Risk factors for medication-induced amenorrhea in first-episode female Chinese patients with schizophrenia treated with risperidone
2013-12-11HaizhiCHENMincaiQIANXinhuaSHENShengliangYANGJianhongYANGJuanfangSONGXiaocongFEIBaipingTAOBaohuaSONGLihuaRENZhongxiaSHEN
Haizhi CHEN, Mincai QIAN*, Xinhua SHEN, Shengliang YANG, Jianhong YANG, Juanfang SONG,Xiaocong FEI, Baiping TAO, Baohua SONG, Lihua REN, Zhongxia SHEN
陈海支 钱敏才* 沈鑫华 杨胜良 杨剑虹 宋娟芬 费小聪 陶百平 宋宝华 任丽华 沈仲夏
• Original article •
Risk factors for medication-induced amenorrhea in first-episode female Chinese patients with schizophrenia treated with risperidone
Haizhi CHEN, Mincai QIAN*, Xinhua SHEN, Shengliang YANG, Jianhong YANG, Juanfang SONG,Xiaocong FEI, Baiping TAO, Baohua SONG, Lihua REN, Zhongxia SHEN
1.Introduction
Amenorrhea is a common side effect of treatment with antipsychotic medication that can affect both the fertility and compliance of female patients with schizophrenia.[1-6]Defining amenorrhea as three months or longer without menstrual periods in individuals who previously had regular menstrual cycles, a study in China reported amenorrhea in 11 to 35% of female patients being treated with various antipsychotic medications and found that risperidone was associated with higher rates of amenorrhea than other antipsychotics.[7]Amenorrhea is presumed to be caused by medicationinduced hyperprolactinemia, but only a minority of antipsychotic-treated patients with hyperprolactinemia develop amenorrhea.[1,2,8-10]Other reports find that some female patients with schizophrenia have low levels of estrogen (estradiol), another potential cause of amenorrhea.[8,11,12]
The risk factors and protective factors that increase or decrease the likelihood of amenorrhea in females treated with antipsychotic medications remain unknown. Identifying high-risk patients prior to the selection of an antipsychotic would help clinicians avoid the use of medications that are most likely to result in amenorrhea. Information about risk status for amenorrhea would also be useful in deciding whether or not to provide concurrent treatment with metformin,which has recently been proven to be effective in the treatment of antipsychotic-induced amenorrhea.[13]The current study uses a nested case-control design to compare pretreatment levels of reproductive hormones in first-episode, drug-naïve female patients (with regular menses) with schizophrenia who do and do not subsequently develop amenorrhea during an initial 12-week course of treatment with risperidone.
2.Methods
2.1 Patient enrollment
The enrollment of patients is shown in Figure 1.Females admitted to the inpatient service at the Third People’s Hospital of Huzhou City from July 2009 to October 2011 who met ICD-10 criteria for schizophrenia,who were in the first episode of illness (i.e., duration of illness of 3 to 12 months) and who had never received antipsychotic medication were potential subjects for the study. Inclusion and exclusion criteria included the following: Han nationality, 20 to 40 years of age, regularmenstrual cycles of 28 to 30 days for the six months prior to admission, most recent menstrual cycle 7 to 14 days prior to admission (i.e., in the endometrial proliferation phase of the menstrual cycle), total score of the Positive and Negative Syndrome Scale (PANSS)[14]≥ 60 on admission, no significant physical diseases, no history of substance abuse, no history of allergies, no history of menstrual abnormalities (including polycystic ovary syndrome), and no abnormalities on routine admission laboratory examinations (including routine blood chemistry, urinalysis, thyroid function tests, and abdominal ultrasound).
