Dual Channel Rhodamine Based Colorimetric Fluorescent Probe for Cu2+in Living Cells
2013-09-29JIANGLiZHANGBoSUNJianYINGuiSHANGTongMingWANGLingWANGRuiYong
JIANG LiZHANG BoSUN JianYIN Gui*,SHANG Tong-MingWANG LingWANG Rui-Yong
(1School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210093)(2School of Chemistry and Environmental Engineering,The Laboratory of Precious Metals Processing Technology and Application,Jiangsu Teachers University of Technology,Changzhou,Jiangsu 213001)(3School of Life Sciences,Nanjing University,Nanjing 210093)
Dual Channel Rhodamine Based Colorimetric Fluorescent Probe for Cu2+in Living Cells
JIANG Li1ZHANG Bo1SUN Jian1YIN Gui*,1SHANG Tong-Ming2WANG Ling3WANG Rui-Yong3
(1School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210093)(2School of Chemistry and Environmental Engineering,The Laboratory of Precious Metals Processing Technology and Application,Jiangsu Teachers University of Technology,Changzhou,Jiangsu 213001)(3School of Life Sciences,Nanjing University,Nanjing 210093)
An easy prepared fluorescence and UV-Vis dual channel probe for copper ion based on rhodamine-B,which exhibit high sensitivity(2.78×10-8mol·L-1)and selectivity over other metal ions in mixed aqueous system(H2O∶CH3CN=1∶1,V/V),was developed and characterized.This probe involving rhodamine-B and triphenylamine together through the hydrazine bridge,could coordinate with Cu2+and result in turn-on response in UV-Vis absorption and fluorescence.Furthermore,the cell stain experiments showed that compound 1 might be used for monitoring Cu2+within biological samples.
dual channel;rhodamine-B;fluorescence;sensitivity and selectivity;heLa cells
Among the essential heavy metal ions(HTM)in human body[1],copper is the third abundant transition ion which plays an indispensable role in fundamental biological process[2-4].On the other hand,Cu2+is also an important environmental pollutants that can be toxic to naturalsystem.However,traditionalinstrumental techniques,such as spectrophotometry,atomic absorption spectrometry,inductively coupled plasma-mass spectroscopy (ICP-MS),inductively coupled plasmaatomic emission spectrometry (ICP-AES)and voltammetry,are intricate,costly and requiring complicated operation.Thus,simple and inexpensive fluorescent sensors and probes are becoming efficacious methods for monitoring copper in aqueous system.
Due to the energy or electron-transfer process and spin-obital coupling effect,Cu2+mostly acts as a fluores-cence quencher[5-8]and most early classic copper sens-ors and probes showed a “turn-off”type response[9-16],while some “turn-on” type fluorescence sensors orprobes have also been proposed recently[17-26].More importantly,copper probes which could be used in aqueous system are in great need in consideration of the application in environmental and biological systems.Recently,series work of Cu2+probes has been success-fullyapplied tobiologicaland environmental systems[27-32], and the practical applications are in great need to be further explored.
As mentioned in Tong et al′s work[18],They replaced salicylaldehyde with benzaldehyde as the control experiment.Due to the no phenol group compound(benzaldehyde)displayed nearly no response to Cu2+,the phenolic hydroxyl group seems indispensable in the the process of coordination.This sensor chelated with metal ions via its carbonyl O,imino N,and phenol O atom.Then in their another report[33],they developed an other similar compound by replacing the donor with a no phenol group,4-(dimethylamino)benzaldehyde,which exhibited significant absorption and fluorescence change toward Cu2+.And a Cu2+-induced oxidation reaction was proposed to explain binding mode.Our target is to evaluate the role of electron donation during the chelation process.So we replace the donor with a strong electron donating group,4-(diphenylamino)benzaldehyde.The results show that the ligand could still chelate with ions in high affinity without a phenol O atom.And the ESI-MS at m/z=873.17 which was ascribed to [1+Cu2++ClO4-]+has excluded possibility of oxidation reaction.We conclude the strong electron donation can increase the electron cloud density of imino N,thus the chelation capacity toward electron-defect metal ions is strengthened remarkably.And the special rigid structure of donor also contributes to the fluorescence response and the stabilities of probe.We believe,such work and results may complete the mechanism of coordination furthermore.
