Social rank and cortisol among female rhesus macaques (Macaca mulatta)
2013-09-20DongDongQINJoshuaDominicRizakXiaoLiFENGXunXunCHUShangChuanYANGChunLuLILongBaoLVYuanYeMAXinTianHU
Dong-Dong QIN, Joshua Dominic Rizak, Xiao-Li FENG, Xun-Xun CHU Shang-Chuan YANG, Chun-Lu LI, Long-Bao LV, Yuan-Ye MA,5,6,* , Xin-Tian HU,5,6,*
1. State Key Laboratory of Brain and Cognitive Science, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, China;2. University of the Chinese Academy of Sciences, Beijing 100049, China;3. Institute of Psychology, Chinese Academy of Sciences, Beijing 100101, China;4. Kunming Primate Research Center of the Chinese Academy of Sciences, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223,China;5. Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China;6. Kunming Primate Research Center, Kunming Institute of Zoology, the Chinese Academy of Sciences, Kunming 650223, China
In many animal societies, stress generated within long-term dominant-subordinate relationships often predicts an increased vulnerability to an array of diseases,e.g., cardiovascular disease, rheumatoid arthritis,respiratory, reproductive, immune related disorders and mental illnesses (Adler et al, 2000; Kawachi & Kennedy,2006; Shively & Clarkson, 1994; Siegrist & Marmot,2004; Wilkinson, 2001; Cohen et al, 1997; Manuck et al,1995; Sapolsky & Share, 1994; Ader & Cohen, 2001;Dhabhar & McEwen, 1999). In response to varying stressors, animals release hormones and glucose into the bloodstream to provide extra energy, which also inhibits digestion, growth, tissue repair and reproduction. This secretion is more commonly referred to as the “fight or flight” reaction, or the stress response, which is accompanied by enhanced cognition, enhanced immune defenses,1blunted pain perception, and sharpened sensory thresholds (McEwen & Lasley, 2004; Sapolsky, 2004b).This stress response helps animals adapt to acute stress and/or short-term reactions to stress. Chronic stress,however, such as that induced by a social hierarchy, leads to excessive activation of this response, which, in turn,has been shown to have pathogenic ends (Sapolsky,2004a).
A number of stress-related physiological studies have shown that animals experiencing more social stress due to dominance rank within a hierarchy exhibit hyperactivity of the hypothalamus-pituitary-adrenal (HPA)axis, including elevated basal levels of glucocorticoids,enlarged adrenal glands accompanied by increased hormone secretion, a repressed stress response to glucocorticoids when dealing with a challenge, an impaired sensitivity of the HPA axis to negative feedback regulation and a delayed recovery from stress (Sapolsky, 2005).
The effects of dominance rank on subordinate and dominant individuals vary between species, and even within species. These variations largely depend on the dominance style, the means of maintaining a despotic dominance, the style of breeding system, rank stability,the availability of coping outlets for subordinates, the ease with which subordinates avoid dominant individuals,the availability of alternative strategies to overt competition, and even individual disposition or personality (Sapolsky, 2005). In some cases, dominant individuals are more socially stressed, such as when species maintain the hierarchy through frequent physical reassertion of dominance, in species that have a cooperative style of breeding system, those with transient periods of major rank instability, and species where dominants perceive neutral interactions as challenging while subordinates take advantage of coping strategies(Sapolsky, 2005). By contrast, these profiles are commonly observed in species with several defining characteristics: among subordinates in species where a despotic hierarchy is maintained through nonphysical intimidation, in species with consistently stable ranks,lower coping outlets available for subordinates, a lack alternative strategies to overt competition, and in species where dominants are adept at exerting social control and are highly affiliative whereas subordinates are poor at exploiting opportunities for social support, and likewise for species kept in an enclosure without sufficient space for subordinates to evade dominant animals (Sapolsky,2005).