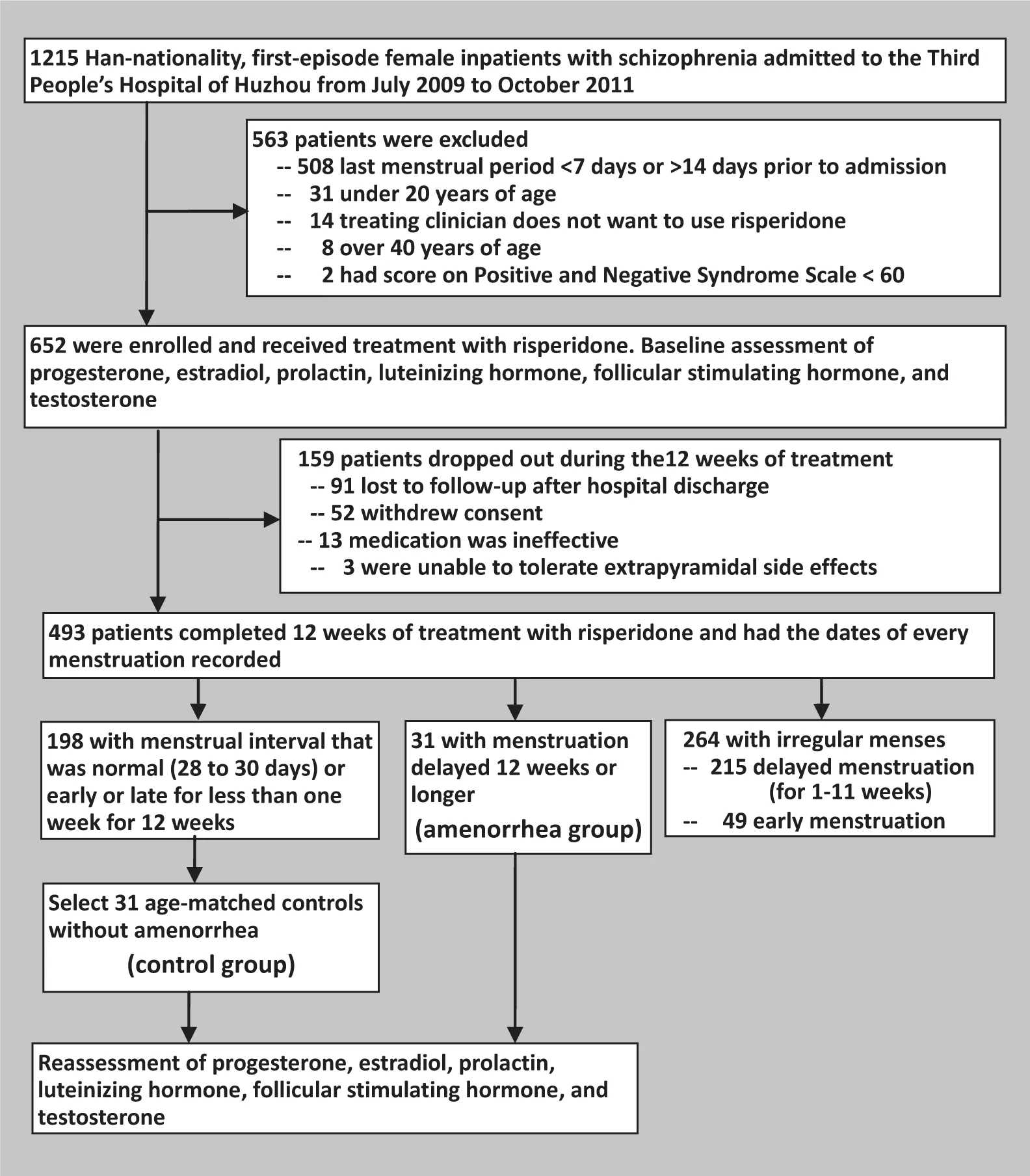
Figure 1.Patient enrollment and follow-up
All patients and their guardians signed informed consent forms to participate in the study. The study was approved by the Medical Ethics Committee of the Third People’s Hospital of Huzhou City.
2.2 Intervention and assessment
Among all 1215 first-episode female patients with schizophrenia, 652 (54%) were enrolled in the study.Most of the 563 (46.3%) excluded patients were excluded because they were not in the middle of their menstrual cycle at the time of admission (i.e., last menstrual period was less than 7 days or more than 14 days from admission) but some were excluded because they did not meet other inclusion or exclusion criteria.Almost all first-episode patients with schizophrenia were initially treated with risperidone, the most widely used antipsychotic medication in China. Initial doses of risperidone were gradually increased to therapeutic levels (2-6mg/d) over the first two weeks of treatment (based on the judgment of the treating clinician). The occurrence of side effects was assessed monthly using the Treatment Emergent Symptom Scale(TESS).[15]If necessary, adjunctive non-antipsychotic medication were administered: clonazepam for serious sleep problems, propranolol for tachycardia, and trihexyphenidyl for parkinsonian side effects.