Herein,profiting from the excellent photophysical properties of Rhodamine-B with high fluorescence,large absorption coefficient and long absorption and emission wavelength,for instance,the new dual channel Rhodamine based “turn-on”type copper probe that we have developed presents high sensitive and selective fluorescence enhancement toward Cu2+in aqueous system(CH3CN-H2O,V/V=1).The long absorption and emission wavelength elongated to visible region make the inexpensive probe useful in “naked-eye”detection at low concentration of copper(4×10-7mol·L-1).As we know,long-wavelength can not only avoid the influence of background fluorescence(<400 nm)[34],but also do less harm to living cells.Thus,we utilized this Cu2+probe in HeLa cells successfully[35].From the cell imagining,we catch sight of the potential of this fluorescence probe in biological systems and environmental systems.
1 Experimental
1.1 Materials and methods
All the reagents were of analytic grade unless noted.1H NMR and13C NMR were measured on a Bruker Ultrashield 300 MHz NMR Spectroscopy.UVVisand fluorescence spectra were recorded on PerkinELmer Lamda 25 UV/Vis Spectrometer.ESI-MS data were recorded on a Finnigan LCQ ESI-MS.HeLa cells were provided by the School of Life Science,Nanjing University.The biological imaging test was carried out with an Olympus FV-1000 laser scanning confocal fluorescence microscope.
1.2 Synthesis of probe and reference compounds(Scheme 1)
Compound 2 To Rhodamine-B(4.98 g,10.4 mmol)methanol solution (8 mL)in a 50 mL round-bottom flask,was added 3 mL hydrazine hydrate.The solution was refluxed and stirred for 3 h.After cooling to the room temperature,pink solid was precipitated and washed by cool methanol until the filtrate turned colorless.Pink powder 2 (2-amino-3′,6′-bis(diethylamino)spiro[isoindoline-1,9′-xanthen]-3-one)was obtained in a yield of 73%and kept without light.1H NMR(300 MHz,CDCl3)δ:7.96~7.93(m,1H),7.48~7.45(m,2H),7.13~7.10(m,1H),6.49~6.43(m,4H),6.31(dd,J1=8.9 Hz,J2=2.6 Hz,2H),3.36(q,J=6.9H,8 Hz),1.18(t,J=6.9 Hz,12H).

Scheme 1 Synthesis of 1,2
Compound 1 To compound 2 (0.45 g,1 mmol)methanol solution (15 mL)in a 50 mL round-bottom flask,was added 4-(diphenylamino)benzaldehyde(0.27 g,1 mmol).The solution was refluxed for 3 h.After cooling to room temperature,straw yellow solid was precipitated.The solid was washed by cool methanol until the filtrate turned colorless and dried in vacuum to get 0.51 g pale yellow powder 1 in a yield of 72%.1H NMR(300 MHz,CDCl3)δ:8.73(s,1H),8.05~7.94(m,1H),7.53~7.39(m,4H),7.24(d,J=7.4 Hz,4H),7.16~7.11(m,1H),7.10~7.01(m,6H),6.95(d,J=8.5 Hz,2H),6.55 (d,J=8.8 Hz,2H),6.44 (d,J=1.7 Hz,2H),6.27(dd,J=8.8,2.1 Hz,2H),3.34(q,J=7.0 Hz,8H),1.18(t,J=7.0 Hz,12H).13C NMR(75 MHz,CDCl3):164.7,153.2,151.49,149.2,148.9,147.5,147.4,147.2,133.1,129.7,129.3,129.2,128.5,128.2,128.1,124.9,123.8,123.4,123.2,122.2,107.9,106.3,97.9,66.0,44.3,12.7ppm.ESI-MS(positivemode):710.33[1+H]+.
1.3 Cell imaging
HeLa cells were grown in Dulbecco′s modified Eagle′s medium (DMEM)supplemented with 10%bovine serum,100 U·mL-1penicillin and 100 μg·mL-1streptomycin at 37 ℃ and 5%CO2.Before staining,cells were washed twice with fresh DMEM,subsequently exposed to the 10 μmol·L-11 solution (900 μL DMEM added with 100 μL of a 100 μmol·L-11 in DMSO)for 10 min at ambient temperature.After washed twice with fresh DMEM,cells were immersed for 10 min with 10 μmol·L-1Cu2+solution (900 μL DMEM added with 100 μL of a 100 μmol·L-1Cu2+in H2O),the DMEM was removed,and the cells were washed twice with fresh DMEM and imaged.
