聚合物-盐双水相技术及研究进展
2014-01-06闫永胜
闫永胜,逯 洋,2,韩 娟,王 赟
(1.江苏大学 化学与化工学院,江苏 镇江 212013;2.吉林师范大学 计算机学院,吉林 四平 136000)
聚合物-盐双水相技术及研究进展
闫永胜1,逯 洋1,2,韩 娟1,王 赟1
(1.江苏大学 化学与化工学院,江苏 镇江 212013;2.吉林师范大学 计算机学院,吉林 四平 136000)
双水相萃取技术作为一种新型的绿色分离/富集技术,具有简单、省时、高效和绿色无污染等优点,已被应用于金属离子的定量分离萃取、生物活性物质的分离纯化以及天然产物的提取等领域.目前的双水相体系主要包括聚合物-聚合物双水相体系、聚合物-盐双水相体系、离子液体-盐双水相体系和小分子有机溶剂-盐双水相体系,但由于有机溶剂易挥发不稳定、离子液体成本较高和两种聚合物体系的粘度较大等问题,影响了这三种双水相体系在工业规模化生产中的应用.而聚合物-盐双水相体系用盐代替聚合物-聚合物双水相体系中的一种聚合物作为成相物质,在降低体系粘度和生产成本的同时,保留了聚合物生物相容性好的优势,被广泛应用于生物活性物质、天然产物及抗生素的分离纯化,具有较高的开发价值和广阔的应用前景.通过分析聚合物-盐双水相体系的理论及应用研究进展,希望对进一步的研究工作有所帮助和启发.
双水相体系;聚合物;盐
1 双水相体系的研究
液-液萃取[1](Liquid-Liquid Extraction,LLE)是根据目标物质在互不相溶的两种液体中的溶解度不同,从而实现在液体间选择性分配的一种分离技术.LLE作为一种传统的分离技术,由于操作容易、使用设备简单而被长期应用于化学化工领域的分离过程.但是,由于LLE技术存在有机溶剂消耗量大、污染环境和安全性低等缺点,已经不能满足当前国际社会对环境保护和绿色生产日趋重视的要求[2-4].双水相萃取(Aqueous Two Phase Extraciton,ATPE)作为一种新型的绿色分离纯化技术,在一定程度上克服了LLE的缺点,被广泛应用于食品化学、环境化学、医疗卫生和生物工程等领域的分离纯化环节.
1.1 概述
早在1896年,贝叶林克就发现在将琼脂水溶液和明胶(或可溶性淀粉)水溶液以一定比例混合时,会形成两相体系,即双水相现象.直到20世纪60年代,双水相体系(Aqueous Two Phase System,ATPS)才被逐步应用于物质的分离操作,关于ATPS的理论研究和应用研究才引起研究者们的关注.1956年,瑞典学者Albertsson成功地应用ATPS实现了叶绿素的分离纯化,开辟了ATPS在分离纯化过程的应用先河;随后,德国的Kula等人又利用ATPS对生物活性物质进行了分离纯化,并取得了成功.此后,ATPE因具有工艺简单、操作条件温和、绿色无毒等优势,被广泛应用于食品工程、环境科学、医药卫生和生物工程等领域,成功的实现了蛋白质[5,6]、酶[7,8]、多肽[9]、氨基酸[10,11]、遗传物质[12,13]、金属离子[14,15]、化工原料[16]、细胞[17]、细胞色素[18]以及抗生素[19-21]的分离纯化.
ATPS是指将两种可以互溶的物质相混合,当体系中两种物质的浓度达到(或超过)临界浓度以后,原来的均一相体系会分成互不相容的两相体系.ATPE的原理与传统的液-液萃取相似,都是根据物质在两种溶液中的溶解度不同而实现选择性分配,最终达到分离的目的.ATPS中的各目标组分,在范德华力、疏水作用、静电作用和界面张力的作用下,选择性富集到上相或下相,从而实现目标组分与杂质组分的选择性分离.
1.2 双水相体系的分类
早期的ATPS研究主要集中在聚合物-聚合物ATPS,随着ATPS研究的不断深入,一系列新型ATPS相继出现,并成功应用于各种生物活性物质的分离纯化.目前,按组成物质不同,ATPS可以分为:聚合物-聚合物ATPS、聚合物-盐ATPS、小分子有机溶剂-盐ATPS和离子液体-盐ATPS,如图1所示.各种ATPS在具有共同特性的同时还有各自的优势与不足,因此,在针对特定的目标物质选择ATPS时,应该深入研究各种体系的特点,综合考虑目标物性质、ATPS的特性、操作条件和成本等因素.

图1 双水相体系的分类
1.3 双水相萃取的特点
ATPE是一种在温和无污染的条件下,使用常见的简便设备,进行短时简单的操作,即能以较高回收率萃取得到高纯度目标产物的新型分离富集技术.与其它已报道的分离方法相比,ATPE具有以下特点:
(1)萃取条件温和,在常温常压下操作,且上、下两相的含水量大(含水量高达70%~90%),不易引起生物活性物质的失活或变性.
(2)体系所用设备简单,传统液-液萃取所用的混合、离心、分离等设备可直接应用于ATPE操作.
(3)两相界面张力小,与普通体系的界面张力103~10-2N·m-1相比,ATPS的界面张力仅为10-6~10-4N·m-1,此外,ATPS两相的密度差也很小,十分有利于物质的扩散,传质速度快.
(4)体系易于线性放大(理论上可放大104倍),各种参数按比例放大的同时,目标物的回收率并不降低,有利于其在工业生产中的应用.
(5)两种成相物质的种类和浓度、体系的温度和pH值等多种因素对目标产物在两相的分配比率都呈显著性影响,因此,可以通过调整各种实验参数达到最佳萃取效果.
(6)绿色无毒无污染.
1.4 影响双水相体系成相能力及物质分配行为的主要因素
双水相体系的成相能力和目标物质在其中的分配行为受诸多因素的影响,目前的研究主要集中在讨论成相聚合物(离子液体、小分子有机溶剂)的类型和浓度、成相盐的类型和浓度、体系的pH值以及温度等因素对体系成相能力和目标物质的分配行为的影响.在对某一种具体目标物质进行分离、富集时,需要根据目标物的性质选用合适的ATPS,并对影响分配系数和萃取效率的各因素进行优化实验,才能确定理想的萃取条件.
(1)成相物质的影响
同一种目标物在不同类型的ATPS中具有不同的分配行为.对于聚合物ATPS,成相聚合物的相对分子量和其在体系中的浓度是影响物质在两相间分配的重要因素.同一种聚合物的疏水性随其相对分子量的增大而增强,物质的分配系数也会随之发生变化.对于离子液体ATPS,离子液体的阳离子烷基链的增长会导致其疏水性增强,物质的分配系数也会随之变化.
(2)盐的影响
成相盐的类型和浓度都会对ATPS的相平衡条件和物质在两相间的分配产生影响.具有相同阴(或阳)离子的盐,其成相能力与阳(或阴)离子的化合价、吉布斯自由能和有效排除体积均有关.盐的类型对被萃物质的分配系数具有重要影响,即使是对于结构相近的目标物,盐的影响效果也不尽相同.
(3)pH值的影响
对于相同的ATPS,pH值的变化会引起体系中目标组分和杂质组分的电性改变,亦会导致被萃物质的电荷发生变化,进而影响各组分在两相体系中的分配行为.
(4)温度的影响
温度对ATPS相平衡和物质分配系数的影响取决于ATPS的类型,目前的研究显示,温度对聚合物-聚合物ATPS、聚合物-盐ATPS、离子液体-盐ATPS的影响较为明显,而对小分子有机溶剂-盐ATPS的影响很微弱,这可能与聚合物、离子液体和小分子有机溶剂的疏水性随温度的变化强弱有关.