As shown in Figure 1, 493 of the 652 patients(75.6%) completed the initial 12 weeks of treatment with risperidone. The main reasons for drop-out were loss to follow-up after hospital discharge, withdrawal of consent, and lack of efficacy of risperidone. The time of each menstrual period in the subjects was regularly recorded during the 12-week period. Based on this information, patients who completed the 12-week course of treatment with risperidone were categorized into three groups: 31 patients (6.3%) had no menstrual periods over the entire 12-week treatment period and,thus, met criteria for amenorrhea[6](the amenorrhea group); 198 patients (40.2%) had regular menses (no more than 6 days early or late) during the 12-week period; and 264 patients (53.5%) had irregular menses over the 12-week period (i.e., at least one menstrual period that was either 7 or more days early or 7 or more days late).
Fasting venous blood samples (taken at 7am)were obtained from all patients before the initiation of treatment with risperidone and from those who completed 12 weeks of treatment with risperidone.(For all subjects, the pretreatment blood sample was taken during the endometrial proliferation phase of patients’ menstrual cycles, but this was only the case for the post-treatment blood samples of subjects who continued to have normal menstrual cycles.) In addition to the routine tests of blood sugar and of liver, renal and thyroid function, the levels of the following reproductive hormones were assayed: progesterone, estradiol,prolactin, luteinizing hormone, follicular stimulating hormone, and testosterone. These assays were conducted using the ACCESS automatic microparticle chemiluminescence immunoassay system and the associated reagent kits (American Beckman Coulter Ltd.). The intra and inter coefficients of variation for the assessments were 5% and 8%.
2.3 Statistical methods
The analysis used a nested case-control design. For each of the 31 cases in the amenorrhea group a control subject was selected from among patients of the same age (within 3 years) who had had normal menstrual periods over the 12 weeks of treatment. These 31 individuals constituted the control group.
Data were analyzed using SPSS13.0 statistic software.The results for several of the hormone measurements were not normally distributed, so both paired t-tests and Wilcoxon’s signed ranked test (for matched data) were used in the univariate analyses. Univariate analyses included: a) assessment of the before versus after changes in the measures of reproductive hormones in the amenorrhea group and, separately, in the control group; b) comparison of baseline values between cases and controls; c) comparison of post-treatment values for cases and controls; and d) comparison of the magnitude of the before versus after change in values in cases versus controls. Multivariate conditional logistic regression was used to determine whether or not any of the pre-treatment measures of reproductive hormones was associated with the occurrence of amenorrhea during treatment. The two-tailed level of significance was set at 0.05.
3.Results
3.1 Characteristics of cases and controls
The 31 patients in the amenorrhea group had a mean(sd) age of 26.3 (5.6) years. At the time of admission their mean duration of illness was 5 (2) months (range,3 to 10 months); 22 of them were married; 17 had borne children; and the mean total PANSS score was 81.6 (16.3). Their mean duration of inpatient treatment was 47.5 (16.3) days. Their mean therapeutic dose of risperidone was 3.3 (0.4) mg/d (range, 2 to 5 mg/d). Their use of adjunctive treatments was as follows: 5 patients used 1 to 2 mg/d of clonazepam for 2 to 6 weeks; 6 patients used 20 mg/d of propranolol for 5 weeks; and 12 patients used 4 mg/d of trihexyphenidyl for 6 weeks.
The 31 patients in the control group had a mean age of 26.6 (6.2) years. At the time of admission their mean duration of illness was 5 (2) months (range, 3 to 10 months); 24 of them were married; 19 had borne children; and the mean total PANSS score was 80.8(18.5). Their mean duration of inpatient treatment was 44.8 (17.6) days. Their mean therapeutic dose of risperidone was 3.3 (0.4) mg/d (range, 2 to 5 mg/d).Their use of adjunctive treatments was as follows: 7 patients used 1 to 2 mg/d of clonazepam for 2 to 7 weeks; 5 patients used 20 to 30 mg/d of propranolol for 5 weeks; and 10 patients used 4 mg/d of trihexyphenidyl for 6 weeks.
There were no statistically significant differences between the two groups in any of these measures.