2 Results and discussion
2.1 Selectivity
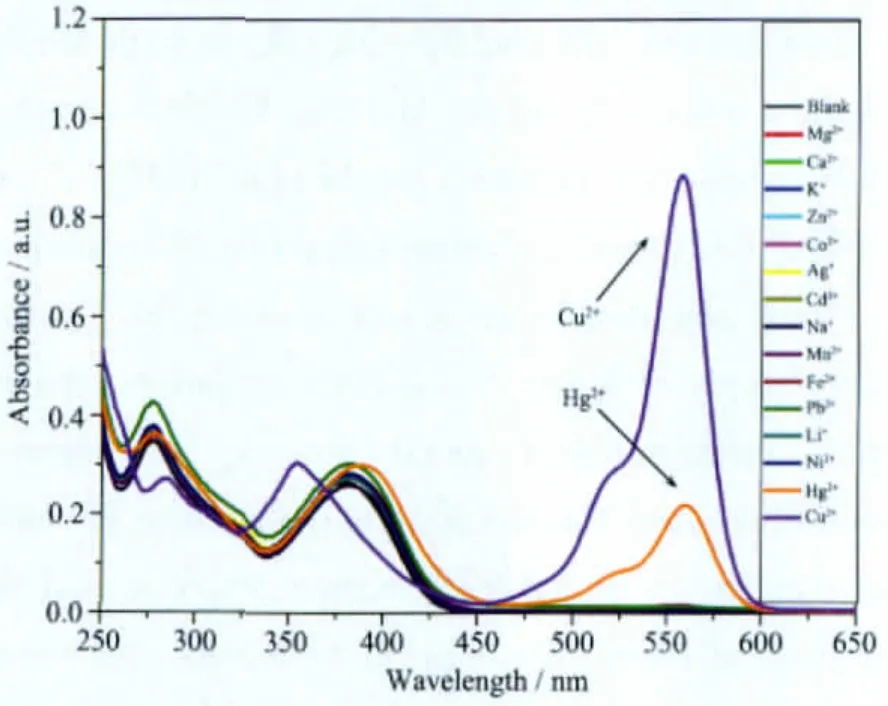
Fig.1 Absorption spectra of 1 (10 μmol·L-1) upon the addition of miscellaneous ions (10 equiv) in CH3CN-H2O system (V/V=1) at 25 ℃
We took compound 1 to carry out UV-Vis and fluorescence experiments firstly.Fig.1 showed the absorption of1 only and in the presence of miscellaneous ions in CH3CN-H2O system(V/V=1).Theaddition of Cu2+obviously caused the appearance of new band at 558 nm.Although,Hg2+seemed to have little absorbance at the same wavelength,it can be still distinguished by the fluorescence measurement as shown in Fig.2 Only Cu2+can trigger the fluorescence response of 1 at 582 nm,while others lead to much smaller fluorescence change.The high enhancement factor was more than 400 fold at fluorescence of 1 upon the addition of Cu2+.Both UV-Vis and fluorescence measurements indicated that 1 showed a great selectivity toward Cu2+over other cations.

Fig.2 Fluorescence intensity of 1(10 μmol·L-1)with the same miscellaneous ions(excitation at 555 nm)(excitation and emission slit 5 nm),Inset shows the amplification of low fluorescence range of the spectra
All these results indicated that compound 1 could be used as potential candidates of facile colorimetric and ratiometric naked eye probe for Cu2+.
2.2 Sensitivity
In order to examine the sensitivity compound 1,we then carry out titration experiments to do quantitative analysis.As shown in Fig.3a,the addition of increasing amounts of Cu2+obviously caused the decrease of the initial band at 381 nm due to 4-(diphenylamino)benzaldehyde moiety and the appearance of new band at 558 nm because of ICT effect[36],both of which indicated that 4-(diphenylamino)benzaldehyde moiety involved in coordination with copper.Besides,the four easily defined isosbestic points at 264,329,367 and 450 nm indicated the presence of a unique complex in equilibrium with the free ligand.The new low-energy band,177 nm red-shift,was responsible for the change of color from colorless to red.
From the absorption titration,the association constant of compound 1 with Cu2+was observed to be 1.3×105L·mol-1.The fluorescence titration of copper was conducted with 10 μmol·L-1ligand 1 in CH3CNH2O system.Upon the addition of increasing concentration of Cu2+,a new emission band peaked at 582 nm with increasing intensity.According to the titration experiment,the detection limit was calculated to be 2.78×10-8mol·L-1.The inset picture in Fig.3b suggested that 1 could serve as a “naked-eye”probe for Cu2+at low concentration(1 only left and 1 with 4×10-7mol·L-1Cu2+right).
