间苯二甲酸和二咪唑基苯构建锌化合物的合成,晶体结构和荧光性质
2013-10-17邓奕芳陈满生张春华邝代治杨颖群
邓奕芳 陈满生 张春华 邝代治 聂 雪 杨颖群 李 薇
(功能金属有机材料湖南省普通高等学校重点实验室,衡阳师范学院化学与材料科学系,衡阳 421008)
0 Introduction
In recent years,metal-organic frameworks(MOFs)with carboxylate-containing ligands have been extensively studied because the carboxylate groups can have varied coordination modes resulting in the formation of different structures[1-3].Generally,the multidentate organic ligands containing coordination sites challenge to control the final products because many factors,such as the coordination geometry of the central metal ions,connection modesoforganic ligands as well as the synthetic method,show great influence on the desirable structures.Among them,the multifunctional auxiliary ligands and metal ions have an important role in the construction of MOFs with distinctive structures[7-9].
On the other hand,mixed ligands with N-donor groups acting as ancillary connectors are another group of effective building units to construct novel coordination polymers,because they have strong coordination affinity and can satisfy the geometric need of metal centers[10-12].In this case,in order to further investigate the influence of organic ligands on the coordination architectures and related properties,we report on the synthesis,crystal structure and properties of a new coordination polymer [Zn2(1,3-BDC)2(dib)(H2O)]n(1)with 1.3-benzenedicarboxylate(1,3-H2BDC),ZnSO4·7H2O as well as rigid N-donor bridging ligands(dib).
1 Experimental
1.1 Materials and instruments
All chemicals and solvents were of reagent grade and used as received without further purification.The dib ligand was synthesized according to the reported method[13].Elemental analysis was carried out on a Perkin-Elmer 2400ギ elemental analyzer.Infrared(IR)spectra were recorded on a Bruker Vector 22 FTIR spectrophotometer by using KBr discs.Thermogravimetric analyses (TGA)were performed on a simultaneous SDT 2960 thermal analyzer under nitrogen with a heating rate of 10 ℃·min-1.The luminescence spectra for the powdered solid samples were measured on an Aminco Bowman Series 2 spectrofluorometer with a xenon arc lamp as the light source.In the measurement of emission and excitation spectra the pass width is 5 nm,all the measurements were carried out under the same experimental conditions.
1.2 Synthesis of the complex
A mixture containing dib (11.0 mg,0.05 mmol),ZnSO4·7H2O (28.8 mg,0.1 mmol),1,3-H2BDC(16.8 mg,0.1 mmol)and NaOH (8.0 mg,0.2 mmol)in 10 mL H2O was sealed in a 16 mL Teflon lined stainless steel container and heated at 150 ℃ for 3 d.After the mixturewasslowlycooled toroom temperature,colorless block crystals of 1 were obtained by filtration and washed with water and ethanol several times with a yield of ca.52%based on dib ligand.Anal.Calcd for C28H20N4O9Zn2(%):C,48.89;H,2.91;N,8.15;found(%):C,48.87;H,2.94;N,8.12.IR main absorption parameters(KBr pellet,cm-1)are as below:3 430.5(s),1 616.6(s),1 580.3(s),1 427.2(m),1 396.4(s),1 346.2(m),1 255.6(s),1 168.8(s),1 143.7(m),1 087.4(s),1 028.0(m),852.5(s),746.9(m),727.1(m),646.5(w),473.0(w).
1.3 X-ray crystallography
A single crystal with dimensions of 0.28 mm ×0.24 mm×0.22 mm was selected and determined by a Bruker Smart ApexⅡCCD diffractometer equipped with a graphite-monochromatic Mo Kα radiation (λ=0.071 073 nm)by using an ω-2θ scan mode at 291(2)K.Out of the total 13 325 reflections collected in the range of 2.32°≤θ≤26.71°,4 927 were independent with Rint=0.047 0,of which 3 596 were considered to be observed(I>2σ(I)).The structure of 1 was solved by direct methods and refined on F2by full-matrixleast-squares method[14].All non-hydrogen atoms were refined anisotropically and the hydrogen atoms were generated geometrically.The crystallographic data and the selected bond lengths and bond angles are given in Table 1 and Table 2.

Table 1 Crystallographic data of the title complex
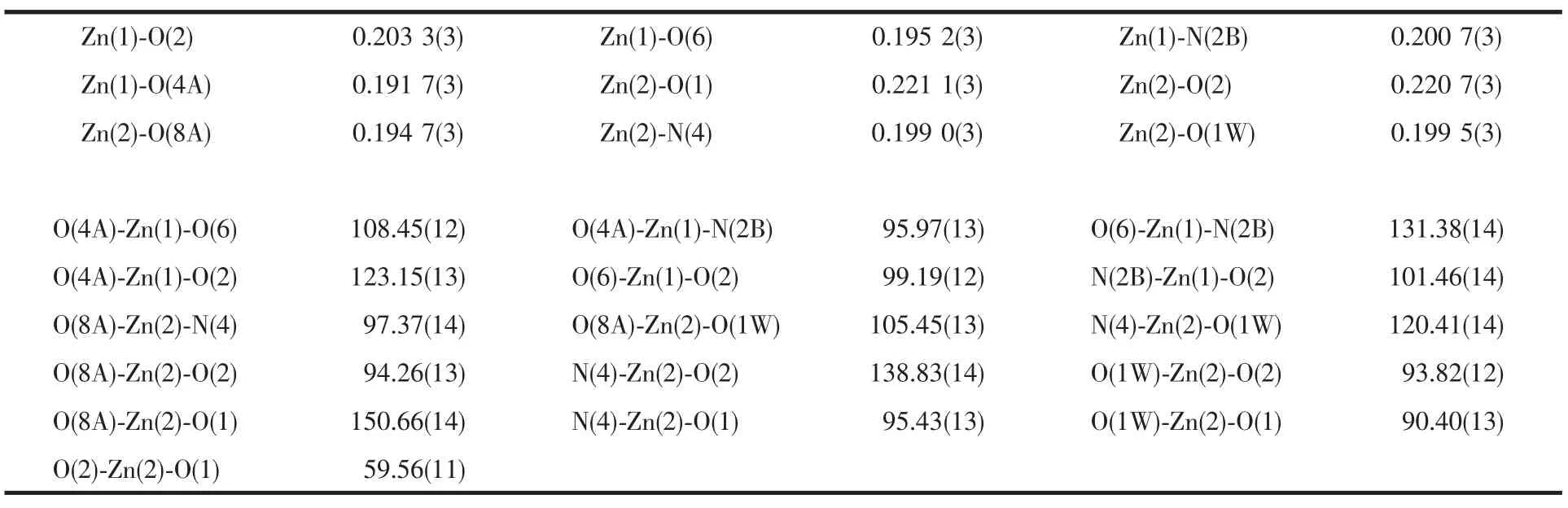
Table 2 Selected bond lengths(nm)and bond angles(°)
CCDC:895702.
2 Results and discussion
2.1 Structure description
The structure of the title complex,1D and 2D nets of the complex and crystalline packing are revealed in Fig.1~Fig.3,respectively.
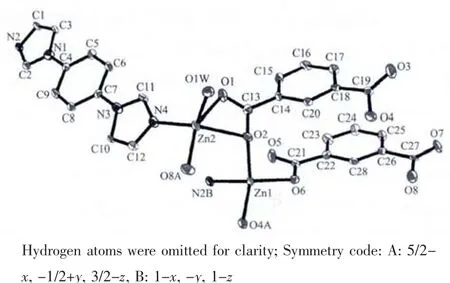
Fig.1 Coordination environments of the Znギ atoms in 1 with the ellipsoids drawn at 30%probability level
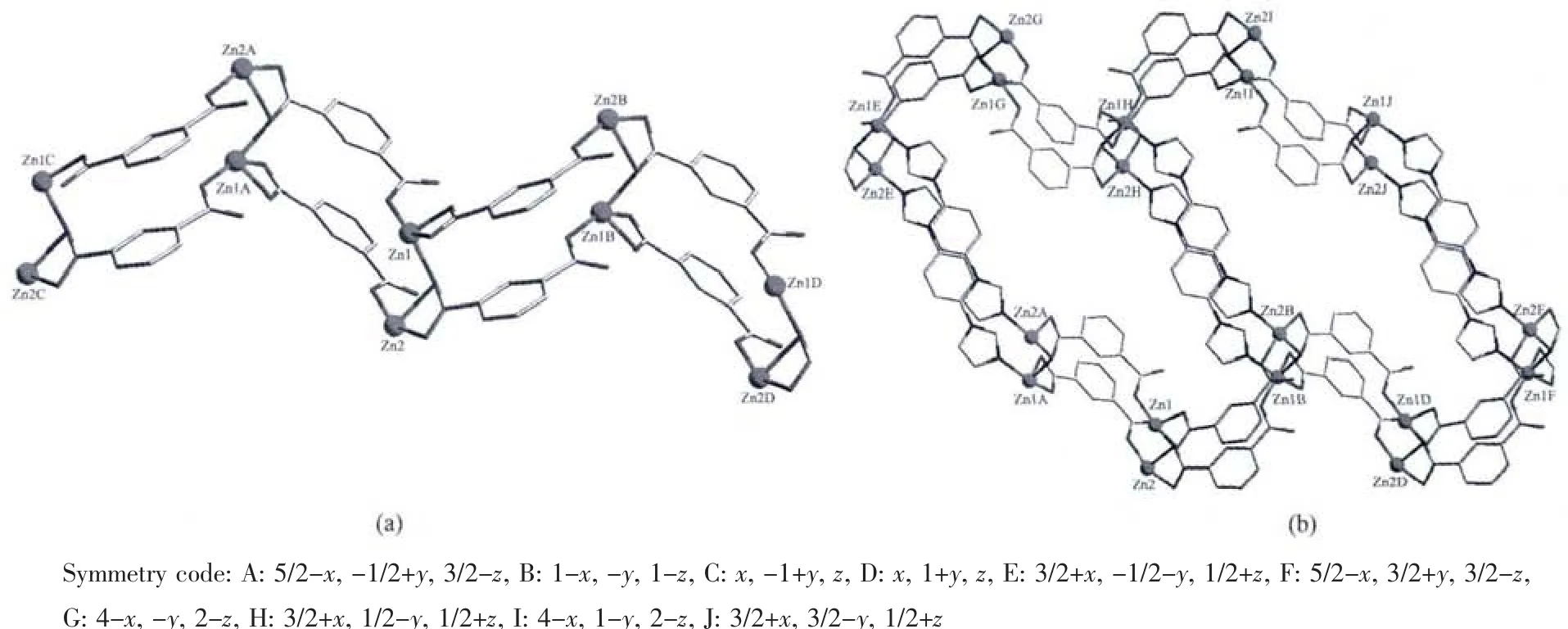
Fig.2 (a)A view of the double 1D chain of 1;(b)Undulated(6,3)2D double-layer framework
Single crystal X-ray diffraction analysis reveals that complex 1 is a unique two-dimensional supramolecular framework in which the asymmetric unit contains two Zn atoms,one dib ligand,two 1,3-BDC2-ligands and one coordinated water molecule.Fig.1 shows the coordination environment of two independent Znギatoms with the atom numbering scheme.It can be clearly seen that Zn1 atom is in a distorted tetrahedral coordination environment with three O atoms from three different 1,3-BDC2-ligands with Zn1-O4A,Zn1-O6,Zn1-O2 bond distances of 0.191 7(3),0.195 2(3),and 0.203 3(3)nm,respectively,and one N atom from dib ligand with Zn1-N2B bond distance of 0.200 7(3)nm (Table 2).However,the other central Zn2 atom shows a distorted squarepyramidal geometry by coordinating to three O atoms from two 1,3-BDC2-ligands,one terminal water molecule and one N atom from the dib ligand.Three O atoms(O1,O2,O8A)and one N atom(N4)constitute the base of the square-pyramid with Zn-O bond lengths varying from 1.947(3)to 0.221 1(3)nm,whereas one O atom(O1w)occupies the apical position with a Zn2-O1w distance of 0.199 5(3)nm(Table 2).It is noticeable that the completely deprotonated 1,3-BDC2-ligands adopt μ2-η1∶η1and μ3-η1∶η2two types of coordination modes to connect Znギ atoms.Firstly,each 1,3-BDC2-ligand links Znギatoms using its two carboxylate groups to form a one-dimensional(1D)double chain which can be seen clearly from Fig.2.Then,the 1D chain is pillared through dib ligands,resulting in a novel two-dimensional (2D)double-layer framework(Fig.2).It is interesting that the bridging O2 atoms display the connection of the double-layer network.Finally,the 2D layers are further linked together through O-H…O hydrogen bonds(O1W…O7A=0.281 2(4)nm,A:5/2-x,-1/2+y,3/2-z;O1W…O6K=0.2812(4)nm,K:-1+x,y,z;and the D-H-A angles are 132°and 153°)to generate a three-dimensional(3D)packing structure.
2.2 Infrared spectroscopy and TG of the complex
The absorption peaks νC=O(COOH)(1 695.0 cm-1)and νC-O(COOH)(1 012.0 cm-1)of the ligand 1,3-H2BDC disappeared in the title complex,while νas(COO)(1 580.3 cm-1)and νs(COO)(1 396.4 cm-1)were found,which indicated that the oxygen atoms of carboxyl group were coordinated with the central ions.And Δν=νas-νs=183.9 cm-1,further showing that the oxygen atoms of carboxyl group were coordinated with the centric ions in the form of bidentate bridge[15],which was identical with the analytical results of crystalstructure.The infrared spectrum ofthe complex also exhibits the strong and broad band of H2O at ca.3 430.5 cm-1[16].TGA were carried out for the synthesized complex,and a weight loss of 2.70%was observed in the temperature range of 80~130 ℃(Calcd.2.62%)due to the loss of solvated water molecules and the anhydrous compositions begin to decompose at 305℃.
2.3 Fluorescence property of the title complex
Inorganic-organic hybrid coordination complexes,especially with d10metal centers, have been investigated for fluorescence properties owing to their potential applications as luminescent materials[17].Thus the luminescent spectra of complex 1,dib as well as free 1,3-H2BDC ligand were investigated in the solid state at room temperature.As shown in Fig.4,complex 1 exhibits an intense broad photoluminescence with maximum emission at 478 nm upon excitation at 410 nm,which is close to the emission at 474 nm of the dib ligand under the same conditions,rather than the free 1,3-H2BDC ligand (448 nm).Therefore,the emission observed in 1 is attributed to the π-π*intraligand photoluminescence due to its resemblance to that of dib ligand.And the enhancement and slight red-shift of 1 compared to that of the dib ligand probably result from the fact that the coordination of Znギions increases the ligand conformational rigidity and thus reduces the loss of energy by thermal vibrational decay[18].
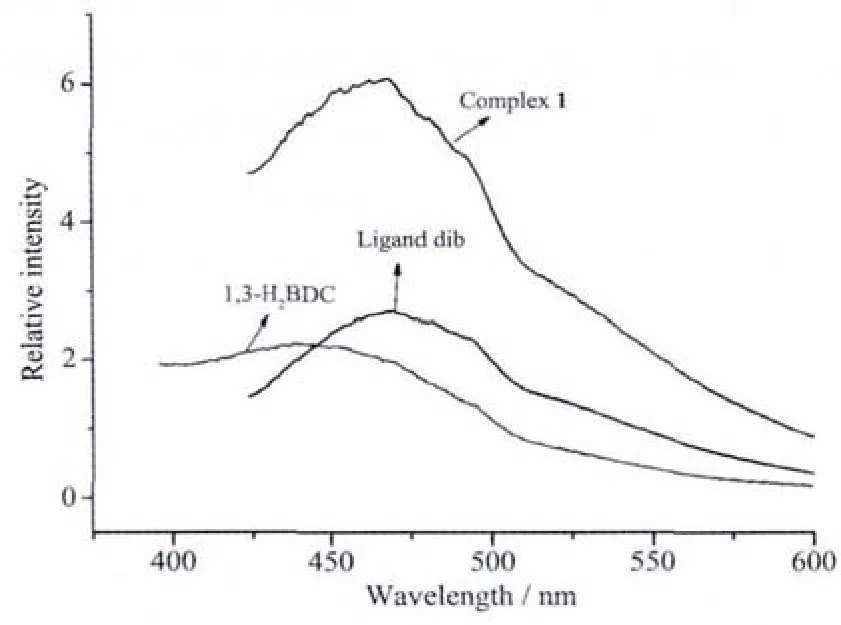
Fig.3 Solid emission spectra of the title complex and ligands
[1]O′Keeffe M,Yaghi O M.Chem.Rev.,2012,112:675-702
[2]Stock N,Biswas S.Chem.Rev.,2012,112:933-969
[3]Cui Y J,Yue Y F,Qian G D,et al.Chem.Rev.,2012,112:1126-1162
[4]Cohen S M.Chem.Rev.,2012,112:970-1000
[5]Ouellette W,Jones S,Zubieta J.CrystEngComm,2011,13:4457-4485
[6]Liu Y Y,Wang Z H,Yang J,et al.CrystEngComm,2011,13:3811-3821
[7]Chen S S,Bai Z S,Fan J,et al.CrystEngComm,2010,12:3091-3104
[8]Guo Z G,Cao R,Li X J,et al.Eur.J.Inorg.Chem.,2007:742-748
[9]Liu T F,Lu J,Shi L X,et al.CrystEngComm,2009,11:583-588
[10]Geng X H,Feng Y L,Lan Y Z.Inorg.Chem.Commun.,2009,12:447-449
[11]Lu Y,Lan Y Q,Xu Y H,et al.J.Solid State Chem.,2009,182:3105-3112
[12]Withersby M A,Blake A J,Champness N R,et al.J.Am.Chem.Soc.,2000,122:4044-4046
[13]Liu G X.Chinese J.Struct.Chem.,2012,31:933-938
[14]Sheldrick G M.SHELXL-97,Programm for the Refinement of Crystal Structure,University of Göttingen Göttingen,1997.
[15]CHEN Zhi-Min(陈志敏),KUANG Dai-Zhi(邝代治),FENG Yong-Lan(冯泳兰),et al.Chemistry(Huaxue Tongbao),2005,68:http://www.hxtb.org/col/2005/pdf/c05081.pdf
[16]Nakamoto K.Infrared and Raman Spectra of Inorganic and Coordination Compounds.5th Ed..New York:John Wiley&Sons,1997.
[17]Vogler A,Kunkely H.Coord.Chem.Rev.,2006,250:1622-1626
[18]Yang W Y,Schmider H,Wu Q G.,et al.Inorg.Chem.,2000,39:2397-2404
