苯并咪唑衍生配体锌-铕(或铽)异金属双核配合物的合成、结构与光学性质
2013-09-21赵顺省石国香冯维旭吕兴强刘向荣
赵顺省 张 召 石国香 冯维旭 吕兴强,* 刘向荣
(1西安科技大学化学与化工学院,西安710054;2西北大学,陕西省物理无机化学重点实验室,西安710069)
1 Introduction
Luminescent lanthanide(Ln)complexes,especially those of europium and terbium,with long-lived and characteristic line like emission bands in visible region,have attracted the interest of scientists in recent decades,1not only because of their potential applications in optoelectronics,2,3but also in the detection of various bioactive molecules and in-vitro imaging.4,5Nevertheless,the absorption coefficients of the lanthanide ions are low due to the parity forbidden f-f transitions and limit the emission of these ions.6One way to overcome this shortcoming is to employ a sensitizing chromophore or antennae as ligand in a lanthanide complex.7For the choice of appropriate ligand for efficient energy transfer to the lanthanide ion after excitation,the binding strength of the ligand,the ultraviolet(UV)absorption properties,absence of high frequency vibration modes,8and location of donor excited states of ligand identity9are important criteria to be considered.Many organic(cyclic or acyclic)ligands10-12and d-block metal complexes13-15have been used as antennae or chromophores for the effective sensitization of near-infrared(NIR)luminescence of Ln3+ions.
Past studies have shown that in the formation of the series of Zn-Ln hetero-metallic complexes16,17from the compartmental Salen-type Schiff-base ligands with the outer O2O2moieties from methoxyl groups,the Zn2+complexes based on the Salentype Schiff-base ligands,as the suitable chromophores,could effectively sensitize the NIR luminescence of the centered Ln3+ions.Moreover,the occupation of pyridine at the axial position of the Zn2+ion is helpful for the strong NIR luminescence,which should be due to the complete avoiding of the luminescent quenching effect arising from OH-,CH-or NH-oscillators around the Ln3+ion.The introduction of heavy atom(Br)on the flank phenyl group is another way to improve the luminescent quantum efficiency of lanthanide ions.As a matter of fact,the utilization of the Salen-type Schiff-base ligands should not be the only way to construct Zn-Ln bimetallic complexes,recently reported Cu-Tb,Cu-Gd bimetallic complexes with single molecular magnet property18and Zn-Nd bimetallic complex19with near-infrared emission illuminate us the further exploration on the synthesis and luminescent properties Zn-Tb and Zn-Eu pairs,since the benzimidazole derivative ligand and its zinc complex emits at blue range,which may match better with the energy level of excited states of Tb3+or Eu3+.Herein,a series of hetero-binuclear ZnLn complexes[ZnLnLn2(Py)(NO3)3]·solvent(Py=Pyridine)based on the benzimidazole derivative ligands HL1(HL1=2-(2-hydroxy-3-methoxyphenyl)benzimidazole)or HL2(HL2=2-(5-bromo-2-hydroxy-3-methoxyphenyl)benzimidazole)were synthesized and characterized.UV-visible absorption spectra,excitation and emission spectra of these complexes were measured and the sensitization and energy transfer for the luminescence of the Ln3+ions in these Zn-Ln complexes were discussed.
2 Experimental
2.1 Reagents and general techniques
All chemicals and solvents purchased commercially were analytical reagents and were used without further purification unless otherwise stated.The benzimidazole derivative ligands HL1and HL2were prepared according to a well-established procedure from the literature.18,19Acetonitrile for photophysical measurement was dried with calcium hydride,distilled and degassed by N2before use.Elemental analyses were performed on a Perkin-Elmer 2400-II elemental analyzer(PerkinElmer Inc.,USA).FTIR spectra were recorded on a P.E.983 FTIR spectrophotometer(PerkinElmer Inc.,USA)in the region 4000-400 cm-1using KBr pellets.UV-visible absorption spectra were recorded with a Cary 300 UV spectrophotometer(Agilent Technologies,USA),and steady-state visible fluorescence,PL excitation spectra on a Photon Technology International(PTI)Alphascan spectrofluorometer(Photon Technology International,Inc.USA)and visible decay spectra on a pico-N2laser system(PTI Time Master).The quantum yield of the visible luminescence for each sample was determined by the relative comparison procedure,using a reference of a known quantum yield(quinine sulfate in 0.05 mol·L-1H2SO4solution,quantum yield Фem=0.546).
