海洋交替假单胞菌活性物质的研究
2013-12-23丁立建严小军
丁立建,何 山,严小军
宁波大学应用海洋生物技术教育部重点实验室,宁波315211
海洋微生物是生物活性物质的重要来源[1-4],交替假单胞菌属(Pseudoalteromonas )是1995 年由Gauthier 等[5]人由Alteromonas 分离出来并建立的一个新的海洋细菌属。近二十年来,在世界范围海洋中,分离到多株交替假单胞菌。它在海洋中的分布非常广泛,分布全球各地,可以生活在海底泥里,也可以共附生一些海洋动植物,研究发现交替假单胞菌属具有保护其宿主比如无脊椎幼虫、海藻和原生动物等抵抗外界不良环境[6]。由于它特殊的生活环境,因此具有特殊的代谢途径,产生许多结构新颖、作用特殊的生物活性物质。据报道它能产生抗肿瘤、抗菌、抗氧化、抗污和抑藻等各种生物活性物质[7-26]。目前国外对该属细菌已经开展探索性研究,并分离出了多株具有活性的海洋假交替单胞菌[9,12,14,16,17]。中国海洋资源十分丰富,海洋环境复杂,但国内对海洋交替假单胞菌研究较少,因此,开展针对海洋交替假单胞菌的研究具有很大的潜力。
1 海洋交替假单胞菌属的生物学特性
海洋交替假单胞菌属(Pseudoalteromonas )是一类轻度嗜盐革兰氏阴性、无芽孢、生鞭毛、需氧的海洋细菌,G+C 含量在37~50 mol%,生长需要海水,生长温度在20 ℃左右,氧化酶阳性,主要以葡萄糖作为碳源,能够生存于贫营养的海洋环境中[5,27]。与大多数可培养的菌属不同,交替假单胞菌属只能在海洋环境里分离得到,如卡内奥赫湾海恬蝓、海绵、海洋无脊椎动物表面、日本海藻共附生的细菌里、南极洋等中分离了海洋交替假单胞菌属,这些发现表明海洋交替假单胞菌在海洋环境的分布是很广泛的。
2 海洋交替假单胞菌属产生的生物活性物质
据报道海洋交替假单胞菌能够产生多种生物活性物质,包括抗菌、抗线虫、抑藻、抗氧化、抗肿瘤等活性物质。
2.1 抗菌
Domonkos 等[7]从卡内奥赫湾海恬蝓中分离了海洋交替假单胞菌Pseudoalteromonas sp. (CMMED 290),其代谢提取物显示了对耐青霉素金黄色葡萄球菌具有显著的抗菌活性。其中提取的亲脂性产物中鉴定了两个新的高度溴化的化合物,pentabromopseudilin 和bromophene。Isnansetyo 和Kamei 等[19]从海洋细菌Pseudoalteromonas phenolica sp. nov. 中得到1 个新的抗生素MC21-A,该化合物对耐青霉素金黄色葡萄球菌有杀菌作用,其作用效果与万古霉素相当,但是作用机制与万古霉素不同,MC21-A 主要是通过透化(permeabilization)细菌细胞膜来发挥作用的。Alim 等[12]通过发酵海洋细菌Pseudoalteromonas phenolica O-BC30T,并利用硅胶柱色谱、高效液相色谱分离纯化得到MC21-B 化合物,通过紫外光谱、红外光谱、质谱和核磁共振波谱分析鉴定结构。此外,通过体外实验,发现它对耐甲氧西林金黄色葡萄球菌有抗性,最小抑制浓度达1 μg/mL。而且还发现对枯草芽孢杆菌抗菌活性很强,最小抑制浓度达4 μg/mL,但对革兰氏阴性细菌和真菌没抗性。2008 年,Wimolpun 等[14]在日本海藻Diginea sp.共附生的细菌里分离了一种交替假单胞菌属,从该海洋细菌培养液提取出能抑制其他海洋细菌生长的代谢物。从这个细菌里分离了两种新的四肽化合物,分别是环-(苯丙,脯,亮,脯)四肽和环-(异亮,脯,亮,丙)四肽,发现对芽孢杆菌和弧菌有生长抑制作用。