2 聚合物-盐双水相体系的研究
ATPS早期的研究主要集中在聚合物-聚合物ATPS,但由于使用两种聚合物不但成本较高而且体系粘度较大,限制了该技术在工业上的应用.为了解决这一问题,研究者们考虑用盐代替一种聚合物作为成相物质,提出了较为廉价且高效的聚合物-盐ATPS.与聚合物-聚合物ATPS相比,聚合物-盐ATPS成本较低,体系粘度小,已被广泛应用于物质的萃取分离过程.
2.1 聚合物-盐双水相体系
自聚合物-盐ATPS问世以来,许多研究者致力于寻找新型的聚合物-盐ATPS和完善目前已知体系的双节线数据和系线数据;探索ATPS的液-液相平衡性质,研究相平衡理论;建立经验或半经验拟合方程,构建ATPS分相过程的热力学模型,为聚合物-盐ATPS的深入发展提供基础数据支撑和理论依据.测定ATPS的基础实验数据,建立系统、准确、完整的相图数据库是关联实验数据、建立经验模型、研究分相机理、构建热力学模型、设计萃取体系的基础和前提.ATPS的双节线数据一般是通过浊点滴定法测得;系线数据可以通过实验测得,也可以利用“杠杆原则”结合双节线最优拟合公式计算得到.目前,聚合物含量的测定一般使用折光率法或紫外可见分光光度法;盐的含量的测定方法则有很多,常用的有原子吸收法、电导率法、滴定法和密度法等.迄今为止,文献报道的聚合物-盐ATPS的相图数据研究列于表1.从表中可以看出,几乎所有的PEG-盐ATPS都有文献报道,已形成一个完整的理论数据体系;而对于其他聚合物,ATPS的基础数据还不够完整.因此,完善目前已知体系的相图数据和构建新的聚合物-盐ATPS对于ATPS的发展具有一定的实际意义.

表1 聚合物-盐双水相体系的相图研究

表1(续)
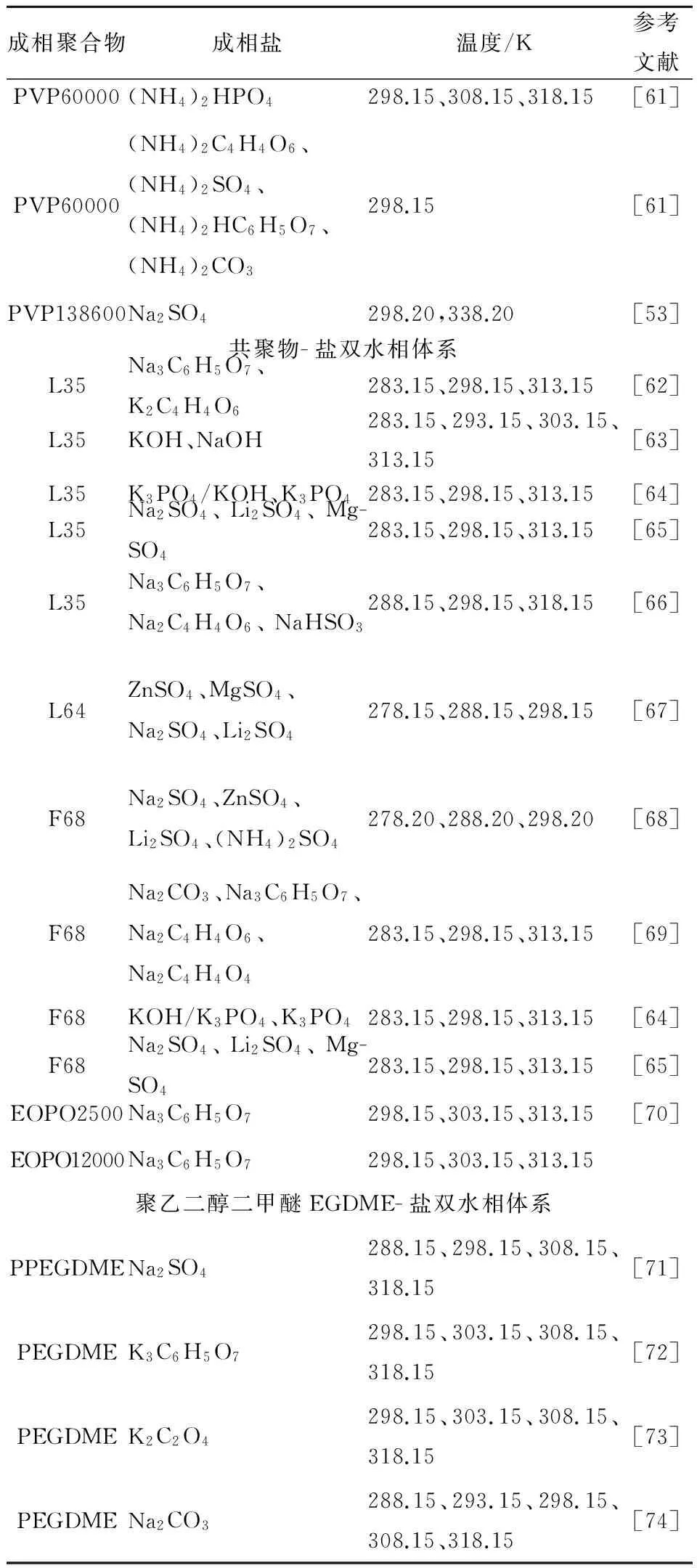
表1(续)
2.2 聚合物-盐双水相体系应用
2.2.1 金属离子的分离
1984年,Zvarova等[75]基于PEG-盐ATPS,成功地实现了Fe(Ⅲ)和Cu(Ⅱ)等金属离子的分离富集,自此ATPS被逐步应用于金属离子的分离过程.近年来,国内外关于利用聚合物-盐ATPS分离金属离子的研究报道见表2所示.研究主要是以不同的显色剂为萃取剂与目标金属离子形成络合物或离子缔合物,该络合物或离子缔合物因不溶于水而富集在ATPS的聚合物上相,从而实现了金属离子的分离富集.聚合物-盐ATPS在金属离子分离过程的应用为金属离子的分离回收开辟了新的思路.

表2 聚合物-盐双水相萃取技术在金属离子分离中的应用
2.2.2 生物分子的分离
1956年,瑞典学者Albertson首次将ATPE技术应用于生物分子的分离纯化,为分离蛋白质等生物活性物质提供了新的技术手段.由于聚合物-盐ATPE技术操作简单、条件温和、富集倍数高、可控因素多,已被广泛应用于蛋白质、核酸、生物酶等物质的分离纯化(表3),并取得了显著成效.