3.2 Comparison of reproductive hormone levels between patients who do and do not develop amenorrhea with risperidone treatment
As shown in Table 1, prior to treatment the levels of estradiol and progesterone in patients who subsequently developed amenorrhea when treated with risperdone were significantly lower than in patients who did not develop amenorrhea with antipsychotic treatment.After 12 weeks of treatment the estradiol level remained lower in the amenorrhea group but the difference was no longer statistically significant. After 12 weeks of treatment the progesterone level in the amenorrhea group and the control group was similar. The baseline and post-treatment levels of prolactin, follicular stimulating hormone, luteinizing hormone, and testosteronewere not significantly different between patients who did and did not developed amenorrhea during treatment with risperidone.

Table 1. Comparison of reproductive hormone concentrations (mean [sd]) between 31 first-episode female patients with schizophrenia who develop amenorrhea during 12 weeks of treatment with risperidone and 31 age-matched patients who do not develop amenorrhea during treatment
In both the amenorrhea group and the control group, 12 weeks of treatment with risperdone was associated with statistically significant drops in the levels of progesterone, estradiol and follicular stimulating hormone, and with a 4-fold increase in the levels of prolactin. The levels of luteinizing hormone and testosterone did not change significantly with treatment.
The drop in the level of progesterone with treatment was significantly smaller in the amenorrhea group than in the control group (4.7 v. 9.7 nmol/L, respectively)and the proportional change from baseline was also much smaller in the amenorrhea group (52% v. 73%,respectively). There was also a trend (p=0.060) of a smaller drop in the level of estradiol with treatment in the amenorrhea group than in the control group (107 v. 150 pmol/L) but the proportional drop from baseline(47% v. 45%, respectively) was similar in the two groups.There were no between-group differences in the magnitude of the changes with treatment of prolactin,follicular stimulating hormone, luteinizing hormone or testosterone.
The conditional logistic regression analysis that simultaneously considered the pre-treatment levels of all six reproductive hormones found that after adjustment for the levels of the other reproductive hormones, low pre-treatment estradiol levels were significantly associated with the subsequent occurrence of amenorrhea during treatment with risperidone.(Table 2)
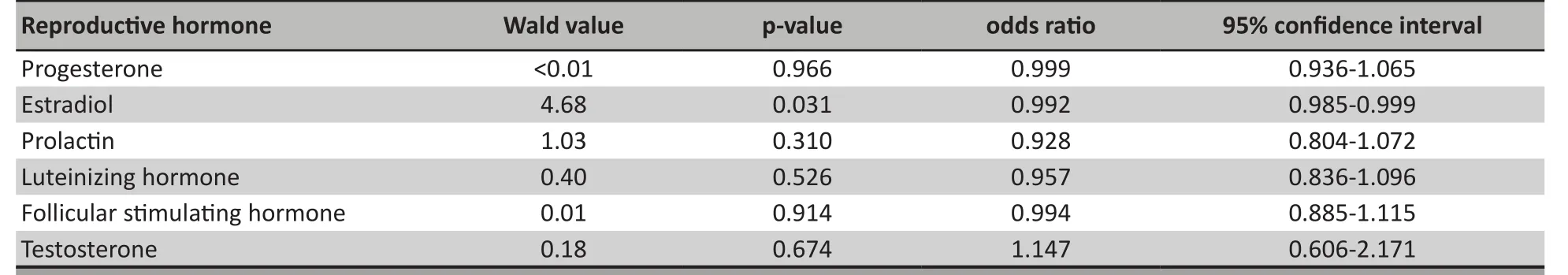
Table 2. Results of conditional logistic regression of the association of pre-treatment levels of reproductive hormones with the subsequent occurrence of amenorrhea during 12 weeks of treatment with risperidone in first-episode female patients with schizophrenia (31 cases and 31 age-matched controls)
4.Discussion
4.1 Main findings
Similar to the findings from other studies[16-18]we found a dramatic increase in prolactin levels in women of childbearing age treated with risperidone, but the pre-treatment and post-treatment levels of prolactin were not different between patients who did and did not develop amenorrhea with treatment. These findings do not support the suggestion that amenorrhea associated with the use of antipsychotic medication is the result of hyperprolactinemia.[8-10]
The most dramatic finding was the significantly lower pre-treatment level of estradiol in the patients who subsequently develop amenorrhea with risperidone treatment. This was a robust finding that persisted even when the pre-treatment levels of the other five reproductive hormones assessed were adjusted for in a conditional logistic regression model. We matched cases with controls by age, and had selected subjects without prior menstrual problems (specifically excluding those with polycystic ovarian syndrome who may have irregular menses[19]) who had regular menses for at least six months prior to admission and who were all in the endometrial proliferation phase of their cycle, so it is unlikely that our results are confounded by differences in the menstrual history or menstrual phase of the subjects.