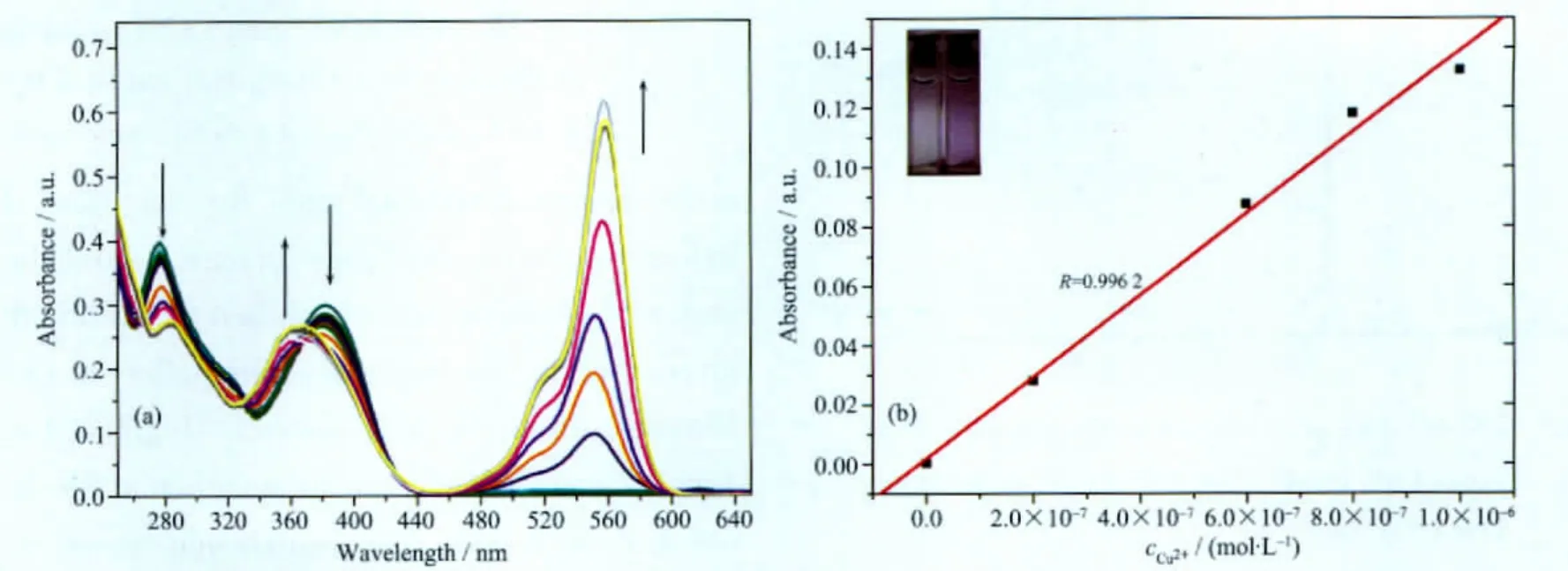
Fig.3 (a)UV-Vis spectra changes of 1(10 μmol·L-1)upon the addition of Cu2+(10-6~5×10-5mol·L-1)in CH3CN-H2O system(V/V=1)at 25 ℃,(b)Absorption intensity at 558 nm of compound 1(10 μmol·L-1)as a function of copper concentration,(0~10-6mol·L-1),Inset:naked eyes detection at 4×10-7mol·L-1
2.3 Fluorescence imaging of living cells
A preliminary study on the Cu2+sensing behavior of 1 in living HeLa cells was carried out by confocal microscopy (Fig.4).The fluorescence imaging of 1-labled cell displayed a very good biological characteristics,demonstrating that 1 is cell permeable(Fig.4a).As determined by fluorescence confocalmicroscopy,labeled HeLa cells exhibited feeble fluorescence in present of 10 μmol·L-1compound 1 (Fig.4b).After the addition of Cu2+ion,the system showed strong fluorescence enhancement from the intracellular region(Fig.4c).These results indicated that 1 might be a potential tool to monitor Cu2+in living cells.