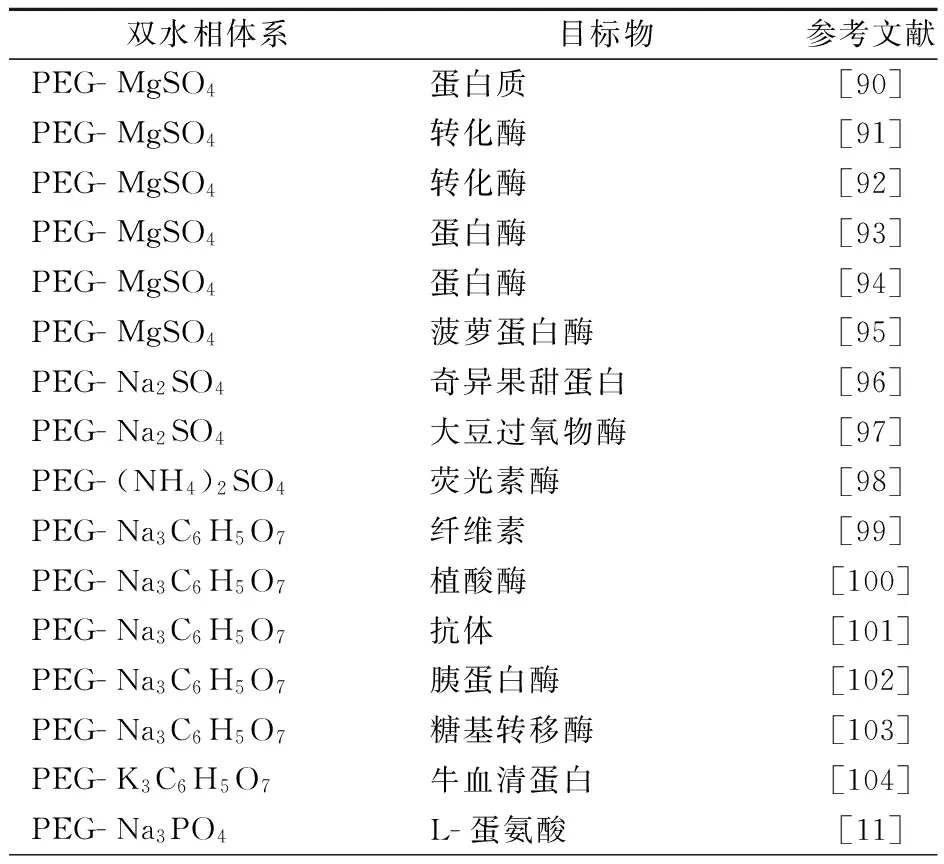
表3 聚合物-盐双水相萃取技术在生物物质分离中的应用
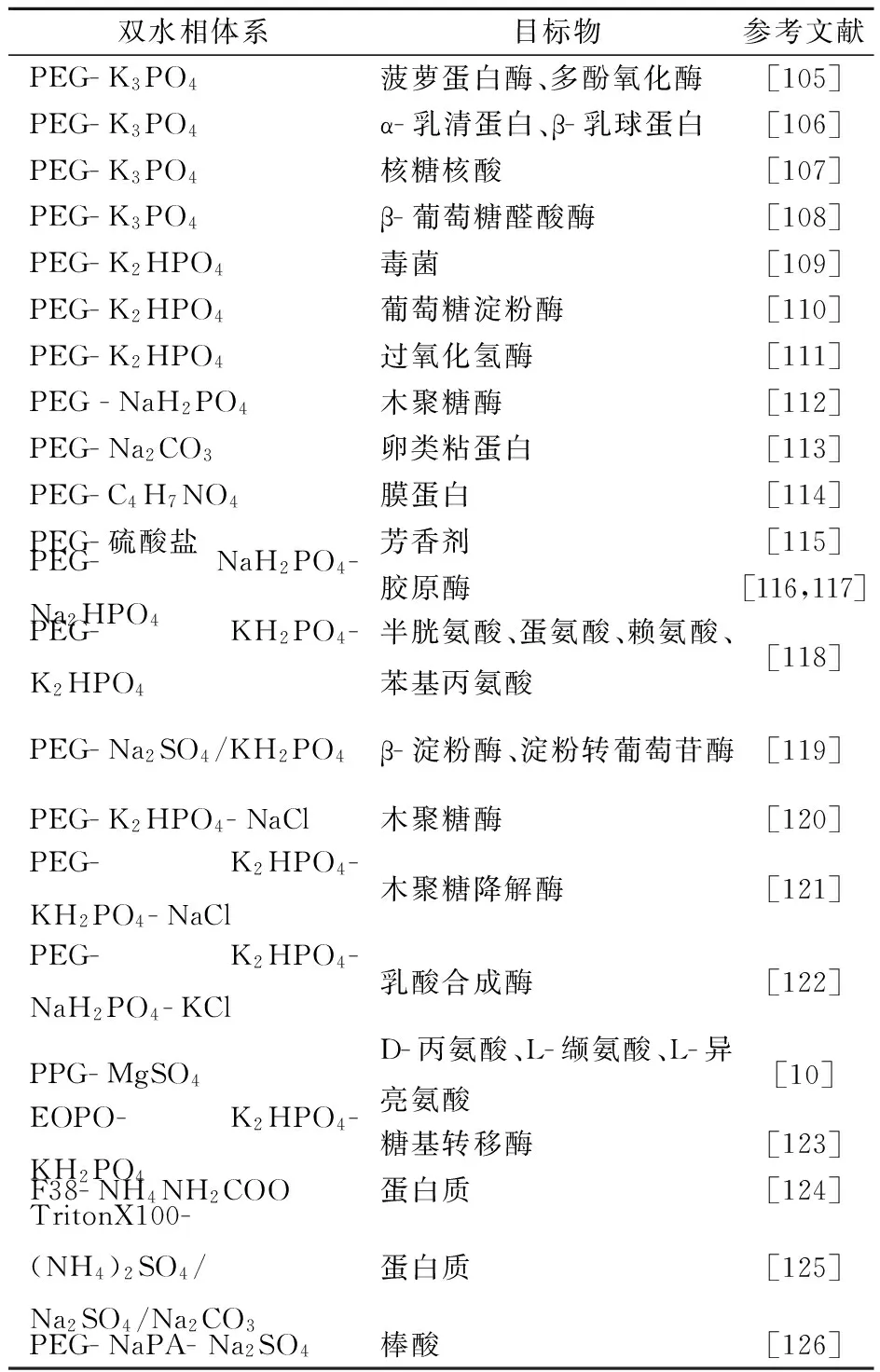
表3(续)
2.2.3 天然产物和中草药有效成分的提取
近年来,ATPE技术作为一种新型的分离技术已经成功的应用于甘草甜素、罂栗碱、培他兰、花青素、植物血凝素和黄酮等多种天然产物的分离提取(表1.6).我国是中草药的发源地,中草药亦是我国的国药,是我国医药学的一个重要而独特的组成部分.但是,由于中草药成分复杂,甚至含有有毒成分,因此必须定向提取、浓缩药材中的某一种或多种有效成分,以提升中成药质量和临床疗效.近几年,国内涌现出大量关于ATPE技术分离纯化中草药的研究和报道,为中草药的提取纯化提供了新的技术方法(表4).

表4 聚合物-盐双水相萃取技术在天然产物和中草药分离中的应用
2.2.4 抗生素的分离提取
抗生素主要是通过生物合成手段得到的,目标产物在转化液中的含量较低,对酸、碱、有机溶剂和温度变化较为敏感,而且容易失活或降解,因此缺乏一种合适的分离与纯化技术已经成为制约抗生素高效、快速生产的“瓶颈”.近年来,研究者们已成功地利用ATPS提取了包括青霉素、头孢菌素、红霉素、四环素等在内的多种抗生素(见表5).ATPE技术能够直接从发酵液中提取抗生素,实现了反应和提取的同步进行,简化了工艺流程,加快了生产过程,提高了生产效率;整个操作过程在常温常压下完成,操作条件温和,能够保持抗生素的分子活性;萃取体系安全、无毒、无有机溶剂残留,不会危害工作人员健康和对环境造成二次污染.

表5 聚合物-盐双水相萃取技术在抗生素分离中的应用
a cholinium-based salt:cholinium chloride,[Ch]Cl; cholinium bicarbonate,[Ch]Bic; cholinium dihydrogencitrate,[Ch]DHcit; cholinium acetate,[Ch]Ac and cholinium dihydrogenphosphate,[Ch]DHph.
3 双水相体系研究的发展趋势
ATPS作为一种新型的绿色萃取分离技术,在具有高效、简单等优势的同时,也存在着一定的不足.未来,ATPS的理论和应用研究工作主要集中在以下几个方面:
(1)完善基础数据,对现有的ATPS,系统的补充实验数据,为ATPS的应用提供数据支持.
(2)扩展ATPS,对可能的成相物质进行特性分析,寻找新的成相物质,扩展体系范围,为ATPS的应用提供新的体系选择.
(3)深化理论研究,建立热力学、动力学模型,为ATPS的应用发展提供理论支撑.
(4)引入智能成相物质(温敏、光敏或pH敏材料),在循环利用成相物质的同时,易于实现目标物与成相物质间的后续分离.