2.2 General procedure for synthesis of[ZnLnL2(NO3)3(Py)]·solvent
To a stirred solution of the precursor HL(0.1 mmol)in absolute MeOH(5 mL),a solution of Zn(CH3COO)2·2H2O(0.011g,0.05 mmol)in methanol(2 mL)was added and stirred for 30 min,Ln(NO3)3·6H2O(0.05 mmol,Ln=Eu,17.9 mg;Ln=Tb,19.3 mg)in acetonitrile(5 mL)and one drop of pyridine were added,then the final mixture was refluxed for 3 h.The respective clear pale yellow solution was then cooled to room temperature and filtered.Diethyl ether was allowed to diffuse slowly into the solution at room temperature and pale yellow crystalline productsof 1-4wereobtainedinafew weeks,respectively.
For[ZnEu(L1)2(NO3)3(Py)]·2Py·THF(1):Yield:31.6 mg,54.1%.Anal.calcd.for C47H45EuN10O14Zn(%):C,41.24;H,2.83;N,11.66;Found(%):C,41.39;H,2.69;N,11.76.IR(KBr,cm-1):3285(w),2944(w),2848(w),1642(s),1608(m),1478(s),1445(w),1423(w),1385(m),1283(w),1254(s),1179(w),1162(s),1091(w),1054(m),1035(w),986(m),933(m),863(w),783(w),740(m),707(w),620(w),557(s).(ESI-MS)(CH3CN)m/z:960.9(100%)[M-2Py-THF]+.
For[ZnTb(L1)2(NO3)3(Py)]·Py·EtOH·H2O(2):Anal.calcd.for C40H40N9O15TbZn(%):C,40.95;H,2.81;N,11.58;Found(%):C,40.48;H,2.79;N,11.49.IR(KBr,cm-1):3301(w),3071(w),2942(w),1624(m),1608(w),1595(w),1535(w),1482(s),1452(m),1426(m),1385(m),1312(w),1285(w),1252(s),1105(w),1099(w),1075(w),1035(w),993(m),930(m),860(m),816(w),783(m),743(s),703(m),638(w),560(m),516(w).ESI-MS(CH3CN)m/z:967.7(100%),[MPy-EtOH-H2O]+.
For[ZnEu(L2)2(NO3)3(Py)]·Py·H2O(3):Anal.calcd.for C38H32Br2EuN9O14Zn(%):C,35.43;H,2.25;N,10.02;Found(%):C,35.50;H,2.27;N,10.20.IR(KBr,cm-1):3419(w),3067(w),2944(w),1646(s),1625(s),1608(m),1596(w),1539(w),1481(s),1386(m),1317(w),1241(m),1120(s),1099(w),1075(w),1035(w),986(m),940(m),937(m),815(w),800(m),766(w),747(m),704(m),616(w),549(s),515(w).ESI-MS(CH3CN)m/z:1118.8(100%),[M-Py-H2O]+.
For[ZnTb(L2)2(NO3)3(Py)]·Py·H2O(4):Anal.calcd.for C38H32Br2TbN9O14Zn:C,35.21;H,2.24;N,9.95;Found(%):C,35.13;H,2.32;N,10.03.IR(KBr,cm-1):3430(w),3067(w),2946(w),2846(s),1614(s),1586(m),1552(w),1512(vs),1468(w),1386(s),1303(w),1264(s),1234(w),1196(w),1168(w),1096(w),1074(w),1024(m),965(w),851(m),814(w),784(w),741(w),649(w),620(w),558(w),513(w),441(w).ESI-MS(CH3CN)m/z:1125.8(100%),[M-Py-H2O]+.
2.3 Crystal structure determinations
Single crystal of suitable dimensions was mounted onto thin glass fibers.All the intensity data were collected at 293 K on a Bruker SMART CCD diffractometer(Mo Kαradiation,λ=0.071073 nm)in Φ and ω scan modes.The structure was solved by direct methods followed by difference Fourier syntheses,and then refined by full-matrix least-squares techniques against F2using SHELXTL.20Absorption corrections were applied using SADABS.21All other non-hydrogen atoms were refined with anisotropic thermal parameters.Crystallographic data and refinement parameters for the complexes are presented in Table 1.Relevant atomic distances and bond angles are collected in Tables 2,3 and 4.CCDC reference numbers are 915907(for 1),915908(for 2),and 915909(for 3).