The two main influences of the stress response are the activation of the sympathetic nervous system and the release of glucocorticoids (such as cortisol or hydrocortisone in primates, and corticosterone in most rodent species) by the HPA axis (Sapolsky et al, 2000).In response to acute stressors, the sympathetic nervous system secrets catecholamine hormones within seconds,making it difficult to obtain accurate measures of the acute stress response. Meanwhile, glucocorticoid levels change over the course of 1-2 minutes, allowing for an extensive study of the correlation between social rank and glucocorticoid changes. While both glucocorticoids and catecholamines are essential for animals to survive acute stressors, they are pathogenic when secreted in excess (Sapolsky et al, 2000). As such, animals of low social rank have been found to present a pathological condition consisting of elevated basal glucocorticoid levels, a slowed on/off “switch” of stress response, and a blunted sensitivity to excessive levels of glucocorticoids.This pathological condition has been observed in cynomolgus monkeys (Adams et al, 1985), talapoin monkeys (Keverne et al, 1982), olive baboons (Sapolsky,1990), squirrel monkeys (Manogue et al, 1975) and lemurs (Schilling & Perret, 1987). Although stress in subordinates has been shown to be related to the hypersecretion of glucocorticoids, there are exceptions reported among macaques (Bercovitch & Clarke, 1995;Chamove & Bowman, 1976; Gust et al, 1991; Gust et al,1993; Van Schaik et al, 1991), squirrel monkeys (Coe et al, 1979; Mendoza et al, 1978; Steklis et al, 1986),marmosets (Saltzman et al, 1994), talapoin monkeys(Keverne et al, 1982) and ring-tailed lemurs (Cavogelli,1999).
As the primary glucocorticoids in humans, nonhuman primates and many larger mammals, cortisol has been used to assess stress response levels in the plasma,saliva, urine, and feces of many species (Cattet et al,2003; Constable et al, 2006; Keay et al, 2006;Millspaugh et al, 2002; Wallner et al, 1999; Whitten et al,1999; Weingrill et al, 2004; Abbott et al, 2003). The most commonly used assays to detect cortisol levels in these samples include radioimmunoassays (RIAs), liquid chromatography-mass spectrometry (LC-MS/MS) and enzyme-linked immunosorbent assays (ELISA) (Gatti et al, 2009). Although plasma samples provide a measurement of cortisol levels at a single point in time,they can only be used to assess acute stress, and are susceptible to physiological fluctuations. Similarly,cortisol levels measured in the plasma peak in the early morning and gradually decrease to their lowest levels in the evening, necessitating multiple blood samples being taken waking until of sleep in order to accurately assess stress levels. Though precise, this methodology is difficult to apply to larger populations, and the compliance of individual participants with the requisite sampling schedule varies (Russell et al, 2011). Likewise, the cortisol measured in blood samples reflect total cortisol,and can thus be affected by changes in levels of cortisolbinding globulin (e.g., by birth control pills or pregnancy), which give the illusion of increased stress levels. Furthermore, the act of blood sampling via venipuncture may be a source of stress and increase plasma cortisol levels (Vining et al, 1983), potentially yielding results that do not reflect an unbiased observation.
Contrary to the more invasive methods used in plasma samples, salivary, urinal and fecal samples can be obtained relatively more easily and the measured cortisol reflects free cortisol. Unfortunately, the concentrations of cortisol still fluctuate significantly throughout the course of the day (Beerda et al, 1996; Cattet et al, 2003; Ekkel et al, 1996; Keay et al, 2006; Mormède et al, 2007;Owen et al, 2005; von der Ohe & Servheen, 2002). To overcome the issue of an animal’s diurnal rhythm,samples must be collected all day (Burch, 1982). More importantly, the cortisol levels measured in these media only reflect short-term stress occurring over hours to days, so without repeated sampling of animals, it is not possible to assess chronic stress levels that occur over weeks or months (Keay et al, 2006; Owen et al, 2005).
Hair offers some interesting alternatives in that it has a fairly predictable growth rate of approximately 1 cm/month. Likewise, hair follicles can be used to track cortisol levels over time, with the most proximal 1 cm segment to the scalp approximates the last month’s cortisol production, the second most proximal 1 cm segment approximates the cortisol production during the month before that, and so on (Wennig, 2000). This makes cortisol measured in hair a biomarker of chronic stress,as demonstrated previously in two studies of rhesus macaques in which hair samples were obtained to assess long-term stress levels (Davenport et al, 2006; Feng et al,2011). Moreover, hair samples are easily transported and stored in envelopes or vials at room temperature for years, and gathering the samples does not affect stress levels of the subjects (Gow et al, 2010; Russell et al,2011).
In the present study, we assessed long-term stress levels by measuring cortisol concentrations in hair samples from female rhesus macaques in order to test the predominant hypothesis of whether stress levels may be related or influenced by the strictness of the dominance hierarchy.