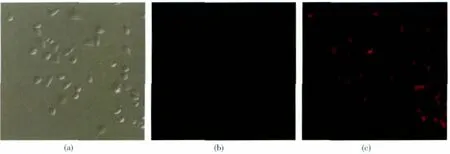
Fig.4 (a)Bright-field image of HeLa cells labeled with 10 μmol·L-11 after 10 min of incubation,(b)Fluorescence image of cells after 10 min treatment with 1,(c)Fluorescence image of cells that are further incubated with 10 μmol·L-1Cu2+for 10 min
2.4 Binding mode
In the ESI-MS data(positive ion mode)(Fig.5)of 1-Cu2+,a peak at m/z=873.17 (calculate value 873.24)corresponding to[1+Cu2++ClO4-]+was clearly observed upon the addition of Cu(ClO4)2,which gave solid evidence for the formation of a 1∶1 complex.Further more,this stoichiometry between 1 and Cu2+was supported by the data of Job′s plots from absorption spectra(Fig.6).

Fig.5 ESI-MS data(positive)of 1 in the presence of 10 equiv Cu2+,Insets:observed isotopic patterns for[1+Cu2++ClO4-]+

Fig.6 Job′plot of 1 and Cu2+with a total concentration of 10 μmol·L-1in CH3CN-H2O system(V/V=1)at 25 ℃,Indicating a 1∶1 metal-ligand ratio
To get an insight view into the binding mode of 1 to Cu2+,1H NMR and13C NMR spectra of 1 and complex 1-Cu2+were measured.Acting as a signal switcher,the spirolactam moiety of rhodamine group envisioned to turn on when Cu2+was bonded.In1H NMR spectra,the active protons broadened after the addition of Cu2+,indicating protons exchange increased.And the active proton of xanthenyl moiety,shifted to downfield,which presenting the electron-deshielding effect of Cu2+on it.Moreover,in the13C NMR spectra(Fig.7)of 1 only and 1 with 2 equiv of Cu2+,the spiro carbon a,which appeared at δ 66.1 ppm,disappeared and moved to downfield,while carbonyl carbon b appeared at δ 164.7 ppm moved to upfield.The shift of two carbons confirmed the formation of an open-ring complex.
Accordingly,a reasonable explanation to these results was proposed that Cu2+induced the spiro ringopen and coordinated with carbonyl oxygen,imino nitrogen so that xanthenyl moiety restored the structure similar to Rhodamine-B and ICT effect to show red fluorescence.

Fig.7 13C NMR(75 MHz)spectra of 1 and 1+2 equivCu2+in CDCl3
3 Conclusions
In conclusion,we have developed a facilitative Rhodamine based colorimetric fluorescent probe for Cu2+via 1:1 binding mode.Compound 1 can be easily synthesized and displayed high sensitivity as low as 2.78×10-8mol·L-1for Cu2+over other ions in aqueous system.The selective recognition of Cu2+gave rise to major color changes from pale yellow to red that was clearly visible to naked eye even at a low concentration of 4×10-7mol·L-1.Therefore,this probe can detect Cu2+via two channels:fluorescence emission and UV-Vis absorption (naked-eye detection).Moreover,from the living cells test,more biological compatible and cell permeable copper probe could be investigated in future.
[1]Maria C L,Maryam H A.Am.J.Clin.Nutr.,1996,63:797s-811s
[2]Uauy R,Olivares M,Gonzalez M.Am.J.Clin.Nutr.,1998,67:952s-959s
[3]Solomon E I,Szilagyi R K,George S D,et al.Chem.Rev.,2004,104:419-458
[4]Gaggelli E,Kozlowski H,Valensin D,et al.Chem.Rev.,2006,106:1995-2044
[5]Varnes A W,Dodson R B,Wehry E L.J.Am.Chem.Soc.,1972,94:946-950
[6]Kemlo J A,Shepherd T M.Chem.Phys.Lett.,1977,47:158-162