3 Results and discussion
3.1 Synthesis and characterization
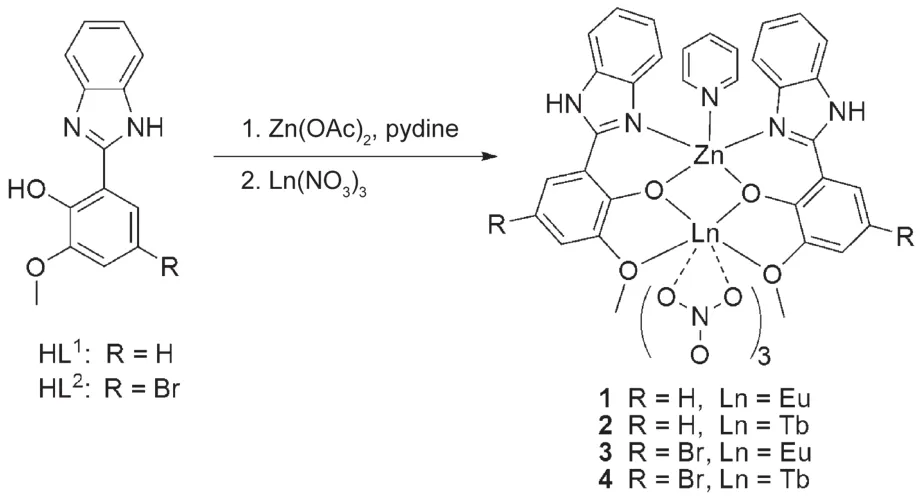
Scheme 1 Assembly of hetero-binuclear complexes
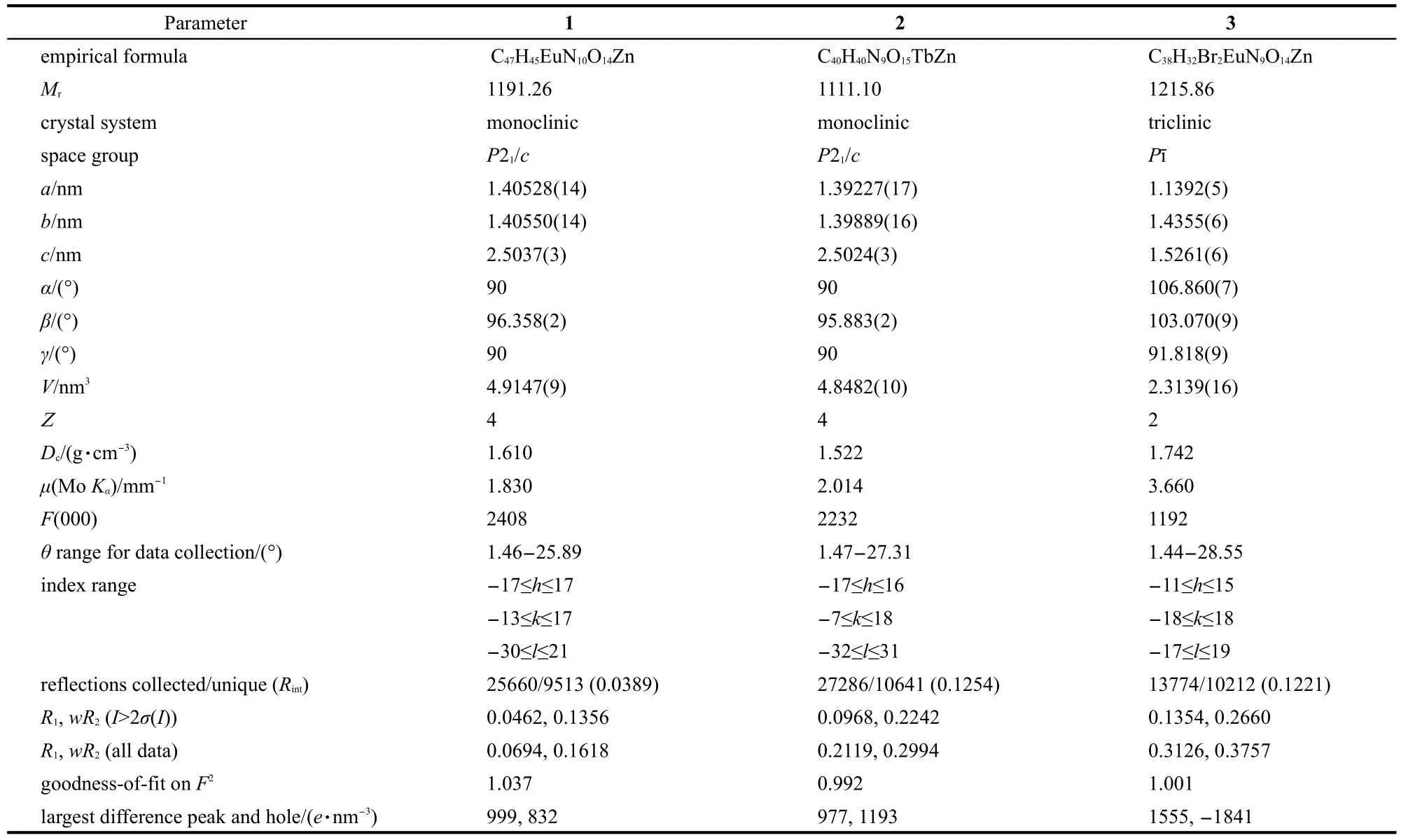
Table 1 Crystallographic data and structure refinement for complexes 1,2,and 3

Table 2 Selected bond lengths and bond angles for complex 1
As shown in Scheme 1,using of HL1or HL2,which are prepared according to the literature method,19to react with the Zn(CH3COO)2·2H2O,subsequently the Ln(NO3)3·6H2O(Ln=Eu or Tb)in the presence of pyridine,results in the formation of two series of the hetero-binuclear Zn-Ln complexes[ZnEu(L1)2(NO3)3Py]·2Py·THF(1),[ZnTb(L1)2(NO3)3Py]·Py·EtOH·2H2O(2),[ZnEu(L2)2(NO3)3Py]·Py·H2O(3),and[ZnTb(L2)2(NO3)3Py]·Py·H2O(4),respectively.For complexes 1,2,and 3,single crystals suitable for X-ray diffraction studies have been obtained by allowing Et2O to diffuse into the respective reaction mixture at room temperature.Two strong absorptions at 1478-1482 and 1314-1320 cm-1shown in the FTIR spectra of complexes 1-4 are typical for the bidentate NO-3groups,and the bands appearing at 766 or 758 cm-1should be assigned to the υ(C-Br)vibration in complexes 3 or 4,respectively.The ESI-MS spectra of the four Zn-Ln complexes(1-4)exhibit the strongest peak at m/z 960.9(1),967.9(2),1118.8(3)or 1125.8(4),respectively,corresponding to the major species[ZnLn(L1)2(Py)(NO3)3](Ln=Eu,1;Ln=Tb,2)or[ZnLn(L2)2(Py)(NO3)3](Ln=Eu,3;Ln=Tb,4),giving the further proof that in the respective dilute MeCN solution the Zn-Ln(Ln=Eu or Tb)molecule keeps indiscrete.
3.2 Structural analysis of the complexes 1-3
X-ray quality crystals of 1 and 2 based on the HL1ligand have been obtained.X-ray diffraction determination indicates that they are isostrucural with the previous reported Cu-Tb,Cu-Gd18and Zn-Nd19bimetallic complexes with different coordinated solvates.Selected bond lengths and bond angles are given in Tables 1 and 2.Complex 1 crystallizes with one independent hetero-binuclear molecules and solvates of THF,H2O,and Py in an asymmetrical unit,as shown in Fig.1.In the crystal,one Zn2+ion coordinates two deprotonated(L1)-ligandsthrough their N,O sites and one Eu3+ion through their O2site,compelling the two ligands to assembly shoulder to shoulder.The Zn2+ion has a five-coordinate environment and adopts a distorted square pyramidal geometry,composed of the inner N2O2core