Release test of alliin/alliinase double-layer tablet by HPLC—Allicin determination
2013-12-23YnLingJingJingZhngQiBingZhngZhongXiWngZongNingYinXinXiLiJinChenLiMingYe
Yn Ling,Jing-Jing Zhng,Qi-Bing Zhng,Zhong-Xi Wng,Zong-Ning Yin,Xin-Xi Li, Jin Chen, Li-Ming Ye,*
aWest China School of Pharmacy, Sichuan University, Chengdu 610041, China
bXinjiang Medical University, Wulumuqi 830000, China
1. Introduction
Garlic (Allium sativum) has been advocated as a therapeutic material for the treatment and prevention of a number of diseases [1]. Nowadays, it presents a growing interest in the nutraceutical industry due to its capacity to form allicin, a powerful antioxidant. Since its discovery by Cavalitto and Bailey [2], allicin has been the best characterized biologically active compound of garlic and shown to possess a variety of biological effects, including antimicrobial [3,4], anti-tumor[5-7], antifungal [8], antigenotoxic [9], and inhibitory immunomodulatory [10]. Allicin is produced by an enzymatic reaction when raw garlic is crushed or chopped. However,the preservation of allicin is complicated by its unstable and reactive nature; once it is generated, it readily changes into other compounds such as allysulfides, vinyldithins, and ajoenes[11].Thus,stabilizing and concentrating allicin need to be considered in depth for further commercialization of garlic products.
In Japan and western countries, garlic products have been popular and marketed in recent years as healthy foods with physiologically beneficial effects on humans. Consequently,the majority of the garlic supplements sold today are garlic powder tablets that are standardized on allicin [12]. However,the quality of garlic products is questionable. Many garlic products appear to undergo harsh processing[13].In the garlic powders, the conversion of alliin into allicin starts when the powder meets water[14].Allinase has been demonstrated to be completely inactive at the acidic pH level found in the stomach[15].
According to the disadvantages mentioned above, we proposed that enteric coated double-layer tablet of alliin/alliinase which was made by extracting stable chemical property of alliin and allinase from fresh garlic and then scientifically screening prescription to overcome unstable property, released active ingredient of allicin in vivo to exert full therapeutic effects. It is critical that allicin release(actually, formation and release) from alliin/alliinase doublelayer tablets can be measured under simulated gastrointestinal conditions. This paper focuses on developing and validating an HPLC assay for quantitative determination of allicin release from double-layer tablet of alliin/alliinase.
2. Experimental
2.1. Apparatus and conditions
HPLC analysis was performed on an Agilent 1100 system consisting of G1311A pump, Diamonsal C18(250 mm×4.6 mm, 5 μm) column, G1315A UV detector and Chem-Stations software (Agilent USA). Dissolution Apparatus (ZRS-12G,Tianda Tianfa Technology Co., Ltd., Tianjing, China) was used for release test. Mobile phase consisted of methanolwater (65:35 v/v) containing formic acid (0.04%, v/v). The injection volume was 10 μL with a flow rate of 1 mL/min and the detector wavelength was 242 nm. Ethylparaben was used as substitute reference standard for allicin release test.
2.2. Chemicals and reagents
Three batches of alliin/alliinase double-layer tablets were provided by pharmaceutics laboratory (West China School of Pharmacy, Sichuan University). Ethylparaben (99.9%) was purchased from Kelun Regents Co., Ltd. (Chengdu, China).Methanol and other solvents were of HPLC grade.
2.3. Standard solutions
Standard solution was prepared by accurately weighting 20.46 mg of ethylparaben, transferring to a 50 mLvolumetric flask and dissolving in 50 mL of mobile phase. The solution was further diluted with mobile phase to get working standard solution with the final concentration of 4.052, 8.104, 20.26,32.42, 81.04, 162.1 and 405.2 μg/mL, which was used for calibration purposes.
2.4. Preparation of sample solution
Twenty alliin/alliinase tablets were finely powdered and weighed about 10 mg for 9 samples into 25 mL volumetric flask,a quantity of ethylparaben standard was added for three levels (0.09, 0.30, 1.50 mg). The solutions were sonicated for 1 min and volumes were made up with the mobile phase.These solutions were used for accuracy and intermediate precision purpose.
