8 Zhang HJ(张红军),Sun JB(孙晶波),Lin HW(林厚文),a new cytotoxic cholesterol sulfate from marine spongeHalichondria rugosa.Nat Prod Res,2007,21:953-958.
9 Naonobu T,Shohei S,Haruaki I,et al.Halichonadins K and L,New Dimeric Sesquiterpenoids from a SpongeHalichondriasp.Organic Letters.2012,14:3498-3501.
10 Xiong F,John RB,M BH,et al.Novel Oxidation Products of Two Sponge-Derived Sesquiterpenes,4α-For--mamedo-and 4α-Isocyanogorgon-11-Ene.Nat Prodt Letters,1997,12:5-83.
11 Junichi T,Irvina N,Tatsuo H,et al.Umabanol,a New Tetracyclic Diterpene from a Marine Spone.Chemistry Letters.1997.489-490.
12 Naoko O,Keiko A,Rie K,et al.Sterol Constituents from Two Edible Mushroom,Lentinula edodes and Tricholoma matsutake.Chem Pharm Bull,2000,48:749-751.
13 Tang JG(汤建国),Shao HJ(邵红军),Liu JK(刘吉开).Chemical constituents of Russula virescens.Chin Tradit Herb Drugs(中草药),2008,39:1776-1778.
14 Yang XL(杨小龙),Zhu YC(朱应成),Fang ST(方圣涛),et al.Chemical Constitutes of Termitomyces schimperi Collected from Africa.Nat Prod Res Dev(天然产物研究与开发),2010,22:972-975.
15 Yasunoria Y,Keiko A,Hiroyuki O,et al.Sterol constituents from five edible mushrooms.Chem Pharm Bull,1998,46:944-950.
16 Yasunori Y,Kaori M,Takeyoshi I,et al.New sterols and triterpenoids from four edible mushrooms.Chem Pharm Bull,2001,49:589-594.