(4)与生物转化结合,利用ATPS及时移走产物,以促进生化反应和增加产率,实现生化反应与转移、分离、纯化同步完成.
(5)与其他技术的集成,弥补单一技术的不足,实现不同技术之间的相互渗透、相互融合和优势互补,为ATPS技术注入新的活力.
[1]C.M.S.S. Neves,S.P.M. Ventura,M.G.Freire,et al.Evaluation of Cation Influence on the Formation and Extraction Capability of Ionic-Liquid-Based Aqueous Biphasic Systems[J].J.Phys.Chem.B,2009,113(15):5194~5199.
[2]J.Pawliszyn.New directions in sample preparation for analysis of organic compounds[J].Trends Anal.Chem.,1995,14(3):113~122.
[3]F.Hernandez,I.Morell,J.Beltran,et al.Multiresidue procedure for the analysis of pesticides in groundwater-application to samples from the comunidad-valenciana,spain[J].Chromatographia,1993,37(5-6):303~312.
[4]A.Balinova.Strategies for chromatographic analysis of pesticide residues in water[J].J.Chromatogr.A,1996,754(1-2):125~135.
[5]J.C. Salgado,B.A.Andrews,M.F.Ortuzar,et al.Prediction of the partitioning behaviour of proteins in aqueous two-phase systems using only their amino acid composition[J].J.Chromatogr.A,2008,1178(1-2):134~144.
[6]H.O.Johansson,F.M.Magaldi,E.Feitosa,et al.Protein partitioning in poly(ethylene glycol)/sodium polyacrylate aqueous two-phase systems[J].J.Chromatogr.A,2008,1178(1-2):145~153.
[7]W.B.Zhi,J.N.Song,F.Ouyang,et al.Application of response surface methodology to the modeling of [alpha]-amylase purification by aqueous two-phase systems[J].J.Biotechnol.,2005,118(2):157~165.
[8]G.Bassani,B.Farruggia,B.Nerli,et al.Porcine pancreatic lipase partition in potassium phosphate-polyethylene glycol aqueous two-phase systems[J].J.Chromatogr.B,2007,859(2):222~228.
[9]C.A.S. Da Silva,J.S.R Coimbra,E.E.G.Rojas,et al.Partitioning of caseinomacropeptide in aqueous two-phase systems[J].J.Chromatogr.B,2007,858(1-2):205~210.
[10]A.Salabat,M.H.Abnosi,A.R.Bahar.Amino acids partitioning in aqueous two-phase system of polypropylene glycol and magnesium sulfate[J].J.Chromatogr.B,2007,858(1-2):234~238.
[11]A.Salabat,R.Sadeghi,S.T.Moghadam,et al.Partitioning of L-methionine in aqueous two-phase systems containing poly(propylene glycol) and sodium phosphate salts[J].J.Chem.Thermodyn.,2011,43(10):1525~1529.
[12]I.P.Trindade,M.M.Diogo,D.M.F.Prazeres,et al.Purification of plasmid DNA vectors by aqueous two-phase extraction and hydrophobic interaction chromatography[J].J.Chromatogr.A,2005,1082(2):176~184.
[13]H.S.Barbosa,A.V.Hine,S.Brocchini,et al.Dual affinity method for plasmid DNA purification in aqueous two-phase systems[J].J.Chromatogr.A,2010,1217(9):1429~1436.
[14]M.R.Helfrich,M.El-Kouedi,M.R.Etherton,et al.Partitioning and assembly of metal particles and their bioconjugates in aqueous two-phase systems[J].Langmuir,2005,21(18):8478~8486.
[15]L.Bulgariu,D.Bulgariu.Extraction of gold(Ⅲ) from chloride media in aqueous polyethylene glycol-based two-phase system[J].Sep.Purif.Technol.,2011,80(3):620~625.
[16]Z.G.Li,H.Teng,Z.L.Xiu.Extraction of 1,3-propanediol from glycerol-based fermentation broths with methanol/phosphate aqueous two-phase system[J].Process Biochem.,2011,46(2):586~591.
[17]M.Kamihira,A.Kumar.Development of separation technique for stem cells[J].Adv.Biochem.Eng.Biotechnol.,2007,106:173~193.
[18]Y.M.Lu,W.J.Lu,W.Wang,et al.Thermodynamic studies of partitioning behavior of cytochrome c in ionic liquid-based aqueous two-phase system[J].Talanta,2011,85(3):1621~1626.
[19]B.Mokhtarani,R.Karimzadeh,M.H.Amini,et al.Partitioning of ciprofloxacin in aqueous two-phase system of poly(ethylene glycol) and sodium sulphate[J].Biochem.Eng.J.,2008,38(2):241~247.
[20]Y.Y.Jiang,H.S.Xia,J.Yu,et al.Hydrophobic ionic liquids-assisted polymer recovery during penicillin extraction in aqueous two-phase system[J].Chem.Eng.J.,2009,147(1):22~26.
[21]H.Li,X.J.Cao.Bioconversion of cephalosporin-G to 7-ADCA in a pH-thermo sensitive recycling aqueous two-phase systems[J].Process Biochem.,2011,46(9):1753~1758.
[22]E.C.De Souza,R.S.Diniz,J.S.Dos Reis Coimbra,et al.Measurements and Modeling of Liquid-Liquid Equilibrium of Polyethylene Glycol 400,Sodium Phosphate,or Sodium Citrate Aqueous Two-Phase Systems at (298.2,308.2,and 318.2) K[J].J.Chem.Eng.Data,2013,58(7):2008~2017.
[23]L.P.Malpiedi,C.Fernández,G.Picó,et al.Liquid-liquid equilibrium phase diagrams of polyethyleneglycol + sodium tartrate + water two-phase systems[J].J.Chem.Eng.Data,2008,53:1175~1178.
[24]J.G.Huddleston,H.D.Willauer,R.D.Rogers.Phase diagram data for several PEG + salt aqueous biphasic systems at 25℃[J].J.Chem.Eng.Data,2003,48:1230~1236.
[25]P.Gonzalez-Tello,F.Camacho,G.Blazquez,et al.Liquid-liquid equilibrium in the system poly(ethylene glycol) + MgSO4+ H2O at 298 K[J].J.Chem.Eng.Data,1996,41(6):1333~1336.
[26]W.L.Zhang,Y.T.Hu,Y.Wang,et al.Liquid-liquid equilibrium of aqueous two-phase systems containing poly(ethylene glycol) of different molecular weights and several ammonium salts at 298.15K[J].Thermochim.Acta,2013,560:47~54.
[27]R.S.Diniz,E.C.Souza,J.S.R.Coimbra,et al.Liquid-liquid equilibria of aqueous two-phase systems containing sodium hydroxide plus poly(ethylene glycol) of (1450,4000,or 10 000) g.mol(-1) at (288.2,298.2,and 308.2) K[J].J.Chem.Eng.Data,2012,57(2):280~283.
[28]B.G.Alvarenga,L.S.Virtuoso,N.H.T.Lemes,et al.Measurement and correlation of the phase equilibrium of aqueous two-phase systems composed of polyethylene(glycol) 1500 or 4000 + sodium sulfite + water at different temperatures[J].J.Chem.Eng.Data,2014,59(2):382~390.
[29]T.A.Graber,H.Medina,H.R.Galleguillos,et al.Phase equilibrium and partition of iodide in an aqueous biphasic system formed by (NH4)2SO4+ PEG + H2O at 25℃[J].J.Chem.Eng.Data,2007,52:1262~1267.
[30]R.Govindarajan,M.Perumalsamy.Phase equilibrium of PEG 2000 + triammonium citrate + water system relating PEG molecular weight,cation,anion with effective excluded volume,gibbs free energy of hydration,size of cation,and type of anion at (298.15,308.15,and 318.15) K[J].J.Chem.Eng.Data,2013,58(11):2952~2958.