2.5. Substitute reference standard for allicin
There is no reference standard of allicin available because it cannot be preserved for a long time due to its extremely unstable property and easy decomposition. By referring to the European Pharmacopoeia 7.0‘‘garlic powder'',butyl hydroxybenzoate,was chosen as an internal standard to determine allicin content of garlic powder.We also studied allicin and butyl hydroxybenzoate under established chromatographic conditions. There is a large difference in retention time between allicin and butyl hydroxybenzoate. That retention time can be shortened by changing organic phase of mobile phase, but retention time of allicin can be shortened largely, which can affect the separation of allicin from adjacent impurities. Furthermore, Yao et al. [16] studied chromatographic retention and separation condition of diallyl ammonia, methylparaben, propyl hydroxybenzoate, and ethylparaben with allicin.It was found that the diallyl ammonia peak was severely deformed, that methylparaben could not be separated from allicin, and that large differences in retention time existed between butyl hydroxybenzoate and allicin. Therefore, this research selected ethylparaben as a reference standard to determine the amount of allicin release from formulations using external standard method because of its good separation from allicin as well as adjacent impurities.
2.6. Detection of wavelength
The European Pharmacopoeia 7.0 ‘‘garlic powder'' states that 1 mg butyl hydroxybenzoate equals to 8.65 mg allicin. Ethylparaben is equal to propyl hydroxybenzoate in response factor,but a big difference exists between allicin and ethylparaben in response factor. Therefore, Yao selected the second largest absorption wavelength of 242 nm as the detection wavelength, where the absorption was lower than that of 254 nm, which could reduce the adjusting factor of allicin against ethylparaben and diluting steps during determination in order to improve the accuracy of measurement.
2.7. Optimization for release test
2.7.1. Selection of release test method
Complying with Appendix XD of Chinese Pharmacopoeia 2010 edition Volume II, the rotating basket method and the paddle method (small-glass method) were investigated respectively under the conditions of 100 mL phosphate buffer(pH=6.8) as release medium at the rotational speed of 100 r/min.
2.7.2. Selection of rotational speed and sampling time for release test
Small glass-method was used to investigate the rotational speed of 75 r/min and 100 r/min respectively under the conditions of 100 mL phosphate buffer (pH=6.8) as release medium.
2.7.3. Selection of release medium volume
Small glass apparatus with 100 mL release medium was used,referring to the first method of the release test method 2 in Appendix XD, Chinese Pharmacopoeia 2010 edition.
2.8. Method validation
The developed method was validated as per the International Conference on Harmonization (ICH) [17] guidelines with respect to linearity and range, specificity, precision, accuracy.
3. Results and discussion
3.1. Optimization for release test
The release of the drug substance from the drug product, the dissolution of the drug under physiological conditions and the permeability across the gastrointestinal tract are ratedetermining steps that affect the drug absorption from a solid dosage form after oral administration.In vitro dissolution test can be used to predict the release and the dissolution of the drug, hence, predict the in vivo performance of the drug. The release test is, now, routinely employed for lot-to-lot quality control of pharmaceuticals in solid dosage forms. We developed the release testing condition for alliin/alliinase doublelayer tablet which is not officially available in pharmacopoeia.
3.1.1. Selection of release test method
Complying with Appendix XD of Chinese Pharmacopoeia 2010 edition Volume II, the rotating basket method and the paddle method (small-glass method) were investigated respectively under the conditions of 100 mL phosphate buffer(pH=6.8) as release medium at the rotational speed of 100 r/min.The release curve suggested that the paddle method was better than the rotating basket method in release extent and rate (Fig.1).
3.1.2. Selection of rotational speed and sampling time for release test
The rotational speeds of 75 r/min and 100 r/min were investigated respectively under the conditions of 100 mL phosphate buffer (pH=6.8) as release medium, applying the small glassmethod. The rotational speed at 50 r/min was not taken into account because alliinase is a macromolecule with the molecular weight ranging from 80 kDa to 100 kDa, whose dissolution in buffer solution can be increased by high rotational speed. As shown in release curve, the result suggested that allicin release extent and rate from each tablet with the rotational speed of 100 r/min were higher than that at 75 r/min (Fig.2). Therefore, selection of the rotational speed of 100 r/min was more reasonable.