MATERIALS AND METHODS
Subjects
In total, 20 female rhesus macaques (Macaca mulatta) living in five breeding groups (n=5 in each group, where one monkey in the group was male) at the Kunming Primate Research Center, Chinese Academy of Sciences were used in this study. The monkeys ranged from 12 to 20 years of age (14.75±1.89 years), and were housed in colonies with access to a connected indoor(2.61×2.46×2.58 m)-outdoor (2.67×2.66×2.67 m) cage.All animals were given commercial monkey biscuits twice a day with tap water ad libitum, and were fed with fruits and vegetables once daily. All subjects had lived in their respective social groups at least 1 year prior to initial observation. All animal procedures were approved by the Institutional Animal Care and Use Committee of Kunming Institute of Zoology and were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Experimental design
Animal behaviors were video recorded using a focal follow technique and analyzed to calculate the linearity of hierarchies and dominance rank for each monkey(Altmann, 1974). After completion of the video recordings, hair samples were obtained to measure the cortisol levels. Afterward, correlations between social rank and hair cortisol levels were calculated.
Behavior sampling
The monkeys were given 7 days to acquaint themselves with the observers and cameras prior to recording and sampling, at which time a digital camera fixed on a tripod was set up in front of the colony to record one of the monkeys in the cage. Observers kept as far away as possible (minimum of 5 meters) from enclosures to avoid disturbing the animals during recording. Fourteen 1-hour recordings were collected for each monkey throughout a 7 day period. Two recordings were collected per day, one taken for two hours in the morning (9:00-11:00) and another segment in the afternoon (14:00-16:00). All video recordings were stored on a hard disk before being reviewed and interpreted by three technicians. The three viewers analyzed each video recording simultaneously and came to a consensus regarding the behavior classification.
Social rank determination
The social ranks for monkeys in each group were calculated according to David’s score (DS), which is based on the consistent outcomes of agonistic encounters(Gammell et al, 2003; Zhao et al, 2011). Aggressive behaviors were divided into extreme aggression and mild aggression. Behaviors that involved physical contact (a bite, slap or grab)were classified as extreme aggression.A stare threat, open-mouth threat, chase, or displacement was considered a mild aggression. Submissive behaviors were also sub-divided into extreme and mild. Extreme submissive behaviors included a scream, a scream threat,crouch, or fleeing. Mild submissions included a lip smack, grimace, submissive present, or moving away(Shively et al, 2005).
The averaged frequencies of agonistic behaviors were used to assign a hierarchal rank for each monkey based on their individual DS. A detailed method for calculating a DS has been described previously(Gammell et al, 2003). Standardized DS scores (DSS)between each group were then produced in the following manner: the smallest DS value (the largest minus value)in a group was added to its absolute value to create a zero value, then other DS values in the same group were added with the same value. The new DS values were then divided by the largest new DS value. For each group, this process created DSSvalues ranging from 0 to 1,indicating the lowest rank (DSS= 0) to the highest rank(DSS= 1) in the respective groups.
Furthermore, an H value calculated using a previously described equation (Singh et al, 1992; Singh et al, 2003) to determine the linearity of the social hierarchy. Calculated H values ranged from 0 to 1,indicating the social hierarchy on a continuum from the total absence of a ranking system (H=0) to a perfect linear order (H=1).
Hair sampling and cortisol extraction
Hair samples from all monkeys were collected between 13:30 and 15:00. At 13:30, each monkey was captured by an experienced technician using a net and removed from the colony. The hair was then taken from the back of the monkey’s neck using a pair of scissors,with particular attention made by technicians to not break or damage the skin. Hair was placed into a small pouch of aluminum foil for protection and stored as previously described (Davenport et al, 2006; Wennig,2000).
The hair cortisol extraction was done as described in detail in previous studies (Davenport et al, 2006; Feng et al, 2011). Briefly, 500 mg of hair sample was washed twice for 3 min each time in 10 mL isopropanol to remove surface contaminants, dried at 37 °C for 8 hours,and then pulverized using a Retsch ball mill (Retsch M400) at 26 Hz for 2.5 min. After, 400 mg of the powdered hair was weighed and incubated in 8 mL of methanol at room temperature for 24 hours with a slow rotation to extract cortisol. Samples were then centrifuged at 8 000 r/min for 5 minutes, and 4 mL of the supernatant was pipetted into a centrifuge tube and dried under a stream of nitrogen gas. The precipitate was reconstituted with 0.5 mL of phosphate buffered saline solution and stored at −20 °C until assayed. The cortisol concentration in each sample was quantified with a radioimmunoassay kit(Cortisol RIA DSL-2000, America). The cortisol RIA was performed at the Radioimmuno Laboratory of the Second Affiliated Hospital of the Kunming Medical College. The cortisol extraction and RIA analysis were performed under a double-blind design, with each hair sample tested twice and the mean of the two hair cortisol values used to reduce measurement error.
