由5-硝基间苯二甲酸和1,4-双(咪唑基-1-甲基)-苯配体构筑的镍配位聚合物的水热合成及晶体结构
2013-10-17李秀梅纪建业牛艳玲王志涛
李秀梅 纪建业 牛艳玲 王志涛
(通化师范学院化学系,通化 134002)
0 Introduction
The design and construction ofmetal-organic coordination frameworks have attracted great attention due to their potential applications and fascinating structures[1-4].Great interests have been focused on self-assembly in the supramolecular architectures by exploiting coordinative bonding,hydrogen bonding,ππ stacking interaction and other weak intermolecular interactions[5-8].Among the frameworks reported so far,polycarboxylate ligands such as terephthalate,isophthalate,1,3,5-benzenetricarboxylate and 1,2,4,5-benzenetetracarboxylate have been proved particularly effective for the constructions of 1D,2D and 3D coordination polymers[9-11].Moreover,po1ycarboxylic acids with various functional groups such as amine,hydroxyl,sulfonate and nitro groups have formed many novel inorganic-organic hybrid materials[12-13].On the other hand,the employment of mixed ligands during the self-assembly process has gradually become an effective approach,which is expected to obtain frameworks with more diverse structural motifs compared to using only one type of ligands.Therefore,the auxiliary ligands such as imidazole-containing ligands,are introduced into the reaction systems so as to tune up the metal coordinated sites[14-16].Taking all the above discussion into account,we utilized the 5-nitroisophthalic acid (H2NIPH) and polycyclic aromatic bidentate bridging ligands,bix,to generate coordination polymers.The 5-nitroisophthalate(NIPH)is known as a versatile ligand,in which the two carboxylate groups can form covalent bonds with metal centers,while the nitro group can also act as a hydrogen-bond acceptor constructing strong hydrogen bonds in the assembly of coordination polymers.To our knowledge,metal coordination polymers with 5-nitroisophthalate have been reported[17-20].
Presented here are the synthesis and structure of a novel nickelギcoordination polymer[Ni(NIPH)(bix)(H2O)2]nwith one-dimensional chain-like structure.The packing in 1 gives rise to three-dimensional structurebased on weak coordination bond and hydrogen bonding interactions.
1 Experimental
1.1 General procedures
All reagents were purchased commercially and used without further purification.Elemental analyses(C,H and N)were measured on a Perkin-Elmer 2400 CHN Elemental Analyzer.IR spectrum was recorded in the range of 4 000~400 cm-1on an Alpha Centaurt FT/IR Spectrophotometer using a KBr pellet.TG studies were performed on a Perkin-Elmer TGA7 analyzer.The UV spectrum was obtained on a Shimzu UV-250 spectrometer in the 200~400 nm range.
1.2 Synthesis of[Ni(NIPH)(bix)(H2O)2]n
The title compound was prepared from a mixture of Ni(OAc)2·4H2O (0.050 g, 0.2 mmol),H2NIPH(0.042 g,0.2 mmol),bix(0.048 g,0.2 mmol)and H2O(18 mL)in a 30 mL Teflon-lined autoclave under autogenous pressure at 140 ℃ for seven days.After cooling to room temperature,green block crystals were collected by filtration and washed with distilled water in 41% yield (based on Ni).Anal.Calcd.for C22H21N5NiO8(%):C,48.74;H,3.90;N,12.92.Found(%):C,48.20;H,3.42;N,12.57.IR (KBr,cm-1):3120 w,2 361w,1 655w,1 599m,1 546w,1 528w,1 453w,1 437w,1 361s,1 339w,1 289w,1 234w,1 121w,1 088 m,1 035w,1 015m,948w,914w,876w,837w,805w,788w,759w,736w,726w,717s,660m,623w,580w,526w,473w,459w.
1.3 Structure determination
A single crystal of the title compound with dimensions of 0.408 mm×0.207 mm×0.170 mm was mounted on a Bruker CCD diffractometer equipped with a graphite-monochromatic Mo Kα (λ=0.071 073 nm)radiation using an ω scan mode at 296(2)K.In the range of 3.82°<2θ<52.02°,a total of 10 767 reflections were collected and 2 128 were independent with Rint=0.034 5,of which 1 703 were observed with I>2σ(I).The correction for Lp factors was applied.The structure was solved by direct methods with SHELXS-97 program[21]and refined by full-matrix least-squares techniques on F2with SHELXL-97[22].All non-hydrogen atoms were refined anisotropically and the hydrogen atoms of organic ligands were generated geometrically.The final R=0.032 4 and wR=0.081 9(w=1/[σ2(Fo2)+(0.041 2P)2+1.630 0P],where P=(Fo2+2Fc2)/3).S=1.049,(Δρ)max=278 e·nm-3,(Δρ)min=-370 e·nm-3and (Δ/σ)max=0.000.The selected bond parameters are given in Table 1.
CCDC:857178.
2 Results and discussion
2.1 IR spectrum
The COO-is coordinated with its asymmetric and symmetric stretching appearing at 1 599 cm-1(ν(OCO)assym)and 1 361 cm-1(ν(OCO)sym)[23],respectively.The Δν(ν(OCO)assym-ν(OCO)sym)are 238 cm-1(>200),showing the presence of monodentate linkage of carboxylates in the dianions.Thus the carboxylates coordinate to the metal as monodentate ligands via the carboxylic carboxylate groups[24].The absence of characteristic bands around 1 700 cm-1in compound 1 attributed to the protonated carboxylic group indicates the complete deprotonation of NIPH ligand upon reaction with Ni ions[25].In addition,X-ray diffraction analysis further indicates the monodentate coordination manners of carboxylate groups and the deprotonation of NIPH ligands.
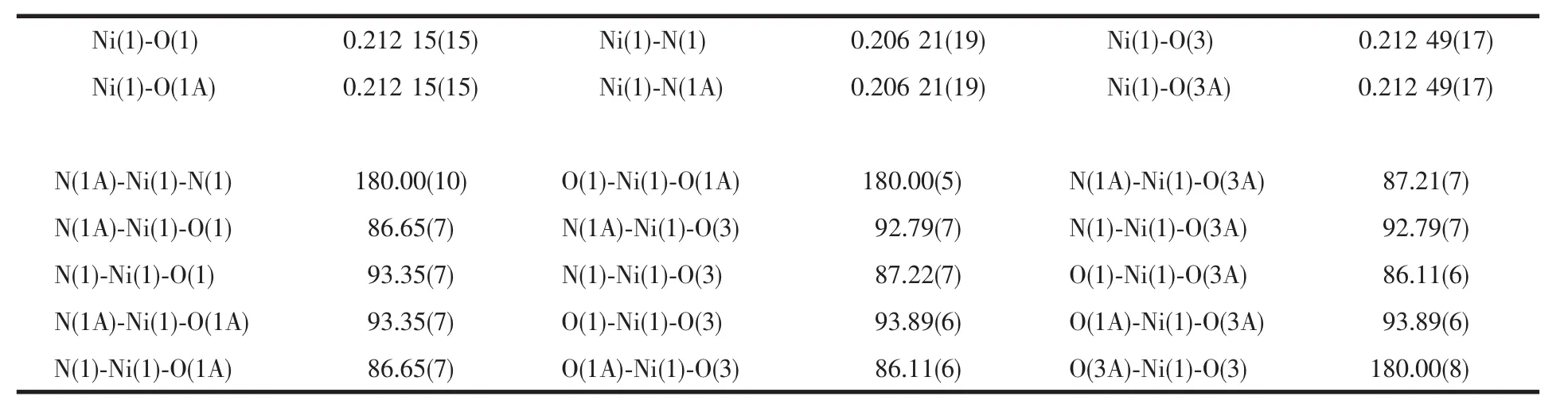
Table 1 Selected bond lengths(nm)and bond angles(°)
2.2 Description of the structure
A single crystal X-ray diffraction study reveals that complex 1 crystallizes in orthorhombic system with space group Pnma and the asymmetric unit contains Niギatoms,one NIPH ligand,one bix ligand and two coordinated water molecules,as shown in Fig.1.Ni(1) is six coordinated with slightly distorted octahedralcoordination geometry defined by two nitrogen donors(N1 and N1A)from two different bix molecules,two carboxylate oxygen atoms (O1,O1A)from two different NIPH ligands and two coordinated water molecules(O3,O3A).The bond distances of Ni-O in compound 1 fall in the 0.212 15(15)~0.212 49(17)nm range,and Ni-N bond length is 0.206 21(19)nm,which are in the normal range and the coordination angles around Ni atom are in the range 86.11(6)°~180.00(10)°.The completely deprotonated NIPH ligands display one kind ofcoordination mode,namely monodentate bridging mode,and bix ligands adopt classic bidentate bridging mode.
As shown in Fig.2,the Ni atoms are bridged by NIPH and bix ligands into a binuclear unit with a Ni…Ni separation of 1.064 5 nm.The dimeric Ni2units are linked by two NIPH ligands and two bix ligands to generate a one-dimensioal[Ni2(NIPH)2(bix)2(H2O)4]ndouble chain.Since bix is flexible ligands,it is not difficult to possess the same pitch in the molecular self-assembly process.As a result,two different kinds of chains are formed.For each infinite single chain contains two units of Ni-ligands,and the adjacent double chains share Ni atoms to develop into twodimensional sheets.

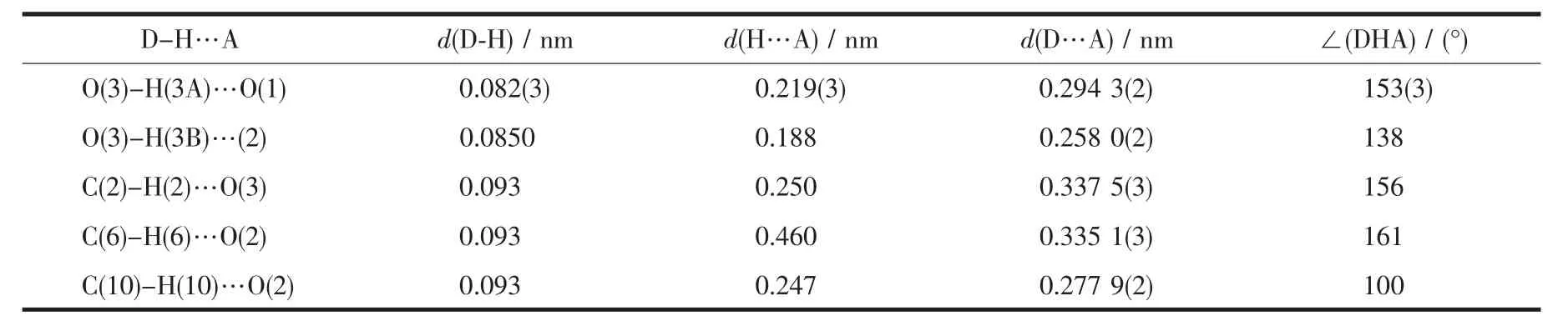
Table 2 Hydrogen bonds for compound 1
Hydrogen bonding interactions are usually important in the synthesis of supramolecular architectture[26].There are persistent O-H…O and CH…O hydrogen bonding interactions in the complex(Table 2),which play an important role in stabilizing the network structure and controlling the orientation of NIPH ligands.Therefore,through hydrogen bonds interactions,the two-dimensional sheets are further extended into athree-dimensionalsupramolecular framework(Fig.3).

2.3 Thermal analysis
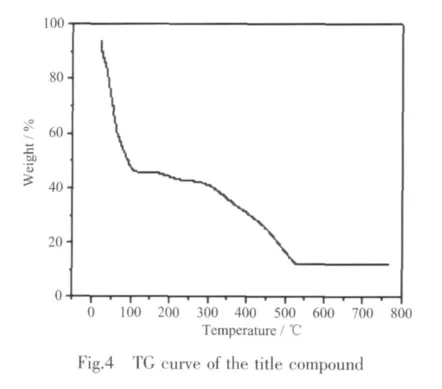
TG curve of 1 show that the first weight loss of 45.9%from 22 to 115 ℃ corresponds to the removal of two coordinated water molecules and NIPH ligand(calcd:44.6%).Upon further heating,an obvious weight loss (45.4%)occurs in the temperature range of 115~530 ℃,corresponding to the release of bix ligands(calcd:44.4%).After 530 ℃ no weight loss is observed,which means the complete decomposition of 1.The residual weight should be NiO.
2.4 UV spectrum
The UV spectra for the title compound,H2NIPH and bix ligands have been investigated in the solution state.For bix ligand,there is no absorption band,while both the title compound and H2NIPH have one absorption band at about 274 nm,which should be assigned to the n→π*[27]transition of H2NIPH.However,after H2NIPH coordinating to the Ni2+ion,the absorption intensity slightly decreases.It is clearly that the absorption band in H2NIPH remains in the same position with that in the title compound,showing that they are not affected basically by the metal coordination.
王 雪 男,1979年3月出生于辽宁省锦州市,现为中国科学院国家授时中心导航与通信研究室研究员.从事导航技术研究工作.
Reference:
[1]Rao C N R,Natarajan S,Vaidhyanathan R.Angew.Chem.Int.Ed.,2004,43(12):1466-1496
[2]Moulton B,Zaworotko M J.Chem.Rev.,2001,101(6):1629-1658
[3]Li H,Eddaoudi M,Yaghi O M,et al.Nature,1999,402(6759):276-279
[4]Hagrman P J,Hagrman D,Zubieta J.Angew.Chem.Int.Ed.,1999,38(18):2638-2684
[5]Yaghi O M,Li H,Davis C,et al.Acc.Chem.Res.,1998,31(8):474-484
[6]Humphrey S M,Mole R A,Wood P T,et al.J.Chem.Soc.,Dalton Trans.,2004,11:1670-1678
[7]Xu J Q,Xu D Q,Tang A Q,et al.Sci.China Ser.B:Chem.,
[8]Ye K Q,Wu Y,Wang Z,et al.Chin.Sci.Bull.,2005,50(5):417-420
[9]Li X M,Niu Y L,Wang Q W,et al.Chinese J.Struct.Chem.,2010,29(7):1122-1126
[10]Zarracino R G,Quinonesm J R,Hopfl H T.Inorg.Chem.,2003,42(12):3835-3845
[11]Murugavel R,Krishnamurthy D,Sathiyendiran M.J.Chem.Soc.,Dalton Trans.,2002,1:34-39
[12]Sun D F,Cao R,Sun Y,et al.Chem.Commun.,2003,13:1528-1529
[13]Tao J,Yin X,Jiang Y B,et al.Inorg.Chem.Commun.,2003,6(9):1171-1174
[14](a)Liu Y Y,Ma J F,Yang J,et al.Inorg.Chem.,2007,46:3027-3037(b)Tian Z F,Lin J Z,Su Y,et al.Cryst.Growth Des.,2007,7:1863-4867(c)Yang J,Ma,J F,Liu Y Y.et al.Eur.J.Inorg.Chem.,2006:1208-1215
[15](a)Sharma C V K,Rogers R D.Chem.Commun.,1999:83-84(b)Wang Q M,Guo G C,Mak T C W.Chem.Commun.,1999:1849-1850(c)Wang C C,Yang C H,Tseng S M,et al.Inorg.Chem.,2003,42:8294-8299
[16](a)Hoskins B F,Robson R,Slizys D A.J.Am.Chem.Soc.,1997,119:2952-2953(b)Wen L L,Lu Z D,Lin J G,et al.Cryst.Growth Des.,2007,7:93-99(c)Huang X C,Zhang J P,Lin Y Y,et al.Chem.Commun.,2004:1100-1101
[17]Tao J,Yin X,Wei Z B,et al.Eur.J.Inorg.Chem.,2004,1:125-133
[18]Luo J H,Hong M C,Wang R,et al.Inorg.Chem.,2003,42(15):4486-4488
[19]Luo J H,Hong M C,Wang R,et al.Eur.J.Inorg.Chem.,2003,14:2705-2710
[20]Abourahma H,Moulton B,Zaworotko M J,et al.J.Am.Chem.Soc.,2002,124(34):9990-9991
[21]Sheldrick G M.SHELXS 97,Program for the Solution of Crystal Structure,University of Göttingen,Germany,1997.
[22]Sheldrick G M.SHELXS 97,Program for the Refinement of Crystal Structure,University of Göttingen,Germany,1997.
[23]Devereux M,Shea D O,Kellett A,et al.Inorg.Biochem.,2007,101:881-892
[24]FarrugiaLJ,WingXA.WindowsProgramforCrystalStructure Analysis,University of Glasgow,Glasgow,UK,1988.
[25]Bellamy L J.The Infrared Spectra of Complex Molecules,Wiley,New York,1958.
[26]Krische M J,Lehn J M.Struct.Bonding,2000,96:3-29
[27]Mohamed G G,El-Gamel N E A.Spectrochim.Acta,Part A,2004,60:3141-3154