from two deprotonated benzimidazole-based(L1)-ligands as the base plane,and one N atom from the coordinated pyridine at the apical position.The Eu3+ion and the Zn2+ion are bridged by two phenoxo oxygen atoms of two(L1)-ligands with the a Zn··Eu separation of 0.3562(2)nm.The Eu3+ion is in a ten-coordinate environment.Besides four oxygen atoms from two deprotonated benzimidazole-based(HL1)-ligands,they complete their coordination environments with six oxygen atoms from three bidentate NO-3anions.The bond lengths(0.2029(5)nm and 0.2044(5)nm)of Zn-N(the benzimidazole(HL1)ligands,N2 and N3)bonds are smaller than that(0.2090(12)nm)of Zn-N(Py,N8)bond while between those(0.2060(4)-0.2078(3)nm)of Zn-O(phenoxo,O2 and O3)bonds.The bond lengths of Eu-O vary during 0.2295(9)-0.2731(4)nm depending on the origin of the oxygen atoms:the bond lengths from oxygen atoms of NO-3anions are longer than those from phenoxo oxygen atoms,while slightly shorter than those from methoxy groups.Besides the intermolecular N4-H4A…N9(-x+1,-y+1,-z+1)hydrogen bonding with the N…N distance of 2.868(2)between one of the solvate Py and an adjacent Zn-Eu molecule(see Table 5 and Fig.S1,in Supporting Information),the other solvate molecules(Py or THF)are not bound to the framework and they exhibit no observable interactions with the host structure.Other heterobimetallic Cu-Ln(Ln=Eu,Tb,Er,Tb,Nd)complexes with similar structure but with different ligand and nonidentical coordination environment of Cu2+have been reported previously.22

Table 3 Selected bond lengths and bond angles for complex 2

Table 4 Selected bond lengths and bond angles for complex 3

Fig.1 Perspective drawing of complex 1
The change of lanthanide ion leads to an isostructural hetero-binuclear Zn-Tb structure from the replacement of Eu3+ion by Tb3+ion.X-ray quality crystals of 2 based on the HL1ligand were obtained and the data of selected crystal properties are given in Tables 1 and 3.Complex 2 is isostructural with 1 containing nonidentical solvates,as shown in Fig.2 and Fig.S2(in Supporting Information).The bond lengths of Zn-N,Zn-O,Tb-O and separation distance of Zn-Tb are similar with those of the complex 1.Intermolecular hydrogen bond of N1-H1A…N9(-x,-y+1,-z)(see Table 5)between the solvate Py and host molecule could also be found in the crystals while the other solvates(EtOH or H2O)being kept unbound.