Data analysis
Data analysis was conducted using SPSS (SPSS inc,Chicago, IL, USA). Spearman correlations were used to assess the effects of age on social rank and hair cortisol levels and evaluate the correlation between social rank and hair cortisol levels. In all analyses, P-values were determined from two-tailed tests, with the significance level set at P<0.05.
RESULTS
Hierarchy linearity and social rank
The linearity of the social hierarchy of five female rhesus macaque breeding colonies was measured using the H values, which were 0.86, 0.49, 0.86, 0.99 and 1.00 among the different groups. Two of the five groups were found to have more stringent linear hierarchies, with H values above 0.90. The remaining three groups with H values less than 0.90 were considered to have a weak linear order in their hierarchy.
Effects of age on social rank and hair cortisol levels
In all the female monkeys (n=20), age did not affect the social rank as measured by DS values (r=-0.14,P=0.57) and DSSvalues (r=-0.13, P=0.58). Similarly,there were no significant relationships between age and hair cortisol levels within the female monkeys (r=-0.06,P=0.82). In despotic female monkeys (n=8), age did not affect the social rank, including DS values (r=-0.18,P=0.67) and DSSvalues (r=-0.18, P=0.67), and there were no significant relationships between age and hair cortisol levels within any female monkeys (r=0.25,P=0.55). Likewise, in less stringent hierarchies (n=12),age did not affect the social rank, including DS values(r=-0.13, P=0.68) and DSSvalues (r=-0.09, P=0.78).Overall, there were no significant relationships between age and hair cortisol levels within all the female monkeys included in our study (r=-0.25, P=0.44).
Correlation between social rank and hair cortisol levels
No significant correlation was discovered in the relationship between social rank and hair cortisol levels within all the female monkeys (n=20) (Figure 1A;r=-0.01, P=0.98; Figure 1B; r=-0.06, P=0.79). However,a unique correlation between social rank and cortisol levels emerged after the female groups were separated into two groups according to their calculated H values.These two groups included monkeys in despotic female groups (n=8) and those in less stringent hierarchies(n=12). No significant correlation between social rank and cortisol levels was found in the despotic female groups (Figure 2A; r=0.35, P=0.40; Figure 2B; r=0.35,P=0.40) but we did find a significant correlation between social rank and cortisol levels within the less stringent hierarchies (Figure 3A; r=-0.62, P=0.03; Figure 3B;r=-0.61, P=0.03).
DISCUSSION
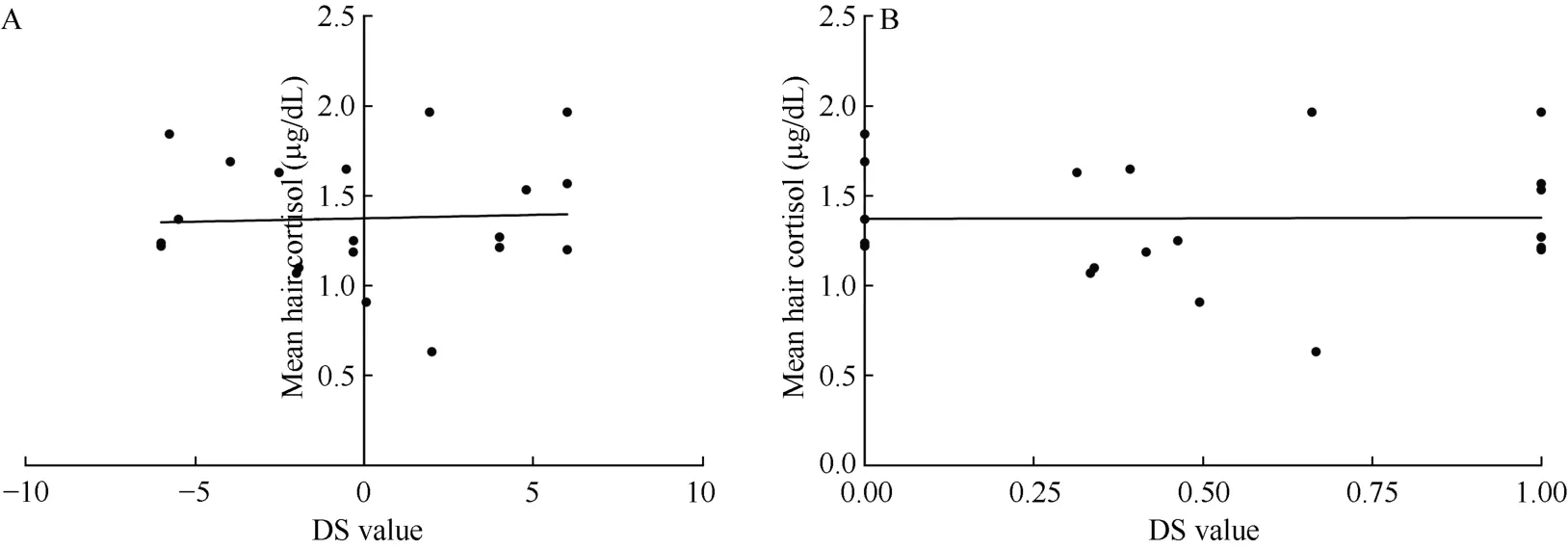
Figure 1 Correlation between social rank and hair cortisol levels in all female monkeys (n=20)X-axes refer to DS value (A) and DSS value (B). Y-axis refers to the mean hair cortisol (μg/dL).