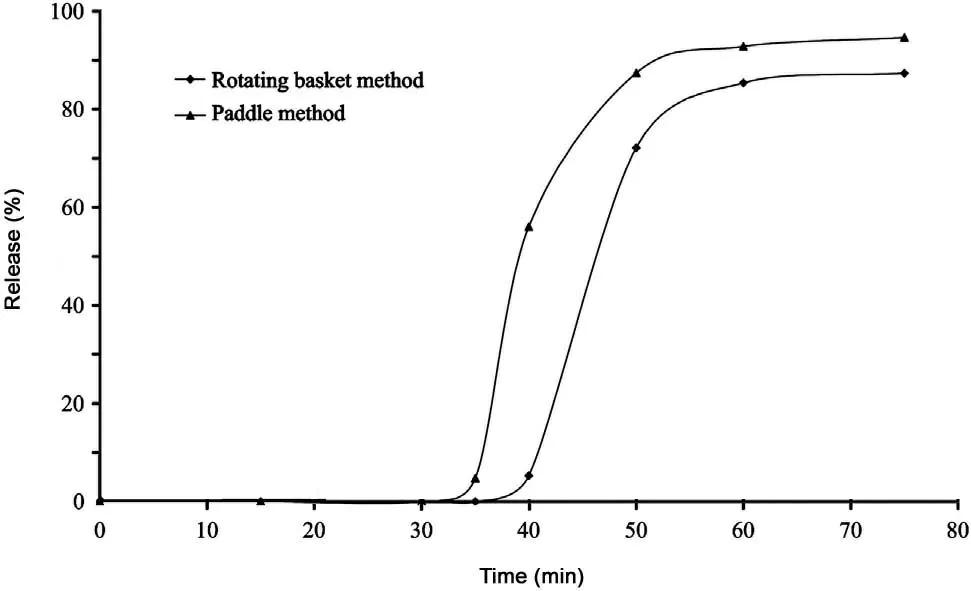
Fig.1 Release curve of the rotating basket and the paddle method.

Fig.2 Release curves of release speed at 100 r/min and 75 r/min.
3.1.3. Selection of release medium volume
Referring to the first method of the release test method 2 in Appendix XD, Chinese Pharmacopoeia 2010 edition, small glass apparatus with 100 mL release medium was used. The reasons for selection of 100 mL release medium are as follows.At 37°C, 1 g raw material of alliin can be fully dissolved in 2 mL deionized water whereas 0.1 g raw material of alliin contained in each alliin/alliinase double-layer tablet can also be fully dissolved in 100 mL release medium, and concentration is far below its saturation concentration. Therefore,100 mL release medium always meets sink condition of release test.
3.2. Developed release test method after optimization
3.2.1. Calculation for allicin release amount
By referring to European Pharmacopoeia 7.0 ‘‘garlic powder''stated and Yao et al. study [16], we used ethylparabe as substituted reference standard for allicin and the external standard method to calculate the release of allicin. The theoretical amount of allicin is 22.89 mg (i.e. labeled amount of allicin) which is calculated based on that the alliinase catalyzes two molecules allin to produce one molecular allicin and the amount of allin contained in the alliin/alliinase double-layer tablet. And also the peak areas of allicin and ethylparabe were measured under the established chromatographic conditions. According to relationship that 1 mg ethylparabe is equivalent to 4.71 mg allicin, the amount of allicin release was to be calculated as Eqs. (1) and (2).
The concentration of allicin release

Accumulative release of allicin (%):

where Cxis the concentration of allicin; Axis the peak area of allicin, Asis the peak area of ethylparabe, V is 100 mL of release medium volume, Csis the concentration of ethylparabe,V1is 1 mL of sample volume for release allicin,Cx1…Cxnare the concentration of allicin at different time and 22.89 is the theoretical release amount of allicin, namely, labeled amount of allicin in the double-layer tablet.