2.2 抗肿瘤
Zheng 等[28]从海绵Hymeniacidon perleve 中分离出海洋交替假单胞菌NJ6-3-1,其发酵物经色谱分析、结构鉴定,得到1 个β-咔啉生物碱Norharman。Norharman 在体外对人宫颈癌细胞HeLa 和胃癌细胞BGC-823 具有显著的细胞毒活性,IC50为5 μg/mL。Domonks 等[13]从夏威夷岛海绵里分离出一株海洋细菌,通过16s rDNA 分子鉴定为交替假单胞菌(Pseudoalteromonas rubra ),为了从该细菌里发现新的海洋药物先导化合物,Pseudoalteromonas rubra 发酵提取亲脂性的代谢物,发现对人类的肿瘤细胞有细胞毒活性,通过现代色谱和波谱技术发现活性成分是一种灵菌红素,并命名为HBPG,该化合物对大肠杆菌、金黄色葡萄球菌、耐甲氧西林葡萄球菌和白色念球菌有很好的抑菌效果。
2.3 抗线虫
Francesco 等[9]从海洋无脊椎动物表面分离出海洋细菌Pseudoalteromonas tunicata D2,通过提取该细菌的代谢产物,鉴定出一个化合物tambjamine YP1,此化合物具有抗菌、抗肿瘤、免疫抑制剂、抗扩散等作用,而且在本研究中首次发现该化合物具有抗线虫的作用。这也恰恰说明了无脊椎动物产生抗线虫的作用是由宿主细菌产生的。
2.4 色素
Ashley Franks 等[16]首次从海洋细菌Pseudoalteromonas tunicata 分离到一种新的黄色色素化合物并通过核磁共振一维和二维图谱以及高分辨率质谱鉴定为tambjamine 生物碱类化合物。这是人们在海洋细菌里的首次发现,可以为人们在海洋里找到新的色素提供一定的基础。
2.5 抗氧化
Maya 等[17]从寒冷的南极洋里发现海洋细菌Pseudoalteromonas haloplanktis TAC12 能产生胞外8种环二肽和2 种多肽。通过制备型液相色谱和和核磁共振分离鉴定其结构。其中一种环二肽是首次发现这类结构,命名为环-(哌可啉,异亮氨酸)二肽。另外一种多肽通过DPPH 抗氧化研究,其抗氧化活性达75%(10 mmol),命名为酪氨酸-缬氨酸-脯氨酸-亮氨酸肽。
2.6 抑藻
Taizo Sakata 等[15]在日本鹿儿岛海湾海水里分离到一株海洋交替假单胞Pseudoalteromonas sp.A1-J11,它能产生三种羟基喹啉类化合物,对弧菌有一定的抗性。通过纸碟法研究其抑藻活性,发现其中一种分离的化合物AVS-03d 对硅藻的生长有很强的抑制。
2.7 其他
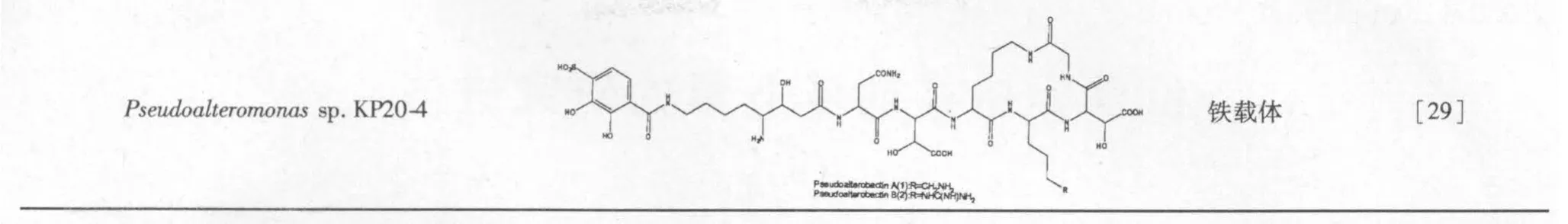
Kaneo 等[29]从帕劳群岛海域上的海绵中分离了一株海洋细菌,通过16SrDNA 测定序列鉴定为Pseudoalteromonas sp.KP20,对其进行40 L 体积的发酵,发酵液过大孔树脂富集,用甲醇洗脱,对馏分进行各种柱层析和HPLC 制备得到一个化合物,运用质谱和核磁共振技术对其结构进行鉴定,最终发现此化合物为铁载体,这是首次在海洋交替假单胞菌里发现。
3 展望

?
?