Figure 2 Correlation between social rank and hair cortisol levels in despotic female groups (n=8)X-axes refer to DS value (A) and DSS value (B). Y-axis refers to the mean hair cortisol (μg/dL).
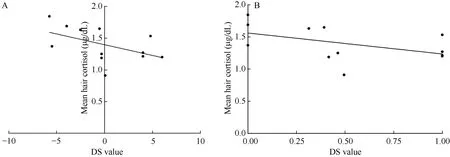
Figure 3 Correlation between social rank and hair cortisol levels in less stringent female groups (n=12)X-axes refer to DS value (A) and DSS value (B). Y-axis refers to the mean hair cortisol (μg/dL).
In the present study, we first examined female rank physiology (as quantified by cortisol levels in hair) in adult rhesus monkeys, then, after finding no relationship between rank and cortisol levels, the groups were separated into two classes according to the linear degree of their hierarchies: despotic groups with a linear hierarchy (H values>.90) and groups with less stringent hierarchies (H values<.90) (Li et al, 2004). After separation, two distinct trends emerged from the analysis.In less stringent groups, there was a negative correlation between social rank and cortisol levels, suggesting that low ranking monkeys were more socially stressed than higher ranking monkeys. Conversely, groups with despotic hierarchies did not display this trend.
Overall, despotic groups were much stricter than the less stringent group hierarchies, which meant that monkeys in the latter groups were relatively more assertive with dominance rank than monkeys in despotic groups. This finding suggests that the despotic patterns of female rhesus monkey culture have a differentiated effect on their rank physiology. Likely, less despotism of female rhesus monkey culture leads to an increased physiological stress for low ranking monkeys, which, in turn, increased release of cortisol by an excessive activation of the HPA axis.
Age may be an important factor that affects social rank and cortisol of animals, but in our study, age had no effect on the social rank and cortisol levels of female monkeys. On the whole, these findings are in line with hypothesis that variations in the strictness of dominance style are the determining factors in rank physiology(Sapolsky, 2005).
A hierarchy is meaningful to the animals within it because it can affect rank-related physiology. The formation of a dominance hierarchy is not only predetermined by differences in the attributes of animals,but is also produced by the dynamics of the social interactions (Chase et al, 2002). Hierarchical structures vary among species. For example, dominance hierarchies in nonhuman primates can be linear (i.e., A>B>C>D) or circular (i.e., A>B>C>A) (Li et al, 2004). In this study, H values were used to calculate the linearity of hierarchies(Singh et al, 1992; Singh et al, 2003). Generally, H values ranging from 0.90 to 1.00 indicate a perfect linear order (Li et al, 2004). The low ranking animals in groups with H values less than 0.90 were found to experience more physiological stressors (as assessed by hair cortisol levels) than higher ranking animals. This phenomena probably occurs because low ranking females in the less stringent groups potentially lack predictive information and/or a sense of control in agonistic encounters with their counterparts, thereby contributing to the physiological stressors generated from such encounters.Moreover, subordinate females have relatively few coping outlets (e.g., being able to displace aggressive frustration onto other females with lower ranking) to alleviate the stress, and this stress may be attributable to the relatively unstable hierarchies (Sapolsky, 2004a).