We also found significantly lower pre-treatment levels of progesterone in patients who subsequently developed amenorrhea and the drop in the level of this hormone with treatment was significantly smaller in individuals who developed amenorrhea than in those who did not develop amenorrhea.However, after adjustment for the other hormone levels in the multivariate analysis, pre-treatment level of progesterone was not an independent predictor of subsequent amenorrhea.
The post-treatment levels of the six reproductive hormones assessed were not significantly different in patients who did and did not develop amenorrhea with treatment. Moreover, with the exception of the change in progesterone levels, the magnitude of the before versus after change in the levels of the hormones was not related to the development of amenorrhea. But the sample was relatively small, so the failure to identify significant relationships may be due to low power to detect clinically important differences between the groups. Further study with larger samples would be needed to assess the interaction effects of estradiol,progesterone, prolactin and the other hormones in the onset and persistence of medication-induced amenorrhea.
4.2 Limitations
There are several factors that need to be considered when assessing these results. a) In choosing a firstonset, drug naïve sample we avoided the complication of prior use of antipsychotic medication, but we found a much lower prevalence of amenorrhea than reported in other studies (only 6.3% after 12 weeks of treatment)so it is possible that the 31 subjects with amenorrhea we identified are not representative of all subjects who would eventually develop amenorrhea after longterm treatment with antipsychotic medication. b) We compared subjects with amenorrhea to those who continued to have normal menstrual periods during 12 weeks of treatment, excluding from the analysis patients who had irregular menses following treatment with risperidone but did not meet criteria for amenorrhea– 53.5% of the total sample. c) Risperidone is reported to result in a higher prevalence of amenorrhea than other antipsychotic medication,[6]so our results may not hold true for other antipsychotic medications. d) Some potential risk factors for amenorrhea (including family history of menstrual abnormalities, diet, BMI, exercise,etc.) were not considered. e) The small sample size made it impossible to interpret some negative findings (e.g.,lack of relationship between changes in hormone levels with amenorrhea) because they could be due to Type II errors. f) No comparison was made between those who were and were not enrolled and between those who were enrolled but did or did not complete the 12 weeks of treatment with risperidone; it is, therefore, difficult to be certain about how representative these results would be for all female patients with schizophrenia.
4.3 Significance
Amenorrhea is a common adverse reaction to the use of antipsychotic medications. Previous research has implicated medication-induced prolactin as the main cause of amenorrhea in patients treated with antipsychotic medication, but in this sample of firstepisode drug naïve patients we found that pre-treatment prolactin levels or the magnitude of the increase in prolactin with antipsychotic treatment was not related to treatment-induced amenorrhea. However, pre-treatment levels of estradiol were predictive of subsequent amenorrhea during treatment with risperidone.
Some antipsychotic medications have a greater risk of inducing amenorrhea than others. If our finding of the predictive power of pre-treatment levels of estradiol is confirmed in larger studies, this information would be of use to clinicians in selecting antipsychotic medications for female patients with schizophrenia; patients at highest risk of developing amenorrhea would be preferentially treated with the medications that are at lowest risk of inducing amenorrhea. Alternatively, patients at highest risk of developing amenorrhea could be concurrently treated with agents such as metformin that have been shown to decrease the risk of medication-induced amenorrhea.
Further work is needed to clarify the complex relationships between antipsychotic medications,reproductive hormones, protective agents and amenorrhea; but this current study is part of a growing corpus of work that is gradually helping clinicians individualize their selection of treatments for patients with schizophrenia.
Conflict of interest
The authors declare that they have no competing interests.
Funding
This work was supported by a grant from the 2011 Huzhou City Program for Science and Technology(No.2011YS28).
Acknowledgements
We thank Professor Chunsheng Wang from the Huzhou Teachers College for his assistance with the design of the study and the analysis of the data.
1. Dai JL. The effect of the anti-psychotic drugs to female patients on the menstrual cycles. Journal of Clinical and Experimental Medicine 2010; 9(6):463-467. (in Chinese)
2. Xin CD. The effect of menstrual cycles with the treatment of risperidone in female schizophrenic patients. J Clin Psychosom Dis 2005; 11(4):363-365. (in Chinese)
3. Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA,Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353(12): 1209-1223.