目前海洋微生物中研究的较多的是放线菌和蓝细菌等门类,海洋交替假单胞菌作为一种新属能够产生多种不同的活性物质,当前还处于初级研究阶段,因此被认为是21 世纪发现新型海洋药物先导化合物的重要来源。近年来基因组学、生物信息学、分子生物学均取得突破性进展,我们认为,如何在传统天然药物化学的基础上结合这些先进的技术,对海洋交替假单胞菌开展研究,是未来具有前景的研究领域:(1)哈佛大学christtopher T. Walsh 教授于2010 年提出“天然产物2.0”的概念,即从基因的角度发现天然产物[30],主要是通过基因工程的技术发现、组合、合成一些隐性基因的天然产物[31-34]。比如Leah C.Blasiak 等[35]通过异源表达的方法,对海洋交替假单胞菌中的一些隐性天然产物基因进行几个模块的表达,通过液相色谱分离技术发现了2 个从未发现过的新物质,这对海洋假交替单胞菌天然产物活性物质的研究提供了一种新思维、新途径。(2)近年了相继出现了组学技术比如基因组学、转录组学、蛋白组学、代谢组学等,他们对海洋细菌的天然产物的研究提供了强有力技术支持。特别是代谢组学技术,通过代谢组学筛选天然产物和研究其生物合成的途径以及代谢调控机制,以期找到更多的新颖的化合物[36-39],为寻找具有药物活性的先导化合物提供更广阔的空间。
1 Bhatnagar I,Kim SK.Immense essence of excellence:marine microbial bioactive compounds. Mar Drugs,2010,8:2673-2701.
2 Simmons TL,et al. Marine natural products as anticancer drugs.Mol Cancer Ther,2005,4:333-342.
3 Molinski TF,et al. Drug development from marine natural products.Nat Rev Drug Discov,2009,8:69-85.
4 Zhang L,et al. Exploring novel bioactive compounds from marine microbes.Curr Opin Microbiol,2005,8:276-281.
5 Gauthier G,et al.Phylogenetic analysis of the genera Alteromonas,Shewanella,and Moritella using genes coding for small-subunit rRNA sequences and division of the genus Alteromonas into two genera,Alteromonas (emended)and Pseudoalteromonas gen. nov.,and proposal of twelve new species combinations. Int J Syst Bacteriol,1995,45:755-761.
6 Holmstrom C,Kjelleberg S. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol Ecol,1999,30:285-293.
7 Feher D,et al. Highly brominated antimicrobial metabolites from a marine Pseudoalteromonas sp..J Nat Prod,2010,73:1963-1966.
8 Pinkerton DM,et al.Antimicrobial and cytotoxic activities of synthetically derived tambjamines C and E-J,BE-18591,and a related alkaloid from the marine bacterium Pseudoalteromonas tunicata.Chem Biodivers,2010,7:1311-1324.
9 Ballestriero F,et al. Identification of compounds with bioactivity against the nematode Caenorhabditis elegans by a screen based on the functional genomics of the marine bacterium Pseudoalteromonas tunicata D2.Appl Environ Microbiol,2010,76:5710-5717.
10 Zhu P,et al. Molecular phylogeny and modular structure of hybrid NRPS/PKS gene fragment of Pseudoalteromonas sp.NJ6-3-2 isolated from marine sponge Hymeniacidon perleve.J Microbiol Biotechnol,2009,19:229-237.
11 Zhu P,et al.Sequencing and modular analysis of the hybrid non-ribosomal peptide synthase-polyketide synthase gene cluster from the marine sponge Hymeniacidon perleve-associated bacterium Pseudoalteromonas sp. strain NJ631. Can J Microbiol,2009,55:219-227.
12 Isnansetyo A,Kamei Y. Anti-methicillin-resistant Staphylococcus aureus (MRSA)activity of MC21-B,an antibacterial compound produced by the marine bacterium Pseudoalteromonas phenolica O-BC30T. Int J Antimicrob Agents,2009,34:131-135.
13 Feher D,et al. A 2-substituted prodiginine,2-(p-hydroxybenzyl)prodigiosin,from Pseudoalteromonas rubra. J Nat Prod,2008,71:1970-1972.
14 Rungprom W,et al.Cyclic tetrapeptides from marine bacteria associated with the seaweed Diginea sp.and the sponge Halisarca ectofibrosa.Tetrahedron,2008,64:3147-3152.
15 Sakata T,et al.Chemical structures of quinolinol compounds produced by marine Pseudoalteromonas sp.A1-J11.Mem Fac Fish Kagoshima Univ,2008,57:29-35.