The introcduction of heavy atoms(Br)still keeps the heterobinuclear Zn-Ln structures from the replacement of the benzimidazole-based ligand HL2.X-ray quality crystals of 3 as the representative of the two Zn-Ln complexes based on the HL2ligand have been obtained,and the tables of selected crystal properties are also given in Tables 1 and 4.Complex 3 crystallizes with one independent hetero-binuclear molecule,one solvate of Py and one solvate of H2O in the asymmetrical unit,as shown in Fig.3.The slightly shorter Zn-N(benzimidazole)bond lengths(0.196(2)-0.214(2))and the slightly longer Zn…Eu separation(0.3641(3))in complex 3 than those of complex 1 and 2,respectively,should be attributed to the electron with-drawing effect of Br atoms introduced on the ligand HL2.Unlike that of the complexes 1 and 2,the intramolecular N2-H2A…N9 hydrogen bond with N…N distance of 0.2720(3)between the solvate Py and the Zn-Eu molecule is also observed(see Table 5).It is worth noting that in the formation of two hetero-binuclear Zn-Ln complexes 1,2,and 3,comparable of that of near-infrared emitting[Zn(HL1)2Nd(NO3)3],19the occupation of Py at the axial position of Zn2+ions is considered to completely avoid the further coordination of solvents around the Ln3+ions of the four Zn-Ln complexes.
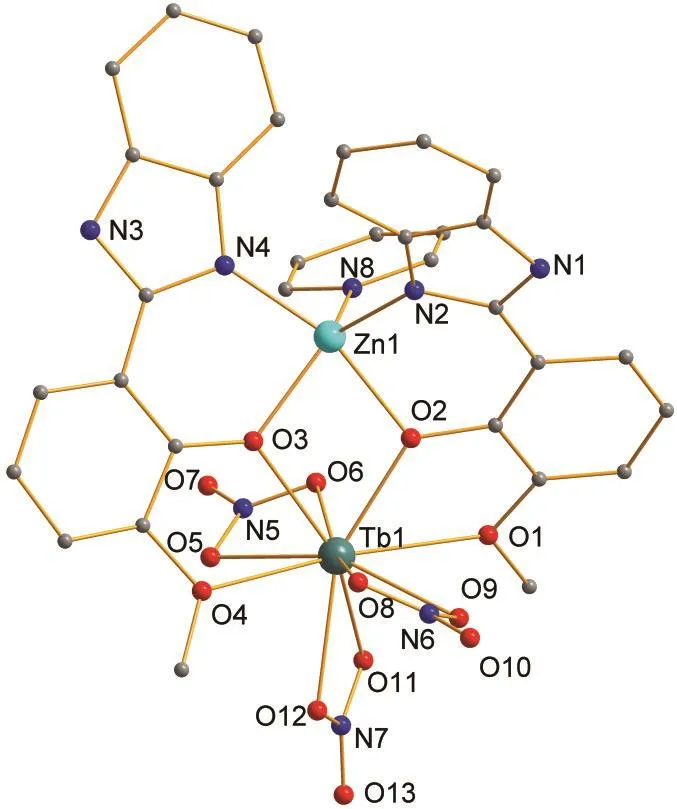
Fig.2 Perspective drawing of complex 2
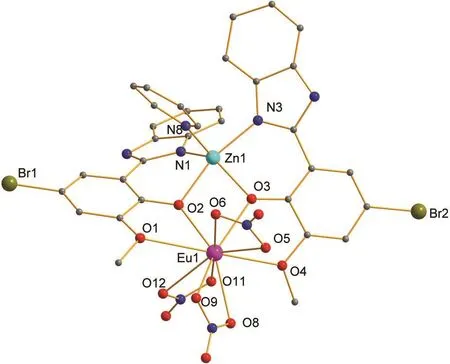
Fig.3 Perspective drawing of complex 3
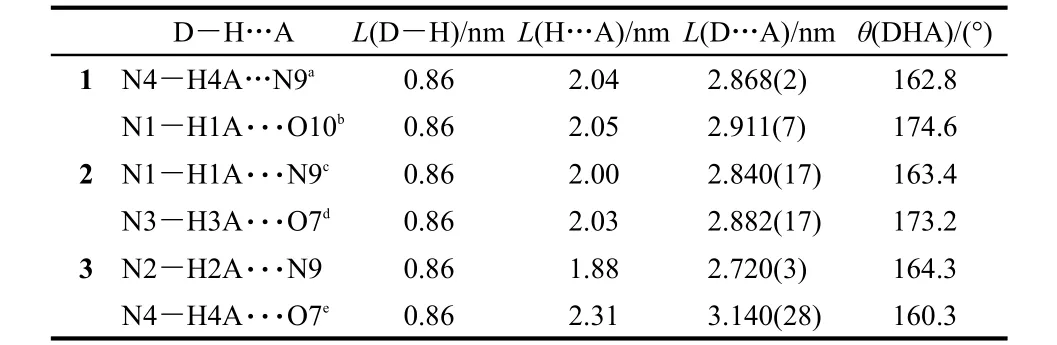
Table 5 Hydrogen bond lengths and angles for complexes 1-3
3.3 Photophysical properties of the complexes
The photophysical properties of the complexes 1-4 have been examined in dilute MeCN solution at room temperature and summarized in Figs.4-6 and Table 6,respectively.As shown in Fig.4,in the UV-visible absorption spectra of the complexes,the similar ligand-centered absorption spectra(229-231,299-301,and 332-334 nm)of complexes 1-4 in the UV-Vis region are observed,which should be assigned to the π-π*transition of the benzimidazole ligands.

Fig.4 UV-visible absorption spectra of complexes 1-4 in 2×10-5mol·L-1CH3CN solution
As shown in Fig.5,the characteristic emissions of the Tb3+ion(5D4→7Fn,n=6,5,4,3)in the green light region have been detected up on photo excitation of the antenna at the range of 275-450 nm in complexes 2 and 4.The emissions at 489,542,584,and 622 nm could be assigned to5D4→7F6,5D4→7F5,5D4→7F4,and5D4→7F3transitions of the Tb3+ion,respectively.The precursor[Zn(L)2(Py)]or Tb(NO3)3does not exhibit the luminescence under the same condition.For complex 2,when monitoring at the two different emission peaks(λem=468 or 542 nm),two similar excitation spectra monitored,which clearly demonstrated that both the residue of the antennae emissions and the characteristic emission of the Tb3+ion are originated from the same π→π transitions of the benzimidazole-based ligand HL1and suggests that the energy transfer from the antenna to the Tb3+ions takes place but not completely.It was worth noting that for complex 4,although there was almost no position shift of the emissions of Tb3+ions in solution at room temperature,each of the emission intensities of complex 4 was distinctively higher than the respective one of complex 2.Moreover,the emission spectrum only shows the characteristic emission of Tb3+,there is no ligand based emission residue observed.This could be attributed to the heavy atom effect of Br on the HL2,which probably promotes of1LC→3LC intersystem crossing and thus increases the energy transfer efficiency between the antennae and Tb3+ion for that the energy transfer takes place mostly from triplet state of the antennae to the lanthanide ions.23
For the Eu3+contained complexes 1 and 3,only ligand centered emission band(ca 468 nm)with low quantum yield(Φem<10-3,much lower than that of the precursor ZnL2Py)in dilute absolute MeCN solution at room temperature(see Fig.6 and Table 6),but no characteristic emission band of Eu3+is observed.From the viewpoint of the energy level match,the energy gap between the energy-donating3LC level(21639 cm-1for theor 21592 cm-1for theof the ligand and the excited5D1state(19020 cm-1)24of Eu3+is suitable for energy transfer,and the characteristic emission of Eu3+would have been sensitized.One explanation is that there may be a deactivation pathway that involves a charge transfer from the Zn(L)2excited state to Eu3+ion.25
We were naturally interested in the efficiency of overall sensitization process which was established by determining luminescent quantum yield of the hetero-bimetallic complexes.The luminescent quantum yields of the complexes were measured by using the quinine sulfate(in 0.05 mol·L-1H2SO4solution,Φem=0.546)as the reference,adjusting the absorbance value to same at 350 nm and comparing the emission area of the complexes and reference.As listed in Table 6,the sensitized efficiency of the characteristic emission of Tb3+is 0.11 for 2 and 0.17 for 4.The luminescent decay curves obtained from timeresolved luminescent experiments can be fitted mono-exponentially with time constant of microseconds(at 542 nm,0.73 ms for 2 and 0.81 ms for 4),and the intrinsic quantum yield ΦLn(ca 0.18 for 2 and 0.20 for 4)of the Tb3+emission may be estimated by ΦLn=τobs/τ0,where τobsis the observed emission lifetime and τ0is the‘natural lifetime’,viz 4 ms for the Tb3+ion,26which indicates the presence of single emitting center for complex in dilute MeCN solutions.27
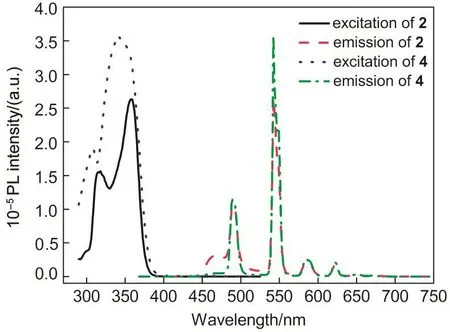
Fig.5 Excitation and emission spectra of complexes 2 and 4 in 2×10-5mol·L-1CH3CN solution

Fig.6 Excitation and emission spectra of complexes 1 and 3 in 2×10-5mol·L-1CH3CN solution
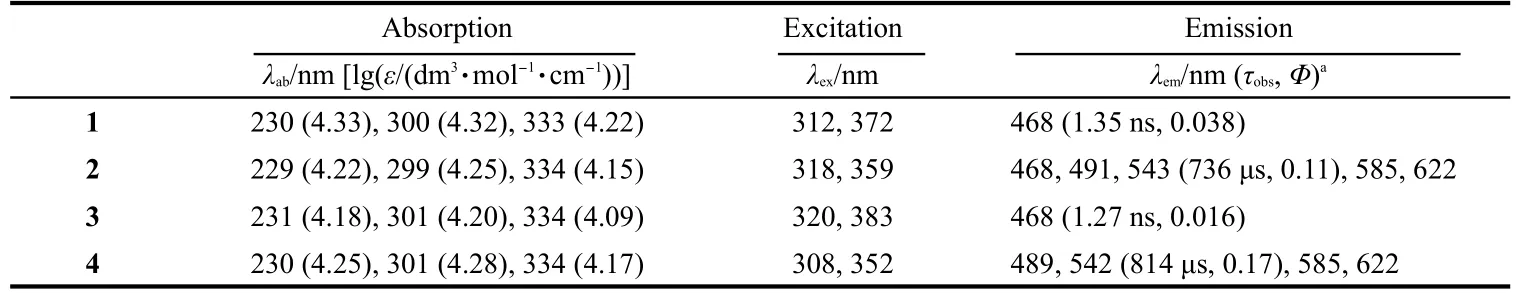
Table 6 Photophysical properties of the complexes 1-4 in 2×10-5mol·L-1CH3CN solution at room temperature
4 Conclusions
In conclusion,with the benzimidazole derived ligand HL1or HL2as the precursor,series of hetero-binuclear Zn-Ln complexes[ZnEu(L1)2(NO3)3Py]·2Py·THF(1),[ZnTb(L1)2(NO3)3Py]·Py·EtOH·2H2O(2),[ZnEu(L2)2(NO3)3Py]·Py·H2O(3),and[ZnTb(L2)2(NO3)3Py]·Py·H2O(4)with two energy donors around the Ln3+ion are obtained,respectively.The results of their photophysical studies show that the strong and characteristic luminescence of Tb3+ions with emissive lifetimes in the microsecond range has been sensitized from the excited state of the benzimidazole-based ligand due to the effective intramolecular energy transfer,and the involvement of heavy atoms(Br)on the ligand promotes the energy transfer and luminescent efficiency.The Eu3+complexes do not show their characteristic emission due to other deactivation pathway.
Supporting Information: Crystal structures of 1,2,and 3 showing the intermolecular or intramolecular hydrogen bonds have been included.This information is available free of charge via the internet at http://www.whxb.pku.edu.cn.
Supplementary Material: The crystallographic data have been deposited at the Cambridge Crystallographic Data Center,CCDC 915907(for 1),915908(for 2),and 915909(for 3).The data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html or from the Cambridge CB21EZ,UK.
(1) Dos Santos,C.M.G.;Harte,A.J.;Quinn,S.J.;Gunnlaugsson,T.Coord.Chem.Rev.2008,252,2512.doi:10.1016/j.ccr.2008.07.018
(2) Bünzli,J.C.G.;Eliseeva,S.V.J.Rare Earths 2010,28,824.doi:10.1016/S1002-0721(09)60208-8
(3) Kido,J.;Okamoto,Y.Chem.Rev.2002,102,2357.doi:10.1021/cr010448y
(4)Law,G.L.;Wong,K.L.;Man,C.W.Y.;Tsao,S.W.;Wong,W.T.J.Biophotonics 2009,2,718.doi:10.1002/jbio.v2:12
(5) Bünzli,J.C.G.Chem.Rev.2010,110,2729.doi:10.1021/cr900362e
(6) Comby,S.;Bünzli,J.C.G.Lanthanide Near-Infrared Luminescence in Molecular Probe and Device.In Handbook on the Physics and Chemistry of Rare Earths;Gschneidner,K.A.,Bünzli,J.C.G.,Pecharsky,V.K.Eds.;Elsevier Science B.V.:Amsterdam,2007;Vol.37,pp 221-224.