4. Yu J, Wang CY, Lin SQ, Zhai QM, Liu RM, Chen XH, et al.Neuroendocrine response in female schizophrenic patients treated with risperidone. Chin J Nerv Ment Dis 2003; 29(6): 445-447. (in Chinese)
5. Tandon R , Jibson MD. Efficacy of newer generation antipsychotics in the treatment of schizophrenia. Psychoneurendocrinology 2003, 28(suppl. 1): 9-26.
6. Ma WL, Lu MK. Survey of amenorrhea induced by antipsychotic medication. Shanghai Arch Psychiatry 2007; 19(4): 224-226. (in Chinese)
7. Su YA, Si TM, Shu L. Antipsychotic drugs and hyperprolactinemia.Foreign Medical Sciences. Section of Psychiatry 2003; 30(3):160-163. (in Chinese)
8. Huber TJ, RoUnik J, Wilhelms J, von zurMuhlen A, Emrich HM,Schneider U, et al. Estradiol levels in psychotic disorders.Psychoneuroendocrinology 2001; 26(1):27-35.
9. Kleinberg DL, Davis JM, De Coster RD, Van Baelen B, Brecher M. Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 1999; 19(1):57-61.
10. Xu GY, Hou J, Ma C, Wu FX, Li DM, Lu XQ, et al. The effect of serum prolactin level of the female schizophrenic patients with the treatment of risperidone and haloperidol. Chin J Nerv Ment Dis 2001; 27(6):454. (in Chinese)
11. Bergemann N, Mundt C, Parzer P, Nagl I, Eckstein-Mannsperger U, Haisch S, et al. Plasma concentration of estradiol in women suffering from schizophrenia treated with conventional and atypical antipsychotics. Schizophr Res 2005; 73(2-3): 357-366.
12. Bergemann N, Parzer P, Runnebaum B, Resch F, Mundt C.Estrogen, menstrual cycle phases, and psychopathology in women suffering from schizophrenia. Psychol Med 2007; 37(10):1427-1436.
13. Wu RR, Jin H, Gao K, Twamley EW, Ou JJ, Shao P, et al. Metformin for treatment of antipsychotic-induced amenorrhea and weight gain in women with first-episode schizophrenia: a double-blind,randomized, placebo-controlled study. Am J Psychiatry 2012;169(8): 813-821.
14. Si TM, Yang JZ, Shu L, Wang XL, Kong QM, Zhou M, et al. The reliability and validity of PANSS and its implication. Chinese Mental Health Journal 2004; 18(1): 45-47. (in Chinese)
15. Shen YC (ed). Psychiatry. 5th ed. Beijing: People’s Health Press,2010: 255-257.
16. Yu DS, Yu L. Psychotropic drugs and hyperprolactinemia. Chinese Journal of New Drugs and Clinical Remedies 2004; 23(12):893-896. (in Chinese)
17. Li Y, Gao JJ. The effect on the endocrine system of anti-psychiatric drugs for female patients. Laboratory Medicine and Clinic 2007;4(8):762-763. (in Chinese)
18. Chen HZ, Niu FR, Qian MC, Yu BR, Shen XH, Yang SG, et al. Effect of aripiprazole on the hyperprolactinmia induced by risperidone in male schizophrenia patients. Chin J Psychiatry 2009; 42(4): 224-227. (in Chinese)
19. Ma YM, Li R, Qiao J, Zhang XW, Wang SY, Zhang QE, et al.Characteristics of abnormal menstrual cycle and polycystic ovary syndrome in community and hospital populations. Chin Med J(Engl) 2010; 123(16): 2185-2189.