[7]Rurack K.Spectrochim.Acta Part A,2001,57:2161-2195
[8]Hu M B,Li H X,Chen L S,et al.Chin.J.Chem.,2009,27:513-517
[9]Fabbrizzi L,Licchelli M,Pallavicini P,et al.Angew.Chem.Int.Ed.Engl.,1994,33:1975-1977
[10]Roy B C,Chandra B,Hromas D,et al.Org.Lett.,2003,5:11-14
[11]Fernandez Y D,Gramatges A P,Amendola V,et al.Chem.Commun.,2004:1650-1651
[12]Mokhir A,Kiel A,Herten D P,et al.Inorg.Chem.,2005,44:5661-5666
[13]Mei Y J,Bentely P A,Wang W.Tetrahedron Lett.,2006,47:2447-2449
[14]Park S M,Kim M H,Choe J I,et al.J.Org.Chem.,2007,72:3550-3553
[15]Weng Y Q,Yue F,Zhong Y R,et al.Inorg.Chem.,2007,46:7749-7755
[16]Kim M H,Noh J H,Kim S,et al.Dyes Pigments,2009,82:341-346
[17]Wen Z C,Yang R,He H,et al.Chem.Commun.,2006:106-108
[18]Xiang Y,Tong A J,Jin P Y,et al.Org.Lett.,2006,8:2863-2866
[19]Mei L,Xiang Y,Li N,et al.Talanta,2007,72:1717-1722
[20]Zhang X,Shiraishi Y,Hirai T.Org.Lett.,2007,9:5039-5042
[21]Lee M H,Kim H J,Yoon S,et al.Org.Lett.,2008,10:213-216
[22]Joseph R,Ramanujam B,Acharya A,et al.Tetrahron Lett.,2009,50:2735-2739
[23]Zhou Y,Wang F,Kim Y,et al.Org.Lett.,2009,11:4442-4445
[24]Wang X L,Zheng W Y,Liu G C,et al.J.Lumin.,2010,130:52-55
[25]Xu Z C,Yoon J,Spring D R.Chem.Commum.,2010,46:2563-2565
[26]Xu Y F,Lu F,Xu Z C,et al.Sci.Chin.Ser.B,2009,52:771-779
[27]Zhao Y,Zhang X B,Han Z X,et al.Anal.Chem.,2009,81:7022-7030
[28]Jiao L J,Li J L,Zhang S Z,et al.New J.Chem.,2009,33:1888-1893
[29]Wang M X,Huang S H,Meng X M,et al.Chem.Lett.,2008,37:462-463
[30]Yu M X,Shi M,Chen Z G,et al.Chem.Eur.J.,2008,14:6892-6900
[31]Zeng L,Miller E W,Pralle A,et al.J.Am.Chem.Soc.,2006,128:10-11
[32]Taki M,Iyoshi S,Ojida A,et al.J.Am.Chem.Soc.,2010:10.1021/ja100714p
[33]Xiang Y,Tong A J.Luminescence,2008,23:28-31
[34]Dujols V,Ford F,Czarnik A W.J.Am.Chem.Soc.,1997,119:7386-7387
[35]He G J,Zhao X W,Zhang X L,et al.New J.Chem.,2010,34:1055-1058
[36]Ueno T,Urano Y,Setsukinai K,et al.J.Am.Chem.Soc.,2004,126:14079-14085
基于罗丹明-B的双通道铜离子荧光探针及其活细胞的应用
姜 李1张 波1孙 剑1尹 桂*,1尚通明2王 玲3王睿勇3
(1南京大学化学化工学院,南京 210093)(2江苏技术师范学院,化学与环境工程学院,贵金属深加工技术及其应用实验室,常州 213001)(3南京大学生命科学学院,南京 210093)
本文介绍了一种紫外-可见和荧光双通道铜离子探针,该探针基于罗丹明-B,通过水合肼为桥梁来连接三苯胺,便利而廉价。其在水体系下对铜离子显示出了良好的选择性和极高的灵敏度(2.78×10-8mol·L-1)。并且,通过初步的活细胞测试,表明该铜离子探针有着良好的生物相容性和细胞穿透性,在生物、医药领域有着非常大的潜力。
双通道;罗丹明;荧光;选择性和灵敏度;海拉细胞
O612.3
:A
:1001-4861(2010)10-1750-06
2010-05-31。收修改稿日期:2010-07-01。
姜 李,男,25岁,硕士研究生;研究方向:离子选择探针的设计与合成。
江苏省自然科学基金(No.BK2006717)资助项目。
*通讯联系人。 E-mail:yingui@nju.edu.cn;Tel:025-83592529;Fax:025-83314502
猜你喜欢
杂志排行
无机化学学报的其它文章
- Solvothermal Synthesis,Crystal Structure and Photoluminescence Property of a Coordination Polymer Based on 1,1′-Ethynebenzene-3,3′,5,5′-tetracarboxylate
- Synthesis,Structure and Fungicidal Activity of Organotin 1H-Tetrazolyl-1-acetates
- Synthesis and Characterization of Tungsten Oxide Nanostructures
- Syntheses,Crystal Structure and Optical Property of Two Bis-ligand Silver(Ⅰ)Complexes Containing Diphenic Acid and Bidentate N-donor Ligands
- Syntheses and Structures of Two Copper(Ⅱ)Complexes with Salicyl Mono-oxime Ligands
- Graphene-RuO2Nanocomposites:Hydrothermal Synthesis and Electrochemical Capacitance Properties
