2,2′-联吡啶-3,3′-二羧酸锰ギ配合物的合成、晶体结构及性能研究
2013-10-17张少华杨颖群匡云飞
张少华 杨颖群 匡云飞
(1衡阳师范学院化学与材料科学系,衡阳 421008)
(2衡阳市西城有机化工厂,衡阳 421008)
0 Introduction
Studies of carboxylic complexes have attracted much attention in the molecular construction and crystalengineering because they have versatile topological structures and potential applications in magnetism,optical device,catalysis and biological fields[1-7].2,2′-bipyridine-3,3′-dicarboxylic acid(H2L)is a class of pyridine carboxylic acid with more than one coordination atoms,and its complexes have been reported in many literature[8-20].In recent years,by using H2L as a ligand,we have also synthesized one carboxylate complex[Cu2(C12H6O4N2)2(2,2′-bipyridine)2]·CH3OH,and studied its magnetic and electrochemical properties[21].Our present work is a continuous study of our research on thesynthesisofcarboxylate complexes by using H2L.Herein,we report the synthesis method,crystal structure and magnetic,fluorescence and electrochemical properties of a new manganeseギ complex[Mn(H2O)(L)(Phen)2]·(H2O)3(1)with L2-anion as a ligand.
1 Experimental
1.1 Materials and instrumentations
H2L was prepared according to the literature method[22]and europiumバnitrate hexahydrate according to the literature method[23].Other agents were of analytical grade and used without further purification.Crystal structure determination was carried out on a BrukerSMART APEX-Ⅱ CCD diffractometer.Magnetic measurements in the range of 2.8~300 K were performed on a MPMS-SQUID magnetometer at a field of 2 kOe on a crystalline sample in the temperature settle mode.Fluorescence spectrum was obtained at room temperature on a WGY-10 fluorescence spectrophotometer.Cyclic voltammogram was measured on a CHI660D electrochemical workstation from Shanghai Chenhua.
1.2 Synthesis of the complex
A mixture of manganese ギ acetate(0.020 g),europiumバ nitrate(0.041 g),H2L(0.052 g)and phen(0.048 g)was dissolved in 6 ml mixed solvent of H2O/DMSO (volume ratio:5∶1).The resulting solution was poured into 20 mL hydro-thermal reaction autoclave and kept at 140 ℃ for 12 h.The resultant solution was filtrated,and the filtrate was stood at room for volatilization slowly.Yellow root-shaped single crystals suitable for X-ray diffraction analysis were obtained after five weeks with a yield of 36%.
1.3 Crystal structure determinations
A crystal with the dimensions of 0.24 mm×0.23 mm×0.22 mm was put on a Bruker SMART APEX-ⅡCCD diffractometer,which was equipped with a graphite-monochromatic Mo Kα radiation(λ=0.071 073 nm)by using ω-φ scan mode at 173(2)K.Of the total 16 166 reflections collected in the range of 1.78°≤θ≤25.01°,5 776 were independent with Rint=0.087 8;3 303 were considered to be observed(I>2σ(I))and used in the succeeding refinement.The crystal structure was solved with a direct method by using SHELXS-97 program[24].The corrections for Lp factors and the empirical adsorption adjustment were applied,while all non-hydrogen atoms were refined with the anisotropic thermal parameters using SHELXL-97 program.The finalrefinements including hydrogen atoms converged to R1=0.055 8,wR2=0.114 7;w=1/[σ2(Fo2)+(0.052 4P)2+0.179 4P],where P=(Fo2+2Fc2)/3,(Δ/σ)max=0.000,S=1.017.Crystallographic data of complex 1 are shown in Table 1.
CCDC:874919.

Table 1 Crystallographic data of complex 1
2 Results and discussion
2.1 Crystal structure analysis
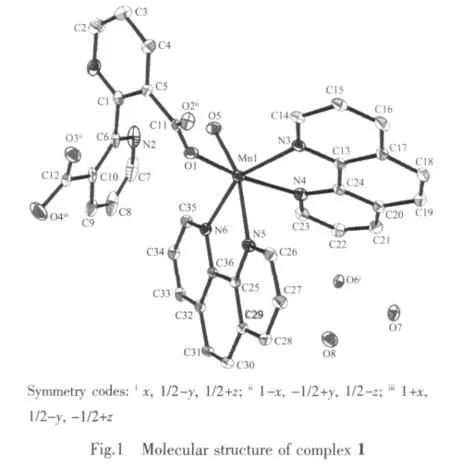
The crystal structure of 1 is revealed in Fig.1.Selected bond lengths and bond angles are shown in Table 2.As shown in Fig.1,the crystal structure of 1 consists of a central Mnギion,one L2-anions,two phens,one coordinated water and three lattice water molecules.The Mnギ ion is coordinated with four nitrogen atoms from two phens,two oxygen atoms from one L2-anion and one water molecule,giving a distorted octahedral coordination geometry in which O(5),N(3),N(5)and N(6)locate at the equator plane,O(1)and N(4)occupy the axial positions.The bond angles of O(5)-Mn(1)-N(3),N(3)-Mn(1)-N(5),N(5)-Mn(1)-N(6)and N(6)-Mn(1)-O(5)are 108.89(11)°,89.53(11)°,73.36(12)°and 88.88(11)°,respectively;and the sum of the above angles is 360.66°.The Mn-N distances are in the normal range of 0.226 7(3)~0.232 9(4)nm.In 1,the bond length of Mn(1)-O(5)is 0.211 9(3)nm,which is shorter than that of Mn(1)-O(1)(0.216 3 nm),indicating that the water molecule has a stronger coordination capability with the Mnギion than the oxygen atom of L2-anion.
In addition,there are strong hydrogen bonding interactions in 1.They could be observed between the adjacent free water molecules,between the free water molecules and the coordinated water molecules as well as between the water molecules and the L2-anions.Selected hydrogen bond lengths and bond angles of complex 1 are listed in Table 3.
2.2 Magnetic properties
The magnetic susceptibility data of 1 under variable temperatures (2.8 to 300 K)were colleted with an applied magneticfield of2 kOe.Thetemperature dependence ofthe molar magnetic susceptibility of 1 is presented in Fig.2 in the form of χmT and 1/χmvs T.As is indicated by Fig.2,the product of χmT changes less from 4.49 cm3·K·mol-1at 300 K to 4.46 cm3·K·mol-1at 14 K.When the temperature drops to about 13 K,the value of χmT is 4.45 cm3·K·mol-1,which is significantly elevated.In addition,the data in the range of 2.8 to 300 K are in a linear relationship between 1/χmvs T,and the linear regression equation is 1/χm=0.222 4T+0.052 7,the correlation coefficient is 1.According to the Curie-Weiss law, χm=C/(T-θ),the Curie constant(C=4.50 cm3·K·mol-1)and Weiss constant(θ=-0.24 K)are obtained.Such magnetic behavior indicates that 1 exhibits weak antiferromagnetic properties at low temperatures.
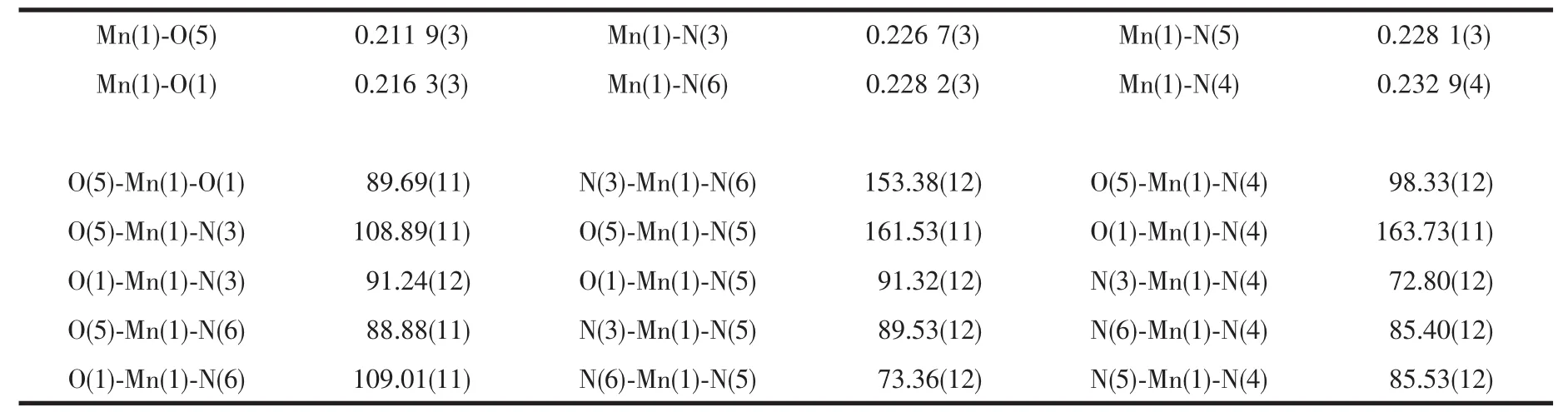
Table 2 Selected bond lengths(nm)and bond angles(°)of complex 1

Table 3 Selected hydrogen bond lengths and bond angles of complex 1

2.3 Fluorescence property
The fluorescence property of 1 was measured in the liquid state at room temperature in the range of 400~750 nm.The optimized excitation wavelength is 656 nm,and the emission spectrum is shown in Fig.3.Complex 1 exhibits one broad emission band around 682 nm.Comparably,under the same conditions,we measured the fluorescence property of the ligands phen and H2L,and the results show that the phen does not give off fluorescent emission,while H2L displays the emission band at about 676 nm.The emission band of 1 is similar to that of H2L(Fig.3),indicating that intraligand excitation is responsible for the emission of 1.However,there is a red shift of the emission for 1 comparing to that of H2L,which is probably due to the coordination between the ligand and Mnギion,because the photoluminescence behavior is closely associated with its local environment[25].

2.4 Electrochemical property
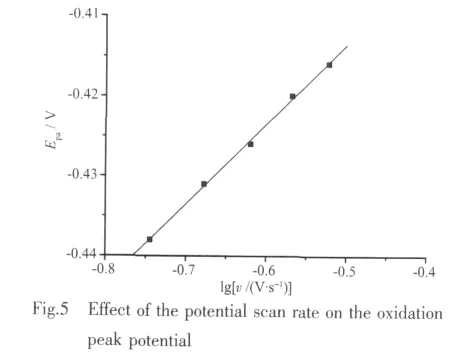
Fig.4 shows the cyclic voltammogram (CV)of 1 in the scan rate range of 0.18~0.30 V·s-1.In the CV measurement of 1,we employed a conventional threeelectrodes system where a platinum electrode was chosen as the working electrode,a saturated calomel electrode (SCE)as the reference electrode,and a glass/C electrode as the counter electrode.1 was dissolved and diluted to 1.2 mmol·L-1with methanol.HAc-NaAc solution(pH 4.0)was used as a buffer solution,and KClis used as the supporting electrolyte.The scanning range is-1.0 ~1.0 V,and the scan rate is 0.18~0.30 V·s-1.As revealed by Fig.4,there is a pair of redox peak in every CV curve,and the electron transfer in the electrode reaction is quasi reversible.In addition,the oxidation peak potential (Epa)shifts to a more positive value with increasing scan rate (v),and it is proportional to lgv.The linear regression equation is Epa(V)=0.099 5lgv-0.363 8 with the correlation coefficient of 0.9983(Fig.5).Based on the slope of Epawith lgv,the number of electrons involved in the reduction of 1 can be evaluated.The n is calculated to be 0.59.Generally,the electron transfer coefficient α is about 0.5 in the totally quasi reversible electrode process.So,the value of n is about 1,indicating that one electron is involved in the oxidation of 1,and the electrode reaction corresponds to Mnバ/Mnギ.

[1]Braverman M A,Supkowski R M,Laduca R L.Solid State Chem.,2007,180(6):1852-1862
[2]Beko S L,Bats J W,Schmidt M U.Acta Crystallogr.,Sect.C:Cryst.Struct.Commun.,2009,65:347-351
[3]WANG Ji-Jiang(王记江),HOU Xing-Yang(侯向阳),CAO Pei-Xiang(曹培香),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28(4):829-832
[4]XU Zhan-Lin(徐占林),HE Yu(贺宇),MA Shuai(马帅),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28(4):851-855
[5]TAN Xiong-Wen(谭雄文),DENG Yi-Fang(邓奕芳),ZHANG Chun-Hua(张春华),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28(4):856-860
[6]GAO Hong-Ling(高洪苓),HU Cong-cong(胡丛丛),ZHANG Hong(张虹),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28(3):632-636
[7]TIAN Jing(田婧),DONG Li-Na(董丽娜),ZHAO Kai(赵凯),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28(1):113-118
[8]Chen Q L,Chen J Z,Wang X W,et al.Acta Sci.Nat.U.Sunyatseni,2009,64:335-338
[9]CHEN Zhi-Min(陈志敏),LI Dong-Ping(李东平),LI Hui-Quan(李慧泉),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(11):1907-1911
[10]Zhang C Z,Mao H Y,Wang J,et al.Inorg.Chim.Acta,2007,360:448-454
[11]Kwak O K,Min K S,Kim B G.Inorg.Chim.Acta,2007,360:1678-1683
[12]Tang Y Z,Wang X S,Zhou T,et al.Cryst.Growth Des.,2006,6:11-13
[13]Wu B L,Zhang H Q,Zhang H Y,et al.Aust.J.Chem.,2003,56:335-338
[14]Zhang H T,Shao T,Wang H Q,et al.Acta Crystallogr.,Sect.C:Cryst.Struct.Commun.,2003,59:259-261
[15]Zhang H T,Shao T,Wang H Q,et al.Acta Crystallogr.,Sect.E:Struct.Rep.Online,2003,59:342-344
[16]Perkovic M W.Inorg.Chem.,2000,39:4962-4968
[17]Tong M L,Yang G,Chen X M.Aust.J.Chem.,2000,53:607-610
[18]Ruiz R,Caneschi A,Gatteschi D,et al.Inorg.Chem.Commun.,1999,2:521-523
[19]Swamy G Y S K,Chandramohan K,Lakshmi N V,et al.Z.Kristallogr.,1998,213:191-194
[20]Zhong Z J,You X Z,Yang Q.Polyhedron,1994,13:1951-1955
[21]Yang Y Q,Chen M S,Li W,et al.Z.Naturforsch B,2011,66b:894-898
[22]YAO Yue-Juan(姚月娟).Thesis for the Master′s Degree of Northwest University(西北大学硕士学位论文).2007.
[23]MA Rui-Xia(马 瑞 霞 ).Thesis for the Master′s Degree of Hebei Normal University(河北师范大学硕士学位论文).2006.
[24]Sheldrick G M.Acta Crystallogr.,1990,A46:467-473
[25]LI Wei-Qi(李伟琦),FENG Xi(封霞),FENG Yun-Long(冯云龙),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(6):873-879