汉族女性精神分裂症首发患者利培酮治疗所致闭经的危险因素
Background:Amenorrhea is a common adverse effect of treatment with antipsychotic medications that influences both fertility and adherence to medication regimens. Most research suggests that medication-induced prolactinemia is the main cause of amenorrhea but few prospective studies have assessed this hypothesis.Aim:Identify risk factors for amenorrhea following treatment with antipsychotic medication.MethodsThe study used a prospective, nested case-control design. First-episode, drug naïve female patients with schizophrenia who were in the middle of their menstrual cycle at the time of admission were enrolled. Serum levels of six reproductive hormones were assessed before and after a 12-week course of treatment with risperidone: progesterone,estradiol, prolactin, follicular stimulating hormone, luteinizing hormone, and testosterone. The hormone levels of 31 patients who had no menstruation during the entire 12 weeks of treatment (the amenorrhea group) were compared to those of 31 age-matched subjects who had normal menstrual periods over the 12 weeks of treatment (the control group).Results:We found a dramatic 4-fold increase in prolactin levels in women of childbearing age treated with risperidone, but the pretreatment and posttreatment levels of prolactin were not different between patients who did and did not develop amenorrhea with treatment. However, there were significantly lower pretreatment levels of estradiol and progesterone in patients who subsequently developed amenorrhea with risperidone treatment than in patients who did not develop amenorrhea. A conditional logistic regression analysis found that pretreatment levels of estradiol remained significantly associated with the development of amenorrhea during treatment even when adjusting for the pretreatment levels of the other five reproductive hormones assessed.Conclusion:These findings do not support the suggestion that amenorrhea associated with the use of antipsychotic medication is the result of hyperprolactinemia. If our finding of the predictive power of pretreatment levels of estradiol is confirmed in larger studies, this information would be of use to clinicians in selecting antipsychotic medications for female patients with schizophrenia; patients at highest risk of developing amenorrhea could be preferentially treated with the medications that are at lowest risk of inducing amenorrhea.
10.3969/j.issn.1002-0829.2013.01.008
Department of Psychiatry, The Third People´s Hospital of Huzhou City, Huzhou, Zhejiang Province, China
*correspondence: qmc@hzsy.com
(received: 2012-01-17, accepted: 2012-05-02)

Dr. Haizhi Chen graduated from Hubei Medical University (now the Medical School of Wuhan University) in 1997 specializing in mental health. He currently works as chief of the Department of Psychiatry at the Third People´s Hospital of Huzhou City.He is a member of the Expert Committee for Psychological Rehabilitation of the National Disabled Person’s Rehabilitation Society. His research interests are clinical treatments in psychiatry and the prevention of adverse reactions to antipsychotic medications.
陈海支 钱敏才* 沈鑫华 杨胜良 杨剑虹 宋娟芬 费小聪 陶百平 宋宝华 任丽华 沈仲夏
湖州市第三人民医院 浙江湖州
*通信作者:qmc@hzsy.com
背景闭经是抗精神病药治疗时常见的不良反应。这一不良反应不仅会影响生育,还会影响患者对药物治疗方案的依从性。多数研究提示药物所致高泌乳素血症是闭经的主要原因,但是极少有前瞻性研究评估这一假设。
目的找出抗精神病药治疗后出现闭经的危险因素。
方法研究采用前瞻性巢式病例-对照设计。将入院时处于月经周期的中间时段、未接受抗精神病药治疗的首发精神分裂症患者纳入研究。分别在利培酮治疗前和治疗 12 周后测定血清中 6 种生殖激素的水平,即孕酮、雌二醇、泌乳素、促卵泡激素、促黄体生成素以及睾酮水平。将利培酮治疗 12 周内未来月经的 31 例患者(闭经组)与年龄匹配、月经仍然规律的 31 例患者(对照组)对照,比较上述激素水平。
结果我们发现育龄妇女利培酮治疗后泌乳素水平升高 4 倍,但无论治疗前还是治疗后闭经组和对照组之间的泌乳素水平差异不明显。然而,治疗前闭经组的雌二醇及孕酮水平均显著低于对照组。条件Logistic回归分析发现,在校正了治疗前其他 5 种生殖激素水平后,治疗前雌二醇水平仍然与治疗期间出现闭经显著相关。
结论本研究结果不支持高泌乳素血症导致抗精神病药物治疗相关的闭经这一观点。如果本研究结果中雌二醇水平的预测作用能够得到大样本研究的证实,那么临床医师可以利用这一信息为女性精神分裂症患者选择合适的抗精神病药物,对易发生闭经的高风险患者而言可以优先选用导致闭经风险低的药物治疗。
猜你喜欢
杂志排行
上海精神医学的其它文章
- SHANGHAI ARCHIVES OF PSYCHIATRY INSTRUCTIONS TO AUTHORS
- Detailed operational regulations are needed to implement the mental health law
- Thinking beyond the mean: a practical guide for using quantile regression methods for health services research
- Case report of Folie à Trois
- Interventions for childhood depression
- Should antidepressants be used to treat childhood depression?