Although extensive research in nonhuman primates has shown that subordinates are more likely to exhibit higher levels of cortisol than dominants, including researches in female cynomolgus monkeys (Adams et al,1985), male squirrel monkeys (Manogue et al, 1975),male olive baboons (Sapolsky, 1990), female talapoin monkeys (Keverne et al, 1982), and male mouse lemurs(Schilling & Perret, 1987), a number of other studies have not found differences in cortisol levels between subordinates and dominants, including studies in female squirrel monkeys (Mendoza & Mason, 1991), male rhesus monkeys (Bercovitch & Clarke, 1995) and female talapoin monkeys (Yodyingyuad et al, 1985). In fact,other investigations have even reported higher cortisol levels in dominants than in subordinate common female marmosets (Saltzman et al, 1998), and in subordinate male and female cotton top tamarins (Snowdon et al,1985).
These nonhuman primate studies mentioned above highlight a great inconsistency between cortisol and rank in a number of species. The inconsistent results are partly due to species variation, but also due to different methodologies. Previous studies utilized plasma or urine samples to measure cortisol levels. Blood sampling requires stressful procedures such as the capture,restraint or venipuncture of the animals, can elevate circulating cortisol levels (Vining et al, 1983), somewhat altering the situation they were intending to measure.Similarly, a shortcoming of urine samples is that they are easily contaminated because of uncontrolled urine excretion. Furthermore, measured concentrations of cortisol in both these media are subject to circadian effects. More importantly, however, these cortisol levels only reflect short-term stress that occur over hours to days, and without the repeated sampling of animals, they cannot assess chronic stress levels that occur over weeks to months long periods (Keay et al, 2006; Owen et al,2005). Cortisol measured in hair, meanwhile, has been considered a biomarker of chronic stress and has proved useful in two studies of rhesus macaques in which hair samples were obtained to assess long-term cortisol and stress levels (Davenport et al, 2006; Feng et al, 2011). To our knowledge, this is the first study using cortisol measurements from hair sampling to evaluate the relationship between social rank and long-term stress in female rhesus monkeys, offering novel insight into longterm stress that arises from social.
Though the realities of nonhuman primate social systems are rather simpler than those of human social systems, humans are similarly considered vulnerable to psychosocial stress stemming from their rank in the socioeconomic status (SES) hierarchy (Brunner, 1997;Sapolsky, 2005). Low SES individuals generally experience worse health outcomes than individuals ranked above them. Potential health related outcomes include an increased risks of cardiovascular disease, rheumatoid arthritis, respiratory, respiratory, reproductive, immune system and psychiatric diseases, as well as an overall increased mortality risk (Adler et al, 2000; Kawachi &Kennedy, 2006; Siegrist & Marmot, 2004; Wilkinson,2001). Similarly, long-term relationships of dominance and subordination stressors generate stressors associated with negative health sequelae in nonhuman primates,such as an increased cardiovascular disease risk (Manuck et al, 1995; Willard & Shively, 2011), depressive behaviors (Shively et al, 2005), HPA axis perturbations(Shively, 1998; Shively et al, 1997), ovarian dysfunction(Shively et al, 1997), reduced hippocampal volume(Willard & Shively, 2011), decreased dopamine-2 receptor function (Shively, 1998), and altered serotonergic function (Willard & Shively, 2011).
Taken together, researches on both human and nonhuman primates both support a role for physiological stress in a SES-related health gradient. As such, there is considerable interest in the development of ranking related stress models to aid the understanding of human SES-related diseases as well as guide the development of new therapeutics (Shively et al, 2005; Willard & Shively,2011).
Abbott D, Keverne E, Bercovitch F, Shively CA, Mendoza SP,Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T,Sapolsky RM. 2003. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates.Hormones and Behavior, 43(1): 67-82.
Adams MR, Kaplan JR, Koritnik DR. 1985. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis.Physiology & Behavior, 35(6): 935-940.
Ader R, Cohen N. 2001. Conditioning and immunity. // Ader R, Felten D, Vohen N, eds. Psychoneuroimmunology. New York: Scademic Press,2: 3-34.
Adler NE, Epel ES, Castellazzo G, Ickovics JR. 2000. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy, White women.Health Psychology, 19(6): 586-532.
Altmann J. 1974. Observational study of behavior: sampling methods.Behaviour, 49(3-4): 227-267.
Beerda B, Schilder MBH, Janssen NS, Mol JS. 1996. The use of saliva cortisol, urinary cortisol, and catecholamine measurements for a noninvasive assessment of stress responses in dogs. Hormones and Behavior, 30(3): 272-279.
Bercovitch FB, Clarke AS. 1995. Dominance rank, cortisol concentrations, and reproductive maