Control Synthesis and Bioconjugation of Core-Shell Structured Gold Nanoparticles
2013-09-29ZHANGHaoRanMANShiQingXUMengZHUXinHaiDepartmentofElectronicEngineeringInstituteofNanoChemistryDepartmentofOncologyTheFirstAffiliatedHospitalJinanUniversityGuangzhou506
ZHANG Hao-RanMAN Shi-Qing*,XU MengZHU Xin-Hai(Department of Electronic Engineering,Institute of Nano-Chemistry,Department of Oncology,The First Affiliated Hospital,Jinan University,Guangzhou 506)
Control Synthesis and Bioconjugation of Core-Shell Structured Gold Nanoparticles
ZHANG Hao-Ran1,2MAN Shi-Qing*,1,2XU Meng3ZHU Xin-Hai3
(1Department of Electronic Engineering,2Institute of Nano-Chemistry,3Department of Oncology,The First Affiliated Hospital,Jinan University,Guangzhou 510632)
A series of Au/SiO2nanoshells was synthesized and the process of their growth was studied in detail.Two competing reaction ways during the process of the Au nanoshell′s growth were found.Utilizing this rule,the plasmon absorption of these nanoshells was continuously tuned from 524 nm to 980 nm.Endostar,a commercial anti-tumor medicine was firstly tried to bioconjugate to the surface of Au/SiO2nanoshells whose absorption peak was at 808 nm.We obtained the new composition Au/SiO2-Endo,and the FTIR spectra proved this successful bioconjugation.By combining the specific function of the starving tumor and a certain target tropism to tumor of Endostar with the easy tunability and favorable photothermal effect of gold nanoshells,it is expected that a novel kind of composite medicine with stronger ablation for tumor can be obtained by the present method.
gold nanoparticle;core-shell;bioconjugation;Endostar
0 Introduction
Recently,considerable efforts have been devoted to the design and constructing of nanometer-and micrometer-sized core-shell structured composite particles with special physical and chemical properties due to their great potential applications in photonic crystals,catalysis,diagnostics,pharmacology[1-5],and surface-enhanced Raman scattering(SERS)[6].Till now,different types of core-shell structured particles have been developed[1-11].Among these reported core-shell structured particles,silica is proven a frequently used agent in core-shell structured materials,either as a core or a shell because it is cheap and easy to tailor its size,morphology,component,and microstructure[6,12-17].
In most recent years,core-shell structured silica coped with noble metal,especially gold,have been studied extensively due to their promising applications in biomedicine[6,16].It has been demonstrated that nanoshell optical resonances may be continuously tuned over a broad region from the ultraviolet to the infrared,including the NIR region where is the transparent window of biological tissues[16,18],just by varying the relative dimensions of the core radius and shell thickness.Gold nanoshells offer appealing properties for biomedical sensing and therapeutic applications including large optical cross-sections exceeding conventional NIR dyes by many orders of magnitude as well as significantly improved photostability resulting from the rigid metallic structure of the nanoshell[3,19].Furthermore,antibodies and other targeting moieties can be readily conjugated to the gold surface of nanoshells,which endows gold nanoshells with target tropism[20-21].Because of the ability to convert light to heat energy that results in a local rise in temperature,gold nanoshell also can be embedded within temperature-sensitive hydrogels to develop a photothermal modulated drug delivery system[22].All of these further broaden the application of Au nanoshell in biomedicine field.
In our present work,NIR absorption-controllable core-shell structured gold nanoparticles,consisting of a silica core with a gold shell,were prepared by a combination of molecular self-assembly and the subsequent electroless plating of gold[23-26].This approach has some advantages that it is relative simple,inexpensive,and easier to control the core diameter and shell thickness of the nanoparticles.More importantly,the absorption of the functional core-shell structured gold nanoparticles can be easily tailored to satisfy the requirement of applying this kind of materials in photothermal therapy.We also tried to biomodify the surface of the gold nanoshells with a kind of commercial medicine,Endostar,which was an endogenous inhibitor of tumor angiogenesis and tumor growth.As we know that angiogenesis,sprouting new blood vessels from existing capillaries,is critical for tumor growth[27-28].Therefore,antiangiogenic molecules offer new promises as novel therapeutic modalities for “starving” the tumors[29].Combined the specific starving effect and its certain targeting tropism of Endostar to tumor with photothermal effect of gold nanoshells,it was expected to obtain a novel kind of compound medicine for tumor therapy.
1 Experimental
1.1 Chemical and procedures
Synthesis of silica cores.Amorphous submicrometer spheres of silica in the size range of 120 nm were synthesized by base catalyzed hydrolysis of tetraethoxysilane(TEOS)via the well-known Stber process[30].This method yielded the colloidal solution of silica particles with a narrow size distribution in the nanosized range,and the particle size of silica depended on the relative concentration of the reactants.In a typical experiment the mixture containing 1.5 mL TEOS (99wt%,A.R.,Beijing Beihua Chemicals Co.,Ltd),10 mL H2O,5 mL NH4OH (25wt%,A.R.)and 25 mL anhydrous ethanol was stirred at room temperature for 8 h,resulting in formation of a white silica colloidal suspension.The surface of silica particles were terminated with amine groups by reaction with aminopropyltriethoxysilane(APTES,97%,Avocado research Chemical,Ltd).Products were centrifugally separated from the suspension and washed with ethanol four times,then dispersed in ethanol.
Coating of SiO2cores with Au shells.Small size of gold colloid (1~2 nm)was synthesized according to Stuff[31].This colloid was aged for two weeks in fridge and then concentrated in low pressure and low temperature.The aminated silica particles were then added to the gold colloid suspension in order to attach the gold colloid to the dielectric nanoparticle surfaces via molecular linkages.The silica nanoparticle covered with islands of gold colloid which served as “seed”,for the final nanoshell would grow up based on those islands of gold colloid,eventually coalescing with neighboring colloid,to form a complete gold shell.The concentration of this seed we used here was 3.89×1012mL-1.Gold nanoshells were then grown by reacting HAuCl4(Signa-Aldrich)with the seeds(50 μL)in thepresence of formaldehyde.And two strategies were designed for controlling the growth of these nanoshells.Firstly the mole ratio of HAuCl4to formaldehyde was set at 1∶100,keep other condition invariable and only change the amount of HAuCl4solution(0.38 mmol·L-1).Secondly,keep the added quantity of HAuCl4solution and other condition constant but vary the amount of formaldehyde solution(37%).
Bioconjugation of the Au/SiO2nanoshells.Endostar(5 g·L-1)waspurchased from Shandong Simcere-Medgenn Bio-pharmaceutical Co.,Ltd.Endostar is an endogenous inhibitor of tumor angiogenesis and tumor growth.It has two pairs of disulfide bonds in a unique nested pattern,which play a key role in its native conformation,stability,and activity[32].For one strategy we treat this protein medicine of Endostar by the conventionalway like antibody.The binding of antibodies to gold nanospheres was first reported in the 1950s since the antibodies are generally bound to negatively charged gold nanospheres by adjusting the pH of the colloidal solution to be just above the isoelectric point of the antibody such that the antibody has a small net negative charge.The antibodies are thus nonspecifically adsorbed onto gold nanoparticles while keeping the nanoparticles negatively changed,providing stability in solution[33-34].A proper amount of Au nanoshells was sufficiently mixed with the Endostar in PBS buffer solution (pH=7.0 ~7.4),and then this solution was kept overnight.The sample was washed by repeated cycles of centrifugation and redispersion in water 6 times to ensure that the residue Endostar was completely removed.Finally,the sample was dried in vacuum under 30 ℃ for the IR measurement.
For another strategy the common protein linker of 2-iminothiolane was used,which could react with the amidogen of protein and release the SH group with the ability to chemically bond onto the metal surface,as shown in Scheme 1.Since thiol chemistry has been widely used for the modification on gold surfaces[35-36],thus the released thiol is used for the same purpose.The 2-iminothiolane was dissolved in the K2CO3solution(pH 8.5),then mixed with proper amount of Au nanoshells and Endostar,the molar ratio of Endostar to 2-iminothiolane was 1∶1.The sample reacted overnight then washed it by repeated cycles of centrifugation and redispersion 6 times in water,followed by drying in vacuum under 30℃to obtain the sample for IR measurement.

Scheme 1 Reaction process of 2-iminothiolane with protein
1.2 Characterization
FTIR spectra were measured by BrukeEquinox-55 infrared spectrophotometer with the KBr pellet technique.
The morphologies of the obtained samples were determined by a Tecnai-10,Philips (purchased from Holland)transmission electron microscope (TEM)at 100 kV acceleration voltage.SamplesforTEM measurement were prepared through ultrasonic dispersion in deionized water and then placing a drop of dispersed suspension on carbon coated copper grid and dried at room temperature.
The UV-Vis absorption spectra range 400~900 nm were recorded on a Cary 5000(VARIAN),and those range 200~1 300 nm were recorded on a UV2550PC(SHIMADZU),all in 1 cm quartz.
2 Results and discussion
In order to systemically investigate the possible factors which affect the formation of the nanoshells and its absorption properties,a series of Au/SiO2samples were synthesized firstly by varying the amount of HAuCl4solution as described in the experimental section.Fig.1 showed the TEM images of aminated SiO2nanoparticles before and after reacting with different amount of HAuCl4solution.As shown in Fig.1(a),the as-prepared aminated SiO2particles were spheric shape with a relatively uniform size of 120 nm.Fig.1(b)was the SiO2particles absorbed with fine gold colloids serving as the “seed” for the further packing or growth of gold shell.Fig.1(c~g)showed the process of Au growth based on the seed by increasing the amount of HAuCl4solution.Inspection of these TEM images,it wasclearthatwhen theamountofHAuCl4isincreasing,firstly,the Au reduced based on those seeds,the Au colloids on the surface of silica became bigger and bigger(Fig.1c~d),when the nearby isolated Au nanoshells connected,the complete spherical Au nanoshells were formed.It was clear that the sample in Fig.1e had already formed a complete Au nanoshell with its absorption peak at 707 nm (Fig.2d)and its thickness was approximately 27 nm.Kept on increasing the added amount of HAuCl4solutions,the Au shells continuously grew,became more and more thick,tight and homogeneous(Fig.1f~g).The thickness of Au shells,df≈32 nm,dg≈35 nm.However,when the increasing amount of HAuCl4solution over a certain degree as reflected in Fig.1(h~i),the integrality of Au nanoshells was not supported,the silica core began to be naked and the Au colloids began to aggregate.

fig.1 TEM images of aminated SIO2 nanoparticles(a);adsorbed with fine Au colloid and serving as te seed(b);and AUu-shelled sampes after reacting with different amount of HAuCL4(0.38 mmol·L-1):(c)3 mL;(d)18 mL;(e)26 mL;(f)32 mL;(g)47 mL;(h)60 mL;(i) mL
The optical absorption spectra of different Au/SiO2nanoshells synthesized by varying the amount of HAuCl4solution were shown in Fig.2,it could be seen that the absorption peaks moved to the red region gradually and the shape of these peaks changed from narrow to broad when increasing HAuCl4amount.However,if 60 mL HAuCl4solution was added,the absorption spectrum presented double peaks located at 850 nm and 683 nm,respectively (Fig.2g).It seemed like that another kind of product was formed besides the desired Au nanoshells,and results in the additional absorption peak at 683 nm.Reviewed the TEM image in Fig.1(h)of the same sample,gold nanoshells began to be wrecked by Au colloids and their aggregation distributed around the silica cores.Reflected in the shape of absorption spectra,the peak located at 850 nm denoted the remnant gold nanoshells while peak located at 683 nm indicated the alien formation of huge goldcolloids or micells.kept on increasing HAuCl4solution to 70 mL the absorption peak changed back to a single peak,and blue shifted to 602 nm as shown in Fig.2h.It might suggest that Au colloids and aggregation had conquered and replaced the formation ofgold nanoshells ulteriorly.Fig.1i gave the visual evidence of such shift,the integral Au nanoshells had been destroyed and the fragment rapped off the silica core,while huge Au colloids or aggregations presented around the silica core.Thus corresponded to the absorption spectrum (Fig.2h),the NIR region peak which denoted the gold nanoshells disappeared while absorption spectrum at 602 nm denoted the gold colloid or aggregation came forth.Throughout the whole design process the only variety was the amount of HAuCl4solutions,which led to the evolution of Au nanoshells:came into being,grew,existed and dissolved.

Fig.2 Optical absorption spectra of Au-shelled samples synthesized by reaction of seed with different amount of HAuCl4(0.38 mmol·L-1)solution:(a)0 mL;(b)3 mL;(c)18 mL;(d)26 mL;(e)32 mL;(f)47 mL;(g)60 mL;(h)70 mL
Colligate these facts mentioned above,it is readily supposed that the nanoshells′growth we have tried to demonstrate actually comprises two competing reaction:(i)Au3+wrere reduced and deposited on the surface of the silica particle to form gold shells as anticipated;(ii)Au3+were reduced to form nucleation of additional gold colloid in the solution,then grew or aggregated.Which reaction would take leading role is mainly decided by the reaction rate,as shown in Scheme 2,the slow reaction rate is fit for the formation of gold nanoshells while the comparatively rapid reaction rate is inclined to form gold colloids and aggregations.Well,in our experiment,the few amount of the HAuCl4solution resulted in the slow reaction rate and led to the growth of the Au nanoshell based on the seeds little by little suchasFig.1(c~g).WhenHAuCl4solutionwasincreased to a certain degree such as 60 mL,it appeared to result in preferential deposition based on self-nucleation,then Au colloidswereproduced in thesolution and aggregated around the silica core accompanying the formation of gold shells as shown in Fig.1h,and the additional peak arised in short wavelength(Fig.2g).Futher increasing HAuCl4solution,like 70 mL,would speed up the kinetics of the gold reduction,the process(ii)conquered the process(i),thus a mass of Au colloids were ruduced in the solution and grew bigger or aggregated and scarcely any Au shells were formed(Fig.1i).Fig.2h also gave spectral evidence of such shift by the replacement of long wavelength with short wavelength.

Scheme 2 Predicted competing reaction ways during the process of Au nanoshells formation that was expected
To verify the hypothesis about the two competing reaction process,another series of samples were prepared,fixed the amount of the seed solution and HAuCl4solution,varied the amount of formaldehyde solution.In this strategy the amount of reductant formaldehyde was the controlling factor of the reaction kinetics,the more formaldehyde added the quicker reaction rate would be.And quick reaction rate preferred the competing process (ii),so Au colloids were produced preferentially in the solution.When the amount of formaldehyde and HAuCl4was consumed to a certain degree,the reaction rate decreased gradually and was insufficient to support the process(ii),then the reaction switched to process(i)and the residual Au3+was reduced based on the seeds(silica nanoparticles modified by nucleation site)to form thinner Au nanoshells.For small nanoparticles of Au colloids were formed in the solution,deposition of the nanoshell gold layer would continue at the expense of these particles.It could be predicted that the more formaldehyde solution was added the more Au colloids would be produced in the solution,then the less residual Au3+would be reduced based on the seeds to form Au shells,and the thinner Au nanoshells would be.According to Mie[37],for the same core dimension,the thinner the gold nanoshell is,the more red-shift of its absorption spectrum will be.Fig.3 exactly manifested such relationship, when increased the amount of formaldehyde the wide peak of these samples red shifted from 865 nm to 980 nm.The shoulder located around 600 nm (Fig.3a~e)indicateed the presence of Au colloids in solution[38],which further proved the validity of the competing reaction to form Au colloids besides Au nanoshells.
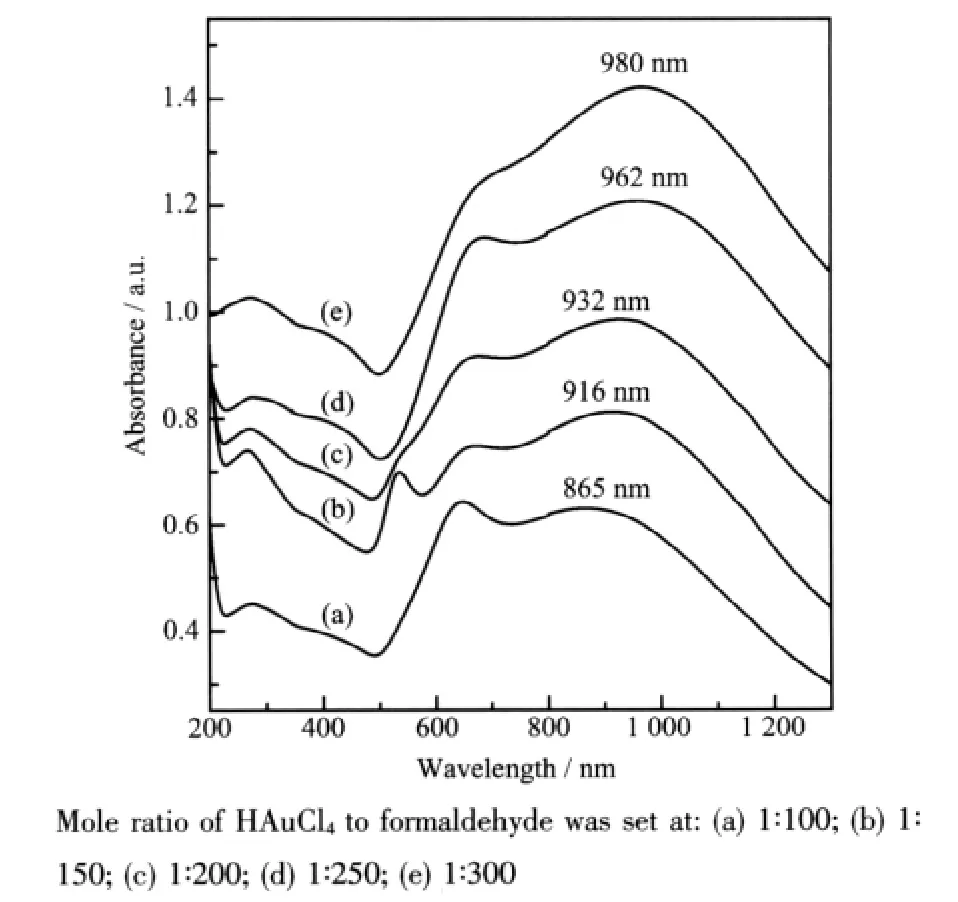
Fig.3 Optical absorption spectra of Au-shell samples synthesized by 60 mL HAuCl4solution(0.38 mmol·L-1)reacting with different amount of formaldehyde solution(37%)
In summary,the controlling method used in this experiment might be taken for an ordinary strategy for monitoring the core radius-shell thickness ratio,and this dimension ratio decided the optical property of the Au nanoshells.The main characteristic of this strategy included a preliminary process of undergoing some selfconsuming which might be called sacrificial reaction.Au nanoshells were formed at the expense of gold colloids(self-consuming),when the reaction kinetics self-adjusted to suit the process(i)then the expected production,Au nanoshells were formed.The density of Au nanoshells here wascontrolled bytheselfconsuming process inversely.
Fig.3 showed spectral evidence of the larger gold colloid presenting in solution in the form of a shoulder located around 600 nm,also evident in TEM images of Fig.4.In addition to the gold nanoshellswere represented in the picture,some comparatively little gold particles were also found in the wings(similar images of other samples were not shown).The gold nanoshells exhibit the broad NIR absorption at 980 nm and the gold colloids exhibit the faintish shoulder peak at 680 nm as shown in Fig.3(e).

Fig.4 TEM images of Au-shell samples synthesized by 60 mL HAuCl4solution(0.38 mmol·L-1)and formaldehyde solution(37%),their mole ratio is 1∶300
For the metal layer of gold nanoshells was grown using the same chemical reaction as gold colloid synthesis,the surfaces of gold nanoshells were virtually chemically identical to those of gold colloids.Thus the methods used to bioconjugate gold colloid were also apt for gold nanoshells.
Fig.5 (a)was the FTIR spectra of Au/SiO2nanoshells,the 1 093 cm-1absorption band could be attributed to the asymmetric stretching of Si-O.Fig.5(b,c)were the FTIR spectra of Au/SiO2nanoshellsbioconjugated with Endostar directly and through the protein linker of 2-iminothiolane respectively,it was clear that in addition to reserving the same patterns of SiO2,they also represented a series of peaks among 1 000~1 500 cm-1and 3 700~4 000 cm-1,which should be attributed to the presence of Endostar.Thus it proved that the protein medicine of Endostar had been bioconjugated to the surface of Au/SiO2nanoshells successfully through the two strategies.However,the asymmetric stretching of Si-O(1093 cm-1)in Fig.5c was weaker than that in Fig.5b,which might indicate the bioconjugation through protein linker provided the better bonding strength than electrostatic strategy,thus the asymmetric stretching of Si-O(1093 cm-1)in Fig.5c was shielded by the bioconjugated layer as shown.In the further study we would like to test the effect of these compound nano-medicines in ablation of cancer cells in vitro.

Fig.5 FTIR spectra of Au nanoshells before(a)and after bioconjugated with Endostar directly(b),and through protein linker of 2-iminothiolane(c)
3 Conclusion
A series of Au/SiO2nanoshells were synthesized by the combination of molecular self-assembly and the subsequent electroless plating of gold.The growth of Au nanoshells was studied in detail,we found that the transitory process actually contained two competing reaction:Au3+was reduced and deposited onto silica surface to form Au nanoshells;high kinetics results in the self-nucleation of Au3+and Au colloids were formed in the solution.This rule facilitated us tuning the plasmon absorption of these nanoshells red shift to 980 nm continuously.We conjugated a commercial antitumor medicine,Endostar,to the surface of Au/SiO2nanoshells in two ways,one strategy was direct bioconjugation,another strategy was using the common protein linker of 2-iminothiolane which could connect Endostar to the surface of Au/SiO2nanoshells,and the two strategies were proven successful.The combination of the specific function for starving tumor and a certain target tropism to tumor of Endostar with the easy tunability and photothermal effect of gold nanoshells,mightprovide apromising candidateforcancer treatment.
[1]Schartl W.Adv.Mater.,2000,12(24):1899-1908
[2]Caruso F.Adv.Mater.,2001,13(1):11-22
[3]Suryanarayanan V,Nair A S,Tom R T,et al.J.Mater.Chem.,2004,14(17):2661-2666
[4]LinAWH,LewinskiNA,RakalinAA,etal.Opt.Eng.,2005:60100L1-60100L8
[5]LizMarzan L M,Giersig M,Mulvaney P,et al.Langmuir,1996,12(18):4329-4335
[6]Xiao G N,Man S Q.Chem.Phys.Lett.,2007,447(4/5/6/7):305-309
[7]XIAO Gui-Na(肖桂娜),MAN Shi-Qing(满石清),LIU Ying-Liang(刘应亮),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2007,23(10):1738-1742
[8]XIAO Gui-Na(肖 桂 娜),MAN Shi-Qing(满 石 清).Chem.J.Chinese Universities(Gaodeng Xuexiao Huaxue Xuebao),2009,30(5):849-854
[9]PENG Hui(彭 卉),MAN Shi-Qing(满石清).Chin.J.Anal.Chem.(Fenxi Huaxue),2010,38(5):611-616
[10]Sertchook H,Avnir D.Chem.Mater.,2003,15(8):1690-1694
[11]Giersig M,Ung T,Liz-Maran L M,et al.Adv.Mater.,1997,9(7):570-575
[12]Salgueirino-Maceira V,Spasova M,Farle M.Adv.Funct.Mater.,2005,15(6):1036-1040
[13]Liu S,Han M.Adv.Funct.Mater.,2005,15(2):961-967
[14]Hardikar V V,Matijevi E.J.Colloid Interface Sci.,2000,221(1):133-136
[15]Chan Y,Zimmer J P,Stroh M,et al.Adv.Mater.,2004,16:2092-2097
[16]Liu Z,Song H,Yu L,et al.Appl.Phys.Lett.,2005,86(11):113109/1-113109/3
[17]YAO Zu-Fu(姚祖福),HUANG Ke-Long(黄可龙),YU Jin-Gang(于金刚),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(12):2164-2168
[18]Sun Y,Xia Y.Anal.Chem.,2002,74(20):5297-5305
[19]YANG Jian-Hui(杨建辉),LU Le-Hui(逯乐慧),ZHANG Hong-Jie(张洪杰).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(8):1191-1199
[20]Hirsch L R,Jackson J B,Lee A,et al.Anal.Chem.,2003,75(10):2377-2381
[21]Fu K,Sun J,Bickford L R,et al.Nanotechnology,2008,19(4):045103-045108
[22]Bikram M,Gobin A M,Whitmire R E,et al.J.Control.Release,2007,123(3):219-227
[23]Loo C,Lin A,Hirsch L,et al.Cancer Res.Treat,2004,3(1):33-40
[24]Hirsch L R,Stafford R J,Bankson J A,et al.Proc.Nati.Acad.Sci.U.S.A.,2003,100(23):13549-13554
[25]Oldenburg S J,Averitt R D,Westcott S L,et al.Chem.Phys.Lett.,1998,288(2/3/4):243-247
[26]HU Yong-Hong(胡永红),RONG Jian-Hua(容建华),LIU Ying-Liang(刘应亮),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2005,21(11):1672-1676
[27]Folkman J.N.Engl.J.Med.,1971,285(21):1182-1186
[28]Folkman J.J.Natl.Cancer Inst.,1990,82(1):4-6
[29]Shi H,Huang Y,Zhou H,et al.Blood,2007,110(8):2899-2906
[30]Stöber W,Fink A,Bohn E.J.Colloid Interf.Sci.,1968,26(1):62-69
[31]Duff D G,Baiker A,Edwards P P.Langmuir,1993,9(9):2301-2309
[32]He Y,Zhou H,Tang H,et al.J.Biol.Chem.,2006,281(2):1048-1057
[33]Sokolov K,Follen M,Aaron J,et al.Cancer Res.,2003,63(9):1999-2004
[34]Sokolov K,Aaron J,Hsu B,et al.Tech.Cancer Res.Treat.,2003,2(6):491-504
[35]Loo C,Lowery A,Halas N J,et al.Nano Lett.,2005,5(4):709-711
[36]Lu H B,Campbell C T,Castner D G.Langmuir,2000,16(4):1711-1718
[37]Bohren C F,Huffman D R.Absorption and Scattering of Light by Small Particles.New York:Wiley,1983.
[38]Link S,El-Sayed M A.J.Phys.Chem.B,1999,103(21):4212-4217
金核壳纳米粒子的控制合成及其生物复合
张浩然1,2满石清*,1,2徐 萌3朱新海3
(1暨南大学电子工程系,2暨南大学纳米化学研究所,3暨南大学第一附属医院肿瘤科,广州 510632)
合成了一系列Au/SiO2核壳纳米粒子,并详细研究了Au纳米壳层的生长过程。发现在金纳米壳层形成的过程中存在着2个竞争反应。利用这一发现,可将金纳米壳层的吸收峰从524 nm连续调谐至980 nm处。恩度是一种临床抗癌药物,我们首次将其生物复合于吸收峰位于808 nm的Au/SiO2壳层表面,得到Au/SiO2-Endo,通过FTIR测试证明该生物复合成功。将恩度特殊的饿杀肿瘤特性以及对肿瘤具有特异识别能力,与Au/SiO2纳米壳层结构的光学可调谐特性以及良好的光热转换能力复合于一体,我们期望得到一种治疗肿瘤效果更强的新型药物。
金纳米粒子;核壳结构;生物复合;恩度
O614.123
:A
:1001-4861(2010)10-1768-08
2010-04-06。收修改稿日期:2010-06-07。
张浩然,女,29岁,博士研究生;研究方向:纳米材料的合成及应用。
国家自然科学基金(No.60477015),广东省自然科学基金(No.9151063201000018)和广东省自然科学基金团队项目(No.05200555)资助。
*通讯联系人。 E-mail:tsqman@jnu.edu.cn,Tel:020-85220658
猜你喜欢
杂志排行
无机化学学报的其它文章
- Solvothermal Synthesis,Crystal Structure and Photoluminescence Property of a Coordination Polymer Based on 1,1′-Ethynebenzene-3,3′,5,5′-tetracarboxylate
- Synthesis,Structure and Fungicidal Activity of Organotin 1H-Tetrazolyl-1-acetates
- Synthesis and Characterization of Tungsten Oxide Nanostructures
- Syntheses,Crystal Structure and Optical Property of Two Bis-ligand Silver(Ⅰ)Complexes Containing Diphenic Acid and Bidentate N-donor Ligands
- Syntheses and Structures of Two Copper(Ⅱ)Complexes with Salicyl Mono-oxime Ligands
- Graphene-RuO2Nanocomposites:Hydrothermal Synthesis and Electrochemical Capacitance Properties