[31]T.Murugesan,M.Perumalsamy.Liquid-liquid equilibria of poly(ethylene glycol) 2000 + sodium citrate + water at (25,30,35,40,and 45) ℃[J].J.Chem.Eng.Data,2005,50(4):1392~1395.
[32]R.M.De Oliveira,J.S.d.R.Coimbra,K.R.Francisco,et al.Liquid-liquid equilibrium of aqueous two-phase systems containing poly(ethylene) glycol 4000 and zinc sulfate at different temperatures[J].J.Chem.Eng.Data,2008,53(4):919~922.
[33]M.E.Taboada,O.A.Rocha,T.A.Graber,et al.Liquid-liquid and solid-liquid equilibria of the poly(ethylene glycol)+sodium sulfate+water system at 298.15 K[J].J.Chem.Eng.Data,2001,46(2):308~311.
[34]S.P.Amaresh,S.Murugesan,I.Regupathi,et al.Liquid-liquid equilibrium of poly(ethylene glycol) 4000 + diammonium hydrogen phosphate + water at different temperatures[J].J.Chem.Eng.Data,2008,53(7):1574~1578.
[35]T.A.Graber,M.E.Galvez,H.R.Galleguillos.Liquid-liquid equilibrium of the aqueous two-phase system water + PEG 4000 + lithium sulfate at different temperatures:experimental determination and correlation[J].J.Chem.Eng.Data,2004,49(6):1661~1664.
[36]Y.Liu,Y.Q.Feng,Y.J.Zhao.Liquid-liquid equilibrium of various aqueous two-phase systems:experiment and correlation[J].J.Chem.Eng.Data,2013,58(10):2775~2784.
[37]Y.P.Jimenez,H.R.Galleguillos.(Liquid+liquid) equilibrium of (NaNO3+PEG 4000+H2O) ternary system at different temperatures[J].J.Chem.Thermodyn.,2011,43(11):1573~1578.
[38]M.T.Zafarani-Moattar,S.Tolouei.Liquid-liquid equilibria of aqueous two-phase systems containing polyethylene glycol 4000 and dipotassium tartrate,potassium sodium tartrate,or di-potassium oxalate:Experiment and correlation[J].CALPHAD,2008,32(4):655~660.
[39]M.T.Zafarani-Moattar,R.Sadeghi.Phase behavior of aqueous two-phase PEG+NaOH system at different temperatures[J].J.Chem.Eng.Data,2004,49(2):297~300.
[40]M.T.Zafarani-Moattar,A.A.Hamidi.Liquid-liquid equilibria of aqueous two-phase poly(ethylene glycol)-potassium citrate system[J].J.Chem.Eng.Data,2003,48(2):262~265.
[41]M.T.Zafarani-Moattar,S.Hamzehzadeh.Liquid-liquid equilibria of aqueous two-phase systems containing polyethylene glycol and sodium succinate or sodium formate[J].CALPHAD,2005,29(1):1~6.
[42]R.Sadeghi,R.Golabiazar.Thermodynamics of phase equilibria of aqueous poly(ethylene glycol) + sodium tungstate two-phase systems[J].J.Chem.Eng.Data,2010,55(1):74~79.
[43]E.V.C.Cunha,M.n.Aznar.Liquid-liquid equilibrium in aqueous two-phase (water + PEG 8000 + salt):experimental determination and thermodynamic modeling[J].J.Chem.Eng.Data,2009,54(12):3242~3246.
[44]M.T.Zafarani-Moattar,R.Sadeghi.Phase diagram data for several PPG + salt aqueous biphasic systems at 25℃[J].J.Chem.Eng.Data,2005,48(5):947~950.
[45]X.H.Zhao,X.Q.Xie,Y.S.Yan.Liquid-liquid equilibrium of aqueous two-phase systems containing poly(propylene glycol) and salt ((NH4)2SO4,MgSO4,KCl,and KAc):experiment and correlation[J].Thermochim.Acta,2011,516(1-2):46~51.
[46]M.T.Zafarani-Moattar,S.Emamian,S.Hamzehzadeh.Effect of temperature on the phase equilibrium of the aqueous two-phase poly(propylene glycol) + tripotassium citrate system[J].J.Chem.Eng.Data,2008,53(2):456~461.
[47]R.Sadeghi,B.Jamehbozorg.The salting-out effect and phase separation in aqueous solutions of sodium phosphate salts and poly(propylene glycol)[J].Fluid Phase Equilib.,2009,280(1):68~75.
[48]R.Sadeghi,B.Jamehbozorg.Effect of temperature on the salting-out effect and phase separation in aqueous solutions of sodium di-hydrogen phosphate and poly(propylene glycol)[J].Fluid Phase Equilib.,2008,271(1-2):13~18.
[49]A.Salabat,H.Dashti.Phase compositions,viscosities and densities of systems PPG425+Na2SO4+H2O and PPG425+(NH4)2SO4+ H2O at 298.15 K[J].Fluid Phase Equilib.,2004,216(1):153~157.
[50]E.L.Cheluget,S.Gelinas,J.H.Vera ,et al.Liquid-liquid equilibrium of aqueous mixtures of poly(propylene glycol) with NaCl[J].J.Chem.Eng.Data,1994,39(1):127~130.
[51]A.Salabat,L.Shamshiri,J.J.Sardrodi.Liquid-liquid equilibrium data,viscosities,and densities of.aqueous mixtures of poly(propylene glycol) with trisodium citrate at 298.15 K[J].J.Chem.Eng.Data,2005,50(1):154~156.
[52]M-K.Shahbazinasab,F.Rahimpour.Liquid-liquid equilibrium data for aqueous two-phase systems containing PPG725 and salts at various pH values[J].J.Chem.Eng.Data,2012,57(7):1867~1874.
[53]N.Fedicheva,L.Ninni,G.Maurer.Aqueous two-phase systems of poly(vinyl pyrrolidone)and sodium sulfate:experimental results and correlation/prediction[J].J.Chem.Eng.Data,2007,52(5):1858~1865.
[54]M.T.Zafarani-Moattar,A.Zaferanloo.Measurement and correlation of phase equilibria in aqueous two-phase systems containing polyvinylpyrrolidone and di-potassium tartrate or di-potassium oxalate at different temperatures[J].J.Chem.Thermodyn.,2009,41(7):864~871.
[55]M.T.Zafarani-Moattar,P Seifi-Aghjekohal.Liquid-liquid equilibria of aqueous two-phase systems containing polyvinylpyrrolidone and tripotassium phosphate or dipotassium hydrogen phosphate:Experiment and correlation.[J].CALPHAD,2007,31(4):553~559.
[56]R.Sadeghi,H R.Rafieia,M.Motamedia.Phase equilibrium in aqueous two-phase systems containing poly(vinylpyrrolidone) and sodium citrate at different temperatures-experimental and modeling[J].Thermochim.Acta,2006,451(1-2):163~167.
[57]R.Sadeghi.Aqueous two-phase systems of poly(vinylpyrrolidone) and potassium citrate at different temperatures--Experimental results and modeling of liquid-liquid equilibrium data[J].Fluid Phase Equilib.,2006,246(1-2):89~95.
[58]M.T.Zafarani-Moattara,R.Sadeghib.Effect of temperature on the phase equilibrium of aqueous two-phase systems containing polyvinylpyrrolidone and disodium hydrogen phosphate or trisodium phosphate[J].Fluid Phase Equilib.,2005,238(1):129~135.
[59]R.Sadeghi.Measurement and correlation of phase equilibria for several PVP+salt aqueous two-phase systems at 303.15K[J].Fluid Phase Equilib.,2005,237(1-2):40~47.
[60]M.Foroutan.Liquid-Liquid equilibria of aqueous two-phase poly(vinylpyrrolidone) and K2HPO4/KH2PO4buffer:effects of pH and temperature[J].J.Chem.Eng.Data,2007,52(3):859~862.
[61]Y.Wang,Y.C.Wu,L.Ni,et al.Liquid-liquid equilibria of polyvinylpyrrolidone + several ammonium salts + water aqueous two-phase systems:experimental and correlation[J].J.Chem.Eng.Data,2012,57(11):3128~3135.
[62]J.P.Martins,A.B.Mageste,M.d.C.H.da Silva,et al.Liquid-liquid equilibria of an aqueous two-phase system formed by a triblock copolymer and sodium salts at different temperatures[J].J.Chem.Eng.Data,2009,54(10):2891~2894.
[63]J.P.Martins,M.d.C.H.da Silva,L.H.M.da Silva,et al.Liquid-liquid phase equilibrium of triblock copolymer F68,poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide),with sulfate salts[J].J.Chem.Eng.Data,2010,55(4):1618~1622.
[64]L.H.M.Da Silva,M.d.C.H.Da Silva,A.F.Mesquita,et al.Equilibrium phase behavior of triblock copolymer + salt + water two-phase systems at different temperatures and pH[J].J.Chem.Eng.Data,2005,50(4):1457~1461.
[65]M.D.H.Da Silva,L.H.M.Da Silva,J.Amim,et al.Liquid-liquid equilibrium of aqueous mixture of triblock copolymers L35 and F68 with Na2SO4,Li2SO4,or MgSO4[J].J.Chem.Eng.Data,2006,51(6):2260~2264.
[66]L.S.Virtuoso,K.A.S.F.Vello,A.A.de Oliveira,et al.Measurement and modeling of phase equilibrium in aqueous two-phase systems:L35 + sodium citrate + water,L35 sodium tartrate +water,and L35 + sodium hydrogen sulfite + water at different temperatures[J].J.Chem.Eng.Data,2012,57(2):462~468.
[67]G.D.Rodrigues,M.d.C.H.da Silva,L.H.M.da Silva,et al.Liquid-liquid phase equilibrium of triblock copolymer L64,poly(ethylene oxide-b-propylene oxide-b-ethylene oxide),with sulfate salts from (278.15 to 298.15) K[J].J.Chem.Eng.Data,2009,54(6):1894~1898.
[68]L.S.Virtuoso,L.M.d.S.Silva,B.S.Malaquias,et al.Equilibrium phase behavior of triblock copolymer + sodium or + potassium hydroxides + water two-phase systems at different temperatures[J].J.Chem.Eng.Data,2010,55(9):3847~3852.
[69]G.D.Rodrigues,L.d.S.Teixeira,G.M.D.Ferreira,et al.Phase diagrams of aqueous two-phase systems with organic salts and F68 triblock copolymer at different temperatures[J].J.Chem.Eng.Data,2010,55(3):1158~1165.
[70]R.Ghahremani,F.Rahimpour.Equilibrium phase behavior of aqueous two-phase systems containing ethylene oxide-propylene oxide of different molecular weight (2500,12000) and sodium citrate salt at various temperatures and pH[J].J.Chem.Eng.Data,2014,59(2):218~224.
[71]M.T.Zafarani-Moattar,D.Nikjoo.Liquid-liquid and liquid-liquid-solid equilibrium of the poly(ethylene glycol) dimethyl ether 2000 + sodium sulfate + water system[J].J.Chem.Eng.Data,2008,53(11):2666~2670.
[72]M.T.Zafarani-Moattar,V.Hosseinpour-Hashemi.(Liquid+liquid) equilibrium of the ternary aqueous system containing poly ethylene glycol dimethyl ether 2000 and tri-potassium citrate at different temperatures[J].J.Chem.Thermodyn.,2012,48(57):75~83.
[73]M.T.Zafarani-Moattar,V.Hosseinpour-Hashemi.Effect of temperature on the aqueous two-phase system containing poly(ethylene glycol) dimethyl ether 2000 and dipotassium oxalate[J].J.Chem.Eng.Data,2012,57(2):532~540.
[74]M.T.Zafarani-Moattar,D.Nikjoo.Phase diagrams for liquid-liquid and liquid-solid equilibrium of the ternary poly(ethylene glycol) dimethyl ether 2000 + sodium carbonate + water system[J].J.Chem.Eng.Data,2009,54(10):2918~2922.
[75]T.I.Zvarova,V.M.Shkinev,G.A.Vorob'eva,et al.Liquid-Liquid Extraction in the Absence of Usual Organic Solvents:Application of Two-Phase Aqueous Systems Based on a Water-Soluble Polymer[J].Mikrochimica Acta,1984,84(3):449~458.
[76]M.Shibukawa,N.Nakayama,T.Hayashi,et al.Extraction behaviour of metal ions in aqueous polyethylene glycol-sodium sulphate two-phase systems in the presence of iodide and thiocyanate ions[J].Anal.Chim.Acta,2001,427:293~300.
[77]L.Bulgariu,D.Bulgariu.Selective extraction of Hg(Ⅱ),Cd(Ⅱ) and Zn(Ⅱ) ions from aqueous media by a green chemistry procedure using aqueous two-phase systems[J].Sep.Purif.Technol.,2013,118:209~216.
[78]L.Bulgariu,D.Bulgariu.Extraction of metal ions in aqueous polyethylene glycol-inorganic salt two-phase systems in the presence of inorganic extractants:correlation between extraction behaviour and stability constants of extracted species[J].J.Chromatogr.A,2008,1196-1197:117~124.
[79]黄振钟,吴欣欣,陈莉莉等.PEG-(NH4)2SO4-8Q5SAC双水相分光光度法测定工业废水中的微量铜[J].江西师范大学学报,2006,30(2):145~147.
[80]蔡红,许秀丽,朱军等.偶氮胂Ⅰ双水相萃取分离钍、铀、镧、钒、钼的研究[J].稀有金属,2003,27(2):265~267.
[81]邓凡政,魏迎,陈影等.双水相体系中Cu(Ⅱ),La(Ⅲ),U(VI),Ce(IV)光谱行为及萃取分离[J].光谱学与光谱分析,2004,24(12):1637~1639.
[82]王碧,阮尚全,覃松等.聚乙二醇-硫酸铵体系双水相萃取光度法测定钯[J].四川大学学报,2003,40(1):108~111.
[83]陈玉焕,卢雁,张秀英等.铟在聚乙二醇-硫酸铵双水相体系中的分配[J].化学通报,2003,66(1):63~66.
[84]谢治民,方正军,李勇等.聚乙二醇-硫酸铵双水相萃取分离测定铬(VI)的研究[J].湖南工程学院学报,2008,18(4):69~72.
[85]练萍,李 蕾,薛珺.PEG600-PVP-(NH4)2SO4-H2O双水相体系萃取Cu2+[J].赣南师范学院学报,2003,3:49~50.
[86]R.Patricio Pda,M.C.Mesquita,L.H.da Silva,et al.Application of aqueous two-phase systems for the development of a new method of cobalt(Ⅱ),iron(Ⅲ) and nickel(Ⅱ) extraction:a green chemistry approach[J].J.Hazard.Mater.,2011,193:311~318.
[87]G.D.Rodrigues,M.d.C.H.da Silva,L.H.M.da Silva,et al.Liquid-liquid extraction of metal ions without use of organic solvent[J].Sep.Purif.Technol.,2008,62(3):687~693.
[88]L.R.Lemos,I.J.Santos,G.D.Rodrigues,et al.Copper recovery from ore by liquid-liquid extraction using aqueous two-phase system[J].J.Hazard.Mater.,2012,237-238:209~214.
[89]G.D.Rodrigues,L.R.de Lemos,L.H.da Silva,et al.Application of hydrophobic extractant in aqueous two-phase systems for selective extraction of cobalt,nickel and cadmium[J].J.Chromatogr.A,2013,1279:13~19.
[90]S.Saravanan,J.R.Rao,T.Murugesan,et al.Partition of tannery wastewater proteins in aqueous two-phase poly (ethylene glycol)-magnesium sulfate systems:Effects of molecular weights and pH[J].Chem.Eng.Sci.,2007,62(4):969~978.
[91]T.Karkaʂ ,S.Önal.Characteristics of invertase partitioned in poly(ethylene glycol)/magnesium sulfate aqueous two-phase system[J].Biochem.Eng.J.,2012,60:142~150.
[92]M.C.Madhusudhan,K.S.M.S.Raghavarao.Aqueous two phase extraction of invertase from baker's yeast:Effect of process parameters on partitioning[J].Process Biochem.,2011,46(10):2014~2020.
[93]P.Chaiwut,S.Rawdkuen,S.Benjakul.Extraction of protease from Calotropis procera latex by polyethylene glycol-salts biphasic system[J].Process Biochem.,2010,45(7):1148~1155.
[94]S.Rawdkuen,P.Pintathong,P.Chaiwut,et al.The partitioning of protease from Calotropis procera latex by aqueous two-phase systems and its hydrolytic pattern on muscle proteins[J].Food Bioprod.Process.,2011,89(1):73~80.
[95]S.Ketnawa,S.Rawdkuen,P.Chaiwut.Two phase partitioning and collagen hydrolysis of bromelain from pineapple peel Nang Lae cultivar[J].Biochem.Eng.J.,2010,52(2-3):205~211.
[96]A.L.Ahmad,C.J.C.Derek,M.M.D.Zulkali.Optimization of thaumatin extraction by aqueous two-phase system (ATPS) using response surface methodology (RSM)[J].Sep.Purif.Technol.,2008,62(3):702~708.
[97]M.E.d.Silva,T.T.Franco.Purification of soybean peroxidase (Glycine max) by metal affinity partitioning in aqueous two-phase systems[J].J.Chromatogr.B,2000,743:287~294.
[98]B.S.Priyanka,N.K.Rastogi,K.S.M.S.Raghavarao,et al.Optimization of extraction of luciferase from fireflies (Photinus pyralis) using aqueous two-phase extraction[J].Sep.Purif.Technol.,2013,118:40~48.
[99]P.N.Herculano,T.S.Porto,M.H.C.Maciel,et al.Partitioning and purification of the cellulolytic complex produced by Aspergillus japonicus URM5620 using PEG-citrate in an aqueous two-phase system[J].Fluid Phase Equilib.,2012,335:8~13.
[100]K.Bhavsar,V.Ravi Kumar,J.M.Khire.Downstream processing of extracellular phytase from Aspergillus niger:Chromatography process vs.aqueous two phase extraction for its simultaneous partitioning and purification[J].Process Biochem.,2012,47(7):1066~1072.
[101]A.M.Azevedo,A.G.Gomes,P.A.J.Rosa,et al.Partitioning of human antibodies in polyethylene glycol-sodium citrate aqueous two-phase systems[J].Sep.Purif.Technol.,2009,65(1):14~21.
[102]L.P.Malpiedi,D.Romanini,G.A.Picó,et al.Purification of trypsinogen from bovine pancreas by combining aqueous two-phase partitioning and precipitation with charged flexible chain polymers[J].Sep.Purif.Technol.,2009,65(1):40~45.
[103]H.S.Ng,C.P.Tan,S.K.Chen,et al.Primary capture of cyclodextrin glycosyltransferase derived from Bacillus cereus by aqueous two phase system[J].Sep.Purif.Technol.,2011,81(3):318~324.
[104]Y.M.Lu,Y.Z.Yang,X.D.Zhao,et al.Bovine serum albumin partitioning in polyethylene glycol (PEG)/potassium citrate aqueous two-phase systems[J].Food Bioprod.Process.,2010,88(1):40~46.
[105]B.R.Babu,N.K.Rastogi,K.S.M.S.Raghavarao.Liquid-liquid extraction of bromelain and polyphenol oxidase using aqueous two-phase system[J].Chem.Eng.Process.,2008,47(1):83~89.
[106]J.G.L.F.Alves,L.D.A.Chumpitaz,L.H.M.d.Silva,et al.Partitioning of whey proteins,bovine serum albumin and porcine insulin in aqueous two-phase systems[J].J.Chromatogr.B,2000,743:235~239.
[107]K.Kimura.Simultaneous accumulation of low-molecular-mass RNA at the interface along with accumulation of high-molecular-mass RNA on aqueous two-phase system partitioning[J].J.Chromatogr.B,2000,743:421~442.
[108]K.C.Ross,C.Zhang.Separation of recombinant β-glucuronidase from transgenic tobacco by aqueous two-phase extraction[J].Biochem.Eng.J.,2010,49(3):343~350.
[109]G.M.F.Braas,S.G.Walker,A.Lyddiatt.Recovery in aqueous two-phase systems of nanoparticulates applied as surrogate mimics for viral gene therapy vectors[J].J.Chromatogr.B,2000,743:409~419.
[110]T.d.Gouveia,B.V.Kilikian.Bioaffinity extraction of glucoamylase in aqueous two-phase systems using starch as free bioligand[J].J.Chromatogr.B,2000,743:241~246.
[111]B.Kavakçglu,L.Tarhan.Initial purification of catalase from Phanerochaete chrysosporium by partitioning in poly(ethylene glycol)/salt aqueous two phase systems[J].Sep.Purif.Technol.,2013,105:8~14.
[112]D.Garai,V.Kumar.Aqueous two phase extraction of alkaline fungal xylanase in PEG/phosphate system:Optimization by Box-Behnken design approach[J].Biocatalysis and Agricultural Biotechnology,2013,2(2):125~131.
[113]F.C.De Oliveira,J.S.Dos Reis Coimbra,L.H.M.Da Silva,et al.Ovomucoid partitioning in aqueous two-phase systems[J].Biochem.Eng.J.,2009,47(1-3):55~60.
[114]U.Sivars,J.Abramson,S.Iwata,et al.Affinity partitioning of a poly(histidine)-tagged integral membrane protein,cytochrome bo3 ubiquinol oxidase,in a detergent-polymer aqueous two-phase system containing metal-chelating polymer[J].J.Chromatogr.B,2000,743:307-316.
[115]M.Rito-Palomares,A.Negrete,E.Galindo,et al.Aroma compounds recovery from mycelial cultures in aqueous two-phase processes[J].J.Chromatogr.B,2000,743:403~408.
[116]B.U.Rosso,C.d.A.Lima,T.S.Porto,et al.Partitioning and extraction of collagenase from Penicillium aurantiogriseum in poly(ethylene glycol)/phosphate aqueous two-phase system[J].Fluid Phase Equilib.,2012,335:20~25.
[117]C.A.Lima,A.C.V.F.Júnior,J.L.L.Filho,et al.Two-phase partitioning and partial characterization of a collagenase from Penicillium aurantiogriseum URM4622:Application to collagen hydrolysis[J].Biochem.Eng.J.,2013,75:64~71.
[118]Q.K.Shang,W.Li,Q.Jia,et al.Partitioning behavior of amino acids in aqueous two-phase systems containing polyethylene glycol and phosphate buffer[J].Fluid Phase Equilib.,2004,219(2):195~203.
[119]S.Shahriari,V.Taghikhani,M.Vossoughi,et al.Measurement of partition coefficients of β-amylase and amyloglucosidase enzymes in aqueous two-phase systems containing poly(ethylene glycol) and Na2SO4/KH2PO4at different temperatures[J].Fluid Phase Equilib.,2010,292(1-2):80~86.
[120]M.A.Bim,T.T.Franco.Extraction in aqueous two-phase systems of alkaline xylanase produced by Bacillus pumilus and its application in kraft pulp bleaching[J].J.Chromatogr.B,2000,743:349~356.
[121]S.A.Costa,Jr.A.Pessoa,I.C.a.Roberto.Partitioning of xylanolitic complex from Penicillium janthinellum by an aqueous two-phase system[J].J.Chromatogr.B,2000,743:339~348.
[122]S.Engel,Z.Barak,D.M.Chipman,et al.Purification of acetohydroxy acid synthase by separation in an aqueous two-phase system[J].J.Chromatogr.B,2000,743:281~286.