The theoretical value of 22.89 mg is the total amount of allicin which is produced from alliin by the catalysis of alliinase under ideal conditions. But the actual allicin release value is different from the theoretical allicin release value.The reasons are as follows: (1) The alliin/alliinase double-layer tablet contains 50% alliin raw material; therefore, the alliin reacts not only with alliinase but also with other 50%substance present in alliin raw material, which may lead to release amount of less than 22.89 mg;(2)Alliinase,one type of protein, whose activity is likely to be affected by temperature,micro-environment of reaction, pH value, and the pressure of tablet-press, giving rise to a decreased amount of catalyzed alliin and allicin production accordingly; (3) Recipients and other factors may prevent a small amount of alliin from participating in reaction,thus leading to the decrease of allicin amount generated.
3.2.2. Release test method
By complying with the requirements for release test(Appendix XD II of Chinese Pharmacopoeia 2010 edition Volume II Appendix XD 2), the small-glass-method stated in dissolution test (Appendix XC of Chinese Pharmacopoeia 2010 edition Volume II) was used. Firstly, 100 mL of 0.1 mol/L hydrochloric acid was added in each of 6 vessels as release medium,adjusting the rotational speed to 100 r/min. This process must ensure that each specimen does not have crevasse or disintegration. The acidic medium was chosen in order to simulate the physiological condition of the stomach.After 2 h, the acid was drained from each of the 6 vessels and then 100 mL of preheated phosphate buffer(pH 6.8)was added to each of the 6 vessels as release medium, and the rotational speed was not changed. One milliliter of the solution was sampled at exactly 0, 15, 30, 40, 45, 50, 60, 75 and 90 min, respectively, filtered and the successive filtrate was used as test solution.One milliliter of fresh medium was added into each glass immediately after the sampling. Ten milligrams of ethylparaben was accurately weighed into a 25 mL volumetric flask,diluted with the mobile phase to volume and mixed well as standard solution. The release amount of allicin from each tablet was measured in the same manner and calculated according to Eqs. (1) and (2).
3.3. Method validation
3.3.1. Specificity
The selectivity was studied by determination of the separation between allicin and other components in alliin/alliinase tablets.For the analysis of tablet excipients, the data indicated that these ingredients did not interfere in the analysis.Fig.3 shows a representative chromatogram of allicin, and other components of alliin/alliinase double-layer tablet, which showed no peaks, interfered with allicin. These results proved a good selectivity of the proposed method.
3.3.2. Linearity
The linearity was demonstrated for ethylparaben by preparing a seven-point calibration curve at different concentrations of 4.052, 8.104, 20.26, 32.42, 81.04, 162.1 and 405.2 μg/mL, and each concentration was measured by three replicate injections.The relation between the amount of ethylparaben and its peak area was linear over the concentration range of 4.052-405.2 μg/mL. The linear equation was: y=0.068×103x+41.336 with a correlation coefficient of 0.9999.
3.3.3. Precision
3.3.3.1. Repeatability. The repeatability of the method was determined by calculating the RSD of the peak areas of six replicate injections for the standard solution of ethylparaben,which was found to be 0.62%,suggesting a good repeatability for the analytical method.
3.3.3.2. Intermediate precision. Intermediate precision of the method was also evaluated by analyzing six samples by two analysts in the same laboratory using different HPLC systems.The results showed that the RSD of the percentage of ethylparaben added in the alliin/alliinase tablets for the 12 samples (6 samples from each analyst) was 1.1%, indicating a good intermediate precision of the method.
3.3.4. Stability
The standard solution was analyzed after being exposed at the room temperature for 0, 2, 4, 6 and 8 h and the release solution was analyzed at 0, 1, 2, 3 and 5 h at the room temperature. The results are shown in terms of RSD. The RSD of standard solution and release solution was 1.6% and 2.7%, respectively, which indicated that ethylparaben standard solution was found to be stable within 6 h and allicin release solution was supposed to finish determination within 5 h.
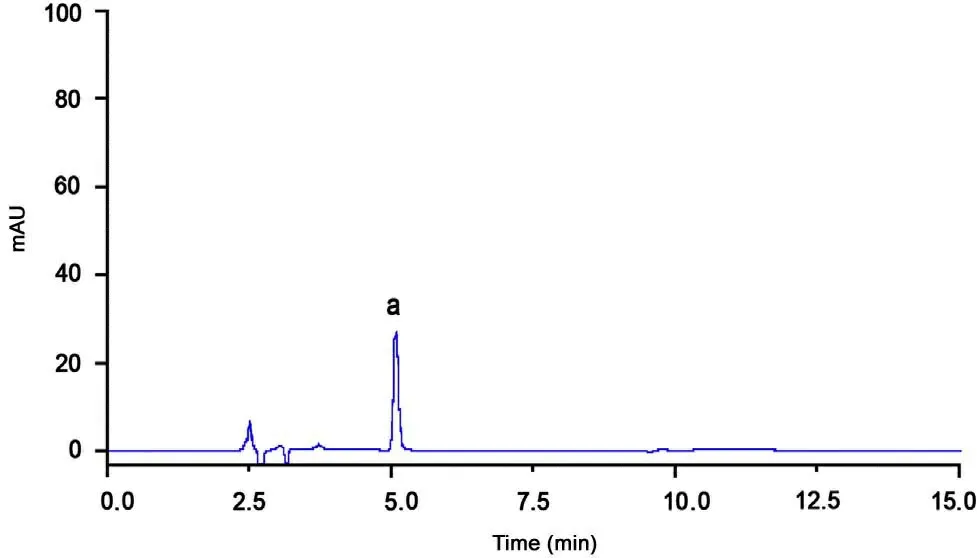
Fig.3 Representative chromatograms of alliin/alliinase sample:(a) allicin.

Fig.4 Release homogeneity curve of the same batch.
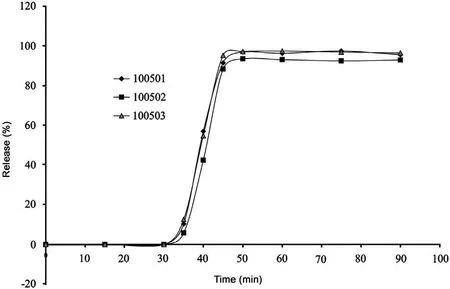
Fig.5 Release homogeneity curve for three batches.
3.3.5. Accuracy
The accuracy was tested as recovery and was determined by spiking the accurate amount of three known levels (0.09, 0.30 and 1.50 mg) of standard to approximate 10 mg powder of alliin/alliinase tablets. The recovery for three levels was 106.3%, 108.3% and 102%, and RSD was 1.3%, 2.3% and 0.16%, respectively. These results indicated a good accuracy for this method.
3.4. Release homogeneity test
Complying with the release test method described in Section 3.2.2, three batches were tested for investigating the homogeneity of allicin release within- and between-batches. The results shown in Fig.4 were the results of allicin release from the same batch and those in Fig.5 were from the three batches.
There was a significant difference between each tablet in allicin release rate and release extent, for preparation process of these tablets was manual, such as tablet pressing and tablet coating. In addition, to prevent stomach acid from inhibiting alliinase, coating thickness of tablet was highly required,which led to a lag-behind release time and a first turning point of allicin release of 35 min when allicin really began to release. Therefore, this study suggested that allicin began to release between 30 and 40 min and the release amount reached more than 75% at exactly 60 min. Sample time of the release test was set at exactly 35 and 60 min.
4. Conclusion
In summary, double-layer tablet of alliin/alliinase, which was not commercially available for garlic standardized on the active compound of allicin, was a new formulation releasing allicin by the reaction of alliin and alliinase in vivo.Therefore,release test in vitro was optimized using the small-glass method with 100 mL release medium and the rotational speed of 100 r/min to rapidly test allicin.And a simple HPLC method for the determination of allicin release amount from alliin/alliinase double-layer tablets was developed and validated.The method shows required linearity,precision,accuracy and selectivity for the quantitative determination of allicin release from alliin/alliinase double-layer tablets.Thus,the developed method can be used for routine analysis for the quality control of allicin.