(7) Sabbatini,N.;Guardigli,M.;Lehn,J.M.Coord.Chem.Rev.1993,123,201.doi:10.1016/0010-8545(93)85056-A
(8) Freund,C.;Porzio,W.;Giovanella,U.;Vignali,F.;Pasini,M.;Destri,S.;Mech,A.;Di Pietro,S.;Di Bari,L.;Mineo,P.Inorg.Chem.2011,50,5417.doi:10.1021/ic1021164
(9) Bünzli,J.C.G.;Comby,S.;Chauvin,A.S.;Vandevyver,C.D.B.J.Rare Earths 2007,25,257.doi:10.1016/S1002-0721(07)60420-7
(10) Bünzli,J.C.G.;Piguet,C.Chem.Soc.Rev.2005,34,1048.doi:10.1039/b406082m
(11) Duan,L.Q.;Qiao,C.F.;Wei,Q.;Xia,Z.Q.;Chen,S.P.;Zhang,G.C.;Zhou,C.S.;Gao,S.L.Acta Phys.-Chim.Sin.2012,28,2783.[段林强,乔成芳,魏 青,夏正强,陈三平,张国春,周春生,高胜利.物理化学学报,2012,28,2783.]doi:10.3866/PKU.WHXB201210122
(12) Zhang,J.;Wang,J.F.;Zhang,J.J.Acta Phys.-Chim.Sin.2012,28,290.[张 洁,王娟芬,张建军.物理化学学报,2012,28,290.]doi:10.3866/PKU.WHXB201112121
(13) Ward,M.D.Coord.Chem.Rev.2007,251,1663.doi:10.1016/j.ccr.2006.10.005
(14) Chen,F.F.;Chen,Z.Q.;Bian,Z.Q.;Huang,C.H.Coord.Chem.Rev.2010,254,991.doi:10.1016/j.ccr.2009.12.028
(15)Ward,M.D.Coord.Chem.Rev.2010,254,2634.doi:10.1016/j.ccr.2009.12.001
(16)Lo,W.K.;Wong,W.K.;Wong,W.Y.;Guo,J.P.;Yeung,K.T.;Cheng,Y.K.;Yang,X.P.;Jones,R.A.Inorg.Chem.2006,45,9315.doi:10.1021/ic0610177
(17)Zhao,S.S.;Lü,X.Q.;Hou,A.X.;Wong,W.Y.;Wong,W.K.;Yang,X.P.;Jones,R.A.Dalton Trans.2009,9595.doi:10.1039/b908682j
(18) Fellah,F.Z.C.;Costes,J.P.;Duhayon,C.;Daran,J.C.;Tuchagues,J.P.Polyhedron 2010,29,2111.doi:10.1016/j.poly.2010.04.010
(19) Shi,G.X.;Feng,W.X.;Zou,D.;Lü,X.Q.;Zhang,Z.;Zhang,Y.;Fan,D.D.;Zhao,S.S.;Wong,W.K.;Jones,R.A.Inorg.Chem.Commun.2012,22,126.doi:10.1016/j.inoche.2012.05.041
(20) Sheldrick,G.M.SHELXS-97:Program for Crystal Structure Refinement;Götingen:Germany,1997.
(21) Sheldrick,G.M.SADABS;University of Götingen:Götingen,1996.
(22)Yang,X.P.;Jones,R.A.;Lai,R.J.;Waheed,A.;Oye,M.M.;Holmes,A.L.Polyhedron 2006,25,881.doi:10.1016/j.poly.2005.09.029
(23) Petoud,S.;Cohen,S.M.;Bünzli,J.C.G.;Raymond,K.N.J.Am.Chem.Soc.2003,125,13324.doi:10.1021/ja0379363
(24) Sayre,E.V.;Freed,S.J.Chem.Phys.1956,24,1213.doi:10.1063/1.1742743
(25)Zhu,X.J.;Wong,W.K.;Wong,W.Y.;Yang,X.P.Eur.J.Inorg.Chem.2011,4651.
(26) Klink,S.I.;Grave,L.;Reinhoudt,D.N.;van Veggel,F.C.J.M.;Werts,M.H.V.;Geurts,F.A.J.;Hofstraat,J.W.J.Phys.Chem.A 2000,104,5457.doi:10.1021/jp994286+
(27) Bünzli,J.C.G.Lanthanide Probe in Life.In Chemical and Earth Sciences,Theory and Practice;Bünzli,J.C.G.,Choppin,G.R.Eds.;Elsievier Science Pub1.B.V.:Amsterdam,1989;ch7,pp 219-293.