16 Franks A,et al.Isolation and structure elucidation of a novel yellow pigment from the marine bacterium Pseudoalteromonas tunicata.Molecules,2005,10:1286-1291.
17 Mitova M,et al.Exocellular peptides from Antarctic psychrophile Pseudoalteromonas Haloplanktis. Mar Biotechnol(NY),2005,7:523-531.
18 Krasikova IN,et al. Elucidation of structure of lipid A from the marine Gram-negative bacterium Pseudoalteromonas haloplanktis ATCC 14393T.Bioorg Khim,2004,30:409-416.
19 Isnansetyo A,Kamei Y. MC21-A,a bactericidal antibiotic produced by a new marine bacterium,Pseudoalteromonas phenolica sp. nov. O-BC30(T),against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother,2003,47:480-488.
20 Kim SJ,Yim JH.Cryoprotective properties of exopolysaccharide(P-21653)produced by the Antarctic bacterium,Pseudoalteromonas arctica KOPRI 21653. J Microbiol,2007,45:
510-514.
21 Franks A,et al. Inhibition of fungal colonization by Pseudoalteromonas tunicata provides a competitive advantage during surface colonization. Appl Environ Microbiol,2006,72:6079-6087.
22 Longeon A,et al.Purification and partial identification of novel antimicrobial protein from marine bacterium Pseudoalteromonas species strain X153. Mar Biotechnol (NY),2004,6:633-641.
23 Mai-Prochnow A,et al.Biofilm development and cell death in the marine bacterium Pseudoalteromonas tunicata.Appl Environ Microbiol,2004,70:3232-3238.
24 Holmstrom C,et al.Antifouling activities expressed by marine surface associated Pseudoalteromonas species. FEMS Microbiol Ecol,2002,41:47-58.
25 Egan S,et al.Correlation between pigmentation and antifouling compounds produced by Pseudoalteromonas tunicata.Environ Microbiol,2002,4:433-442.
26 Kawauchi K,et al.A possible immunosuppressant,cycloprodigiosin hydrochloride,obtained from Pseudoalteromonas denitrificans.Biochem Biophys Res Commun,1997,237:543-547.
27 Bowman JP.Bioactive compound synthetic capacity and ecological significance of marine bacterial genus pseudoalteromonas.Mar Drugs,2007,5:220-241.
28 Zheng L,et al.Antimicrobial screening and active compound isolation from marine bacterium NJ6-3-1 associated with the sponge Hymeniacidon perleve. World Journal of Microbiology and Biotechnolog,21:201-206.
29 Kanoh K,et al.Pseudoalterobactin A and B,new siderophores excreted by marine bacterium Pseudoalteromonas sp. KP20-4.J Antibiot (Tokyo),2003,56:871-875.
30 Walsh CT,Fischbach MA.Natural products version 2.0:connecting genes to molecules.J Am Chem Soc,2010,132:2469-2493.
31 Zelyas NJ,et al. Alanylclavam biosynthetic genes are clustered together with one group of clavulanic acid biosynthetic genes in Streptomyces clavuligerus. J Bacteriol,2008,190:7957-7965.
32 Dairi T,et al.Development of a self-cloning system for Actinomadura verrucosospora and identification of polyketide synthase genes essential for production of the angucyclic antibiotic pradimicin.Appl Environ Microbiol,1999,65:2703-2709.
33 Martin MF,Liras P.Organization and expression of genes involved in the biosynthesis of antibiotics and other secondary metabolites.Annu Rev Microbiol,1989,43:173-206.
34 Malpartida F,Hopwood DA. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host.Nature,1984,309:462-464.
35 Blasiak LC,Clardy J.Discovery of 3-formyl-tyrosine metabolites from Pseudoalteromonas tunicata through heterologous expression.J Am Chem Soc,2010,132:926-927.
36 Robinette SL,et al.NMR in metabolomics and natural products research:two sides of the same coin. Acc Chem Res,2012,45:288-297.
37 Bayet-Robert M,et al.Biochemical disorders induced by cytotoxic marine natural products in breast cancer cells as revealed by proton NMR spectroscopy-based metabolomics.Biochem Pharmacol,2010,80:1170-1179.
38 Kim HK,et al.Metabolomics:a tool for anticancer lead-finding from natural products.Planta Med,2010,76:1094-1102.
39 Rochfort S.Metabolomics reviewed:a new "omics" platform technology for systems biology and implications for natural products research.J Nat Prod,2005,68:1813-1820.