[123]H.S.Ng,C.P.Tan,M.N.Mokhtar,et al.Recovery of Bacillus cereus cyclodextrin glycosyltransferase and recycling of phase components in an aqueous two-phase system using thermo-separating polymer[J].Sep.Purif.Technol.,2012,89:9~15.
[124]M.C.De Oliveira,M.A.H.De Abreu Filho,P.d.A.Pessa Filho.Phase equilibrium and protein partitioning in aqueous two-phase systems containing ammonium carbamate and block copolymers PEO-PPO-PEO[J].Biochem.Eng.J.,2007,37(3):311~318.
[125]H-G.Xie,Y-J.Wang,M.Sun.Modeling of the partitioning of membrane protein and phase equilibria for Triton X-100-salt aqueous two-phase systems using a modified generalized multicomponent osmotic virial equation[J].Process Biochem.,2006,41(3):689~696.
[126]J.F.B.Pereira,V.C.Santos,H-O.Johansson,et al.A stable liquid-liquid extraction system for clavulanic acid using polymer-based aqueous two-phase systems[J].Sep.Purif.Technol.,2012,98:441~450.
[127]P.A.G.Soares,C.O.Nascimento,T.S.Porto,et al.Purification of a lectin from Canavalia ensiformis using PEG-citrate aqueous two-phase system[J].J.Chromatogr.B,2011,879(5-6):457~460.
[128]C.S.Porto,T.S.Porto,K.S.Nascimento,et al.Partition of lectin from Canavalia grandiflora Benth in aqueous two-phase systems using factorial design[J].Biochem.Eng.J.,2011,53(2):165~171.
[129]C.O.Nascimento,P.A.G.Soares,T.S.Porto,et al.Aqueous two-phase systems:new strategies for separation and purification of lectin from crude extract of Cratylia mollis seeds[J].Sep.Purif.Technol.,2013,116:154~161.
[130]S.Chethana,C.A.Nayak,K.S.M.S.Raghavarao.Aqueous two phase extraction for purification and concentration of betalains[J].J.Food Eng.,2007,81(4):679~687.
[131]Q.Cao,S.Li,C.He,et al.Extraction and determination of papaverin in pericarpium papaveris using aqueous two-phase system of poly(ethylene glycol)-(NH4)2SO4coupled with high-performance liquid chromatography[J].Anal.Chim.Acta,2007,590(2):187~194.
[132]谢 涛,王雯娟,吴如春等.PEG/(NH4)2SO4双水相体系萃取甘草中的有效成分[J].化学研究与应用,2005,17(2):230~232.
[133]石 慧,陈媛梅.PEG/(NH4)2SO4双水相体系在加杨叶总黄酮萃取分离中的应用[J].现代生物医学进展,2008,8(5):854~857.
[134]赵爱丽,陈晓青,蒋新宇.应用双水相萃取法分离黄芩苷的研究[J].中成药,2008,30(4):498~501.
[135]涛 谢,廖安平,易元龙等.双水相萃取三七中三七皂苷的研究[J].中药材,2008,31(4):535~537.
[136]K.S.Nascimento,P.A.J.Rosa,K.S.Nascimento,et al.Partitioning and recovery of Canavalia brasiliensis lectin by aqueous two-phase systems using design of experiments methodology[J].Sep.Purif.Technol.,2010,75(1):48~54.
[137]Q.Huo,Q.A.Lin,W.Gao,et al.Measurement and correlation of partition coefficients of glycyrrhizic in aqueous two-phase EOPO/K2HPO4systems[J].Asian J.Chem.,2010,22(6):4496~4500.
[138]戚 琦,李 蕾,李 勋等.聚乙二醇800-PVP双水相体系萃取荧光测定阿司匹林肠溶片中水杨酸[J].光谱实验室,2005.01,22(1):103~105.
[139]M.M.Bora,S.Borthakur,P.C.Rao,et al.Aqueous two-phase partitioning of cephalosporin antibiotics:effect of solute chemical nature[J].Sep.Purif.Technol.,2005,45(2):153~156.
[140]C.Aguirre,I.Concha,J.Vergara,et al.Partition and substrate concentration effect in the enzymatic synthesis of cephalexin in aqueous two-phase systems[J].Process Biochem.,2010,45(7):1163~1167.
[141]Y.Ni,J.Zhou,Z.Sun.Production of a key chiral intermediate of Betahistine with a newly isolated Kluyveromyces sp.in an aqueous two-phase system[J].Process Biochem.,2012,47(7):1042~1048.
[142]S.G.Doozandeh,G.Pazuki,B.Madadi,et al.Measurement of cephalexin partition coefficients in PEG+K2HPO4+H2O aqueous two-phase systems at 301.15,306.15 and 311.15K[J].J.Mol.Liq.,2012,174:95~99.
[143]朱自强,关怡新,李 勉.双水相系统在抗生素提取和合成中的应用[J].化工学报,2011,52(12):1029~1048.
[144]M.C.B.Pimentel,A.I.Araújo,Z.M.B.Figueiredo,et al.Aqueous two-phase system for citrinin extraction from fermentation broth[J].Sep.Purif.Technol.,2013,110:158~163.
[145]J.F.B.Pereira,F.Vicente,V.C.Santos-Ebinuma,et al.Extraction of tetracycline from fermentation broth using aqueous two-phase systems composed of polyethylene glycol and cholinium-based salts[J].Process Biochem.,2013,48(4):716~722.
[146]B.Chen,J.Han,Y.Wang,et al.Separation,enrichment and determination of ciprofloxacin using thermoseparating polymer aqueous two-phase system combined with high performance liquid chromatography in milk,egg,and shrimp samples[J].Food Chem.,2014,148:105~111.
[147]X.Xie,Y.Wang,J.Han,et al.Extraction mechanism of sulfamethoxazole in water samples using aqueous two-phase systems of poly(propylene glycol) and salt[J].Anal.Chim.Acta,2011,687(1):61~66.
Polymer-SaltAqueousTwo-PhaseTechnologyanditsResearchProgress
YANYong-sheng1,LUYang1,2,HANJuan1,WANGYun1
(1.School of Chemistry and Chemical Engineering,Jiangsu University,Zhenjiang 212013,China;2.College of Computer,Jilin Normal University,Siping 136000,China)
Aqueous two phase extration is a new and green separation and enrichment technology,and it has some advantages,such as simple,timesaving,efficient,green and eco-friendly,and it has been applied to the quantitative separation and extraction of metal ions,separation and purification of bioactivator and extraction of natural product.The existent aqueous two-phase system included polymer-polymer aqueous two-phase system,polymer-salt aqueous two-phase system,ionic liquid-salt aqueous two-phase system and micromolecule organic solvent-salt aqueous two-phase system.Because organic solvent is volatile and instable,the price of ionic liquid is higher and the viscosity of system containing two polymer is larger,the application of these three types of aqueous two-phase systems in large-scale industrial production was affected.One polymer of polymer-polymer aqueous two-phase system was replaced by salt,and it is polymer-salt aqueous two-phase system.The cost and viscosity of polymer-salt aqueous two-phase system is cheaper,and it has the advantage of good biocompatibility.It is applied in the separation and enrichment of bioactivator,natural product and antibiotic,and has high-exploited value and broad prospects on its application.In this paper,the research progress on the polymer-salt aqueous two-phase system was given,and hoping that is will be of help fo further research.
aqueous two-phase system;polymer;salt
郎集会)
2014-06-07
国家自然科学基金(21076098,21206059);教育部博士点基金(20133227120006);江苏省自然科学基金(BK2011529,BK20141289);国家博士后科学基金(2013M531284)
闫永胜(1962-),男,吉林省东丰县人,现为江苏大学化学化工学院教授,博士,博士生导师.研究方向: 环境化学及环境分析化学.
O642.4
A
1674-3873-(2014)03-0006-11
