苯磺酰苯丙氨酸构筑的一维链状钙ギ配位聚合物的合成、结构表征和抗肿瘤活性
2013-10-17台夕市赵文华李法辉
台夕市 赵文华 李法辉
(1潍坊学院化学化工学院,潍坊 261061)
(2青海师范大学化学系,西宁 810008)
0 Introduction
The chemistry ofmetal-organic coordination polymers has been enriched enormously in the past two decades owing to their interesting framework topologies and their wide range of potential applications in adsorption,separation, catalysis, magnetism and fluorescence[1-7].A large numberofcoordination polymers constructed by transition metal ions with carboxylate ligands have been extensively investigated.These complexes exhibit extraordinary structural diversity and provide facile accessibility to functionalized new materials[8-10].Calcium is an indispensable element in biology.It is involved in several biochemical processes and is an essential cofactor required for the activation of a variety of enzymes[11].Amino acid is also an important physiological active substance in biological processes.To the best of our knowledge,the calciumギcomplex materials with amino acid ligands have been much less extensively studied than other metal complexes.In this paper,we report the synthesis and structure of[Ca(L)2(H2O)2(CH3CH2OH)]n,which was constructed from calcium perchlorate and N-benzenesulphonyl-L-phenylalanine.The antitumor activity against SMMC-7721,A549,WiDr and P388 cancer cells of the Caギcomplex also was investigated.
1 Experimental
1.1 Materials and measurements
Calcium perchlorate,benzene sulfonyl chloride,L-phenylalanine and other chemicals were obtained commercially and used without further purification.
Elementalanalyses were determined on a Elementar Vario III EL elemental analyzer.Infrared spectra were recorded with KBr optics on a Nicolet AVATAR 360 FTIR spectrophotometer in the range of 4,000~400 cm-1.Mass spectrum was performed on VG ZAB-HS Fast-atom bombardment (FAB)instrumrnt.The crystal data were collected on a Bruker smart CCD Area Detector.
1.2 Synthesis of the ligand(L)
The ligand was prepared according to the literature[12].Yield may reach up to over 65% .Anal.Calcd.for C15H15NSO4(%):C,58.96;H,5.00;N,4.52.Found(%):C,59.02;H,4.92;N,4.59.IR νmax(cm-1):νas(COOH):1 659,νs(COOH):1 437,ν(-SO2-NH-):3 247,1 321,1 154.FAB-MS:m/z=306[M+H]+.
1.3 Synthesis of the complex
1.0 mmol (0.305 g)of N-benzenesulphonyl-L-phenylalanine and 1.0 mmol (0.04 g)of sodium hydroxide were added to the 10 mL of 95%CH3CH2OH solution.After being dissolved,0.5 mmol(0.119 5 g)of calcium perchlorate was added to the above solution.The mixture was continuously stirred for 4 h at refluxing temperature.
The mixture was cooled at room temperature,and was collected by filtration.By evaporation in air at room temperature,the single crystal suitable for X-ray determination was obtained from 95%ethanol solution after 7 d.Anal.Calcd.for C32H38CaN2O11S2(%):C,52.59;H,5.24;N,3.83;Found(%):C,52.78;H,5.59;N,3.72%.IR νmax(cm-1):νas(COO):1 586,νs(COO):1400,ν(-SO2-NH-):3 246,1 322,1,153,ν(H2O):3350~3467,ν(Mg-O):423.
1.4 Crystal structure determination
A colourless block single crystal was placed on a glass fiber and mounted on a CCD area detector.Diffraction data were collected by φ-ω scan mode using a graphite-monochromatic Mo Kα radiation (λ=0.071 073 nm)at 291(2)K.A total of 15 466 reflections were collected,of which 6 656 were unique (Rint=0.015 2)and 4 989 were observed with I>2σ(I).The data were corrected for Lp factors.The structure was solved by direct methods and refined by full-matrix least-squares techniques on F2using SHELXL-97[13]and Fourier techniques.All non-hydrogen atoms and hydrogen atoms were refined anisotropically and isotropically,respectively.The final refinement by full-matrix least squares method was converged at R=0.059 6,and wR=0.1431(w=1/[σ2(Fo2)+(0.06P)2+1.99P],P=(Fo2+2Fc2)/3,S=1.108,(Δ/σ)max=0.000).Molecular graphics were drawn with the program package SHELXTL-97 crystallographic software package[14].The most relevant crystal data for complex are quoted in Table 1.
CCDC:890991.
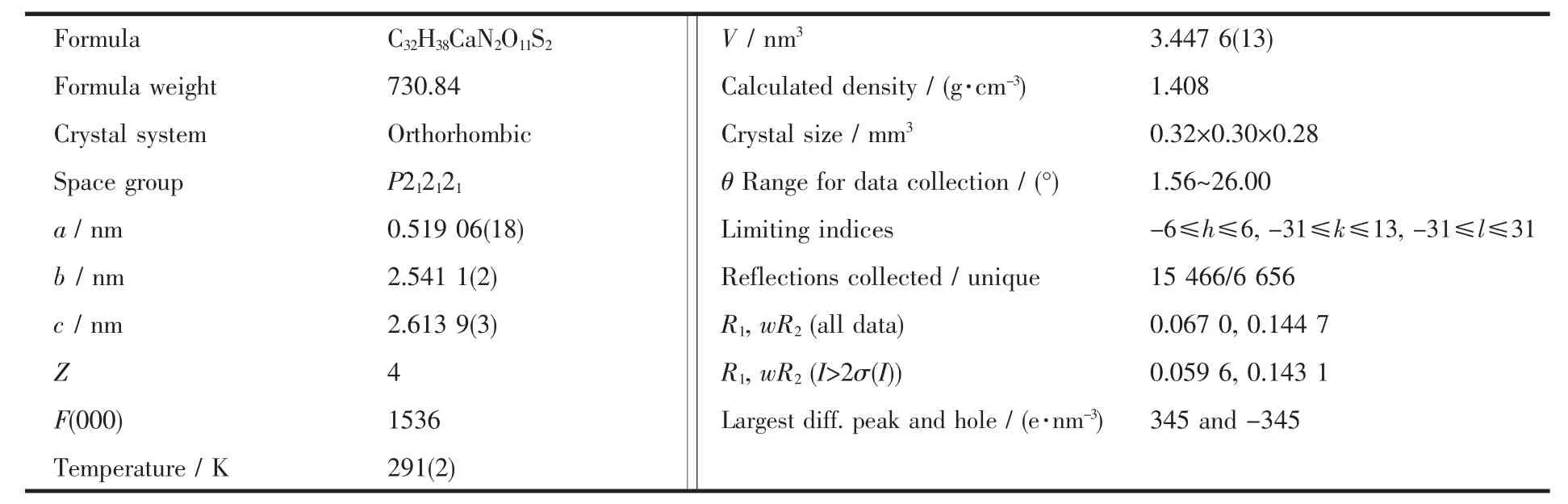
Table 1 Crystal structure parameters of the title complex
1.5 Antitumor activity
SMMC-7721,A549,WiDr and P388 cancer cells were propagated continuously in culture and grown in RPMI 1640 medium with 10%inactivated fetal calf serum and antibiotics.Cell harvested from exponential phase were seeded equivalently into 96 well plates and incubated for 24 h,then compounds studied were added in a concentration gradient.The final concentrations were maintained at c(μg·mL-1)5,10,20,respectively.The plates were maintained at 37℃in a humidified 5%CO2-90%N2-5%O2atmosphere and incubated for 48 h,the MTT solution was added,the following procedure referred to[15].The measurements of absorption of the solution concerned with the number of live cells were performed on spectrophotometer at 570 nm.
2 Results and discussion
2.1 IR spectra
In the IR spectra,the characteristic bands at 1 659 and 1 437 cm-1are assigned to the asymmetric stretching and symmetric stretching of COOH group,respectively.For the complex, the asymmetric stretching and symmetric stretching of COO-group are observed at 1 586 and 1 400 cm-1.It can be explained that the oxygen atoms of carboxylate group take part in the coordination with calcium atom[16].The value of Δν(νas(COO-)-νs(COO-))is 186 cm-1and reveals that the carboxylate groups are coordinated in bidentate fashion,which is consistent with the results of the X-ray analysis.In addition,the broad and strong absorption bands at 3 350~3 467 cm-1correspond to the presence ofwatermoleculesin the complex,which are accordance with the results of elemental analysis.
2.2 Crystal structure

Fig.1 Coordination environment of Ca ギ in the title complex

Table 2 Selected bond lengths(nm)and angles(°)of complex
Colourless block crystals of the Caギcomplex were obtained and its structure was determined by a single-crystal X-ray diffraction study.The selected bond lengths and angles with their estimated standard deviations are listed in Table 2.As depicted in Fig.1,the coordination environment of the Caギatom consists of seven oxygen atoms from the N-benzenesulphonyl-L-phenylalanine ligand,the coordinated water molecules and the coordinated CH3CH2OH molecule,making up a distorted pentagonal pyramid coordination environment.The distances of the Ca-O bonds are in the range of 0.234 2(3)~0.244 8(3)nm.The bonds lengths of Ca-O are consistent with those in reported previously[17-18].The bond lengths and bond angles of benzyl rings in the molecules are within the range of normal values.The benzyl rings(C4-C9 and C25-C30)and the CH3CH2OH are disordered.
The molecular structure is one dimensional chain structure by the interaction of bridged carboxylato groups and result in an 1D coordination polymer(Fig.2).
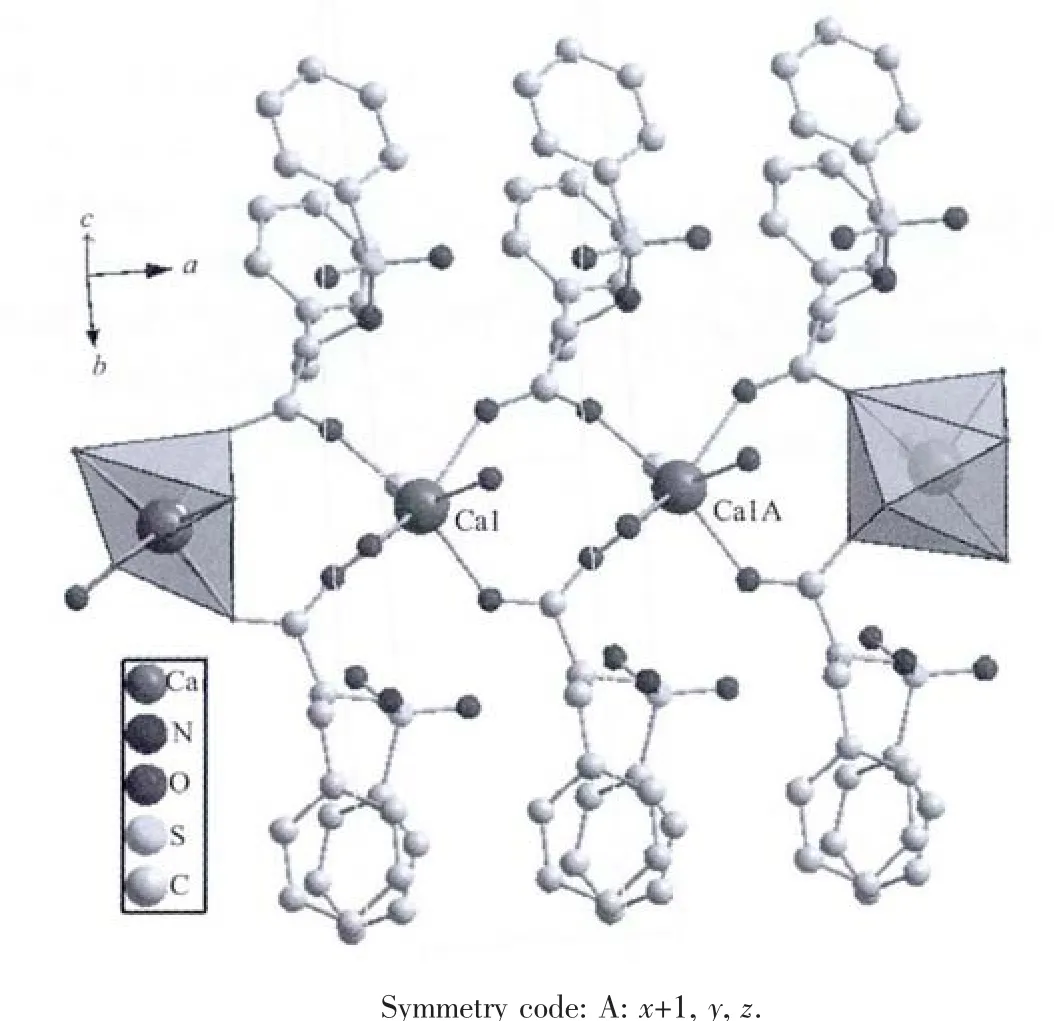
Fig.2 One dimensional chain structure of Ca ギ coordination polymer
2.4 Antitumor activity
The data of the antitumor activities of Caギcomplex and N-benzenesulphonyl-L-phenylalanine are given in Table 3.The concentration of DMSO was controlled under 1%to assure not to affect the results.Ascan beseen,theCaギ complexand N-benzenesulphonyl-L-phenylalanine exerted cytotoxic effect against SMMC-7721,A549,WiDr and P388 cancer cells,however the better cycotoxicity against P388 cancer cell with lower IC50value (<50 μg·mL-1)than other cancer cell,and the complex displays the weaker cytotoxic activity than that of N-benzenesulphonyl-L-phenylalanine.Further structure modification to enhance the cytotoxic activity of the Caギcomplexes is desirable.
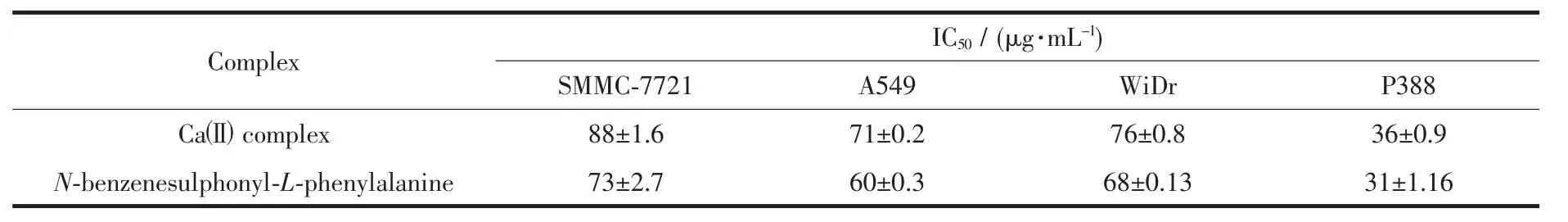
Table 3 Cytotoxicity of Caギcomplex
[1]Garnovskii A D,Nivorozhkin A L,Minkin V I.Coord.Chem.Rev.,1993,126:1-20
[2]Deng H X,Crunder S,Cordova K E,et al.Science,2012,336:1018-1023
[3]Erxleben A,Schumacher D.Eur.J.Inorg.Chem.,2001,2001(10):3039-3046
[4]Lu J W,Huang Y H,Wei H H.Inorg.Chem.Commun.,2007,10(10):1210-1213
[5]Lecren L,Wernsdorfer W,Li Y T,et al.J.Am.Chem.Soc.,2007,129(16):5045-5051
[6]Mala N,Pramendra K S,Ashok K.Appl.Organometal.Chem.,2009,23(11):434-445
[7]Qiao C J,Li J,Xu Y,et al.Appl.Organometal.Chem.,2009,23(10):421-424
[8]TAI Xi-Shi(台夕市),WANG Dong-Fang(王东方),ZHAO Zeng-Bing(赵增兵).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(5):831-834
[9]TAI Xi-Shi(台夕市),FENG Yi-Min(冯一民),KONG Fan-Yuan(孔凡元),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(8):1490-1494
[10]WANG Yan(王 彦),WANG Tao(王 涛),LIU Guang-Xiang(刘光祥),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(8):1467-1471
[11]Yang Y Y,Huang Z Q,Chen X M,et al.Z.Anorg.Allg.Chem.,2003,629:1901-1903
[12]TAI Xi-Shi(台夕市),DU Lian-Cai(杜连彩),ZHAO Zeng-Bing(赵增兵).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(3):575-579
[13]Sheldrick G M.SHELXL-97,Program for Solution of Crystal Structures,University of Göttingen,Germany,1997.
[14]Sheldrick G M.SHELXS-97,Program for Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[15]Dodoff N,Grancharow K,Gugova R,et al.J.Inorg.Biochem.,1994,54:221-233
[16]Nakamoto K.Infrared and Ramen Spectra of Inorganic and Coordination Compounds.3rd Ed.New York:John Wiley and Sons,1978.
[17]TAI Xi-Shi(台夕市),YIN Jie(殷杰),FENG Yi-Min(冯一民),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2007,23(10):1812-1814
[18]Tai X S,Yin J,Hao M Y.Acta Cryst.,2007,E63:m1935