[1] H. Amagase, B.L. Petesch, H. Matsuura, et al., Intake of garlic and its bioactive components, J. Nutr. 131 (2001) 955S-962S.
[2] C.J. Cavallito, J.H. Bailey, Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action, J. Am. Chem. Soc. 66 (1944) 1950-1951.
[3] R.R. Cutler, P. Wilson, Antibacterial activity of a new, stable,aqueous extract of allicin against methicillin-resistant Staphylococcus aureus, Br. J. Biomed. Sci. 61 (2004) 71-74.
[4] P. Canizares,I.Gracia,L.A.Gomez,et al.,Thermal degradation of allicin in garlic extracts and its implication on the inhibition of the in-vitro growth of Helicobacter pylori, Biotechnol. Prog. 20(2004) 32-37.
[5] B.J. Park, S.J. Cho, H.C. Kwon, et al., Caspase-independent cell death by allicin in human epithelial carcinoma cells: involvement of PKA, Cancer Lett. 224 (2005) 123-132.
[6] S. Oommen, R.J. Anto, G. Srinivas, et al., Allicin (from garlic)induces caspase-mediated apoptosis in cancer cells, Eur. J.Pharmacol. 485 (2004) 97-103.
[7] M. Patya, M.A. Zahalka, A. Vanichkin, et al., Allicin stimulates lymphocytes and elicits an antitumor effect: a possible role of p21ras, Int. Immunol. 16 (2004) 275-281.
[8] S.R. Davis, An overview of the antifungal properties of allicin and its breakdown products—the possibility of a safe and effective antifungal prophylactic, Mycoses 48 (2005) 95-100.
[9] Y.H. Siddique, M. Afzal, Antigenotoxic effect of allicin against SCEs induced by methyl methanesulphonate in cultured mammalian cells, Indian J. Exp. Biol. 42 (2004) 437-438.
[10] A. Lang, M. Lahav, E. Sakhnini, et al., Allicin inhibits spontaneous and TNF-alpha induced secretion of proinflammatory cytokines and chemokines from intestinal epithelial cells, Clin.Nutr. 23 (2004) 1199-1208.
[11] B. Iberl, G. Winkler, B. Muller, et al., Products of allicin transformations: Ajoenes and dithiins, characterization and their determination by HPLC, Planta Med. 56 (1990) 202-211.
[12] L.D. Lawson, Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation, Nutr. Cancer 38 (2000) 245-254.
[13] M. Emiko, H. Masakazun, I. Yoshitomo, Simultaneous determination of alliin and allicin in allium plants and their products by liquid chromatography, J. AOAC. Int. 80 (1997) 1052-1056.
[14] I. Arnault, T. Haffner, M.H. Siess, et al., High-performance ionpair chromatography method for simultaneous analysis of alliin,deoxyalliin, allicin and dipeptide precursors in garlic products using multiple mass,J.Pharm.Biomed.Anal.37(2005)963-970.
[15] L.D.Lawson,Z.J.Wang,D.Papadimetriou,Allicin release under simulated gastrointestinal conditions from garlic powder tablets employed in clinical trials on serum cholesterol, Planta Med. 67(2001) 13-18.
[16] Y.Z. Yao, J. Gu, T.J. Hang, Determination of allicin using the substituted reference standard ethylparaben by HPLC method,Chin. J. Anal. Chem. 36 (2008) 1083-1088.
[17] ICH, Q2 (R1) Validation of Analytical Procedures: Text Methodology, ICH Harmonized Tripartite Gulide, 2005.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Cover Figure
- Improved reversed phase liquid chromatographic method with pulsed electrochemical detection for tobramycin in bulk and pharmaceutical formulation
- Fluorescence spectroscopy of osthole binding to human serum albumin
- Simultaneous quantification of prodrug oseltamivir and its metabolite oseltamivir carboxylate in human plasma by LC-MS/MS to support a bioequivalence study
- Accurate quantitation standards of glutathione via traceable sulfur measurement by inductively coupled plasma optical emission spectrometry and ion chromatography
- Determination and stress studies on YK-1101,a potential histone deacetylase, by HPLC-UV and HPLC-TOF/MS methods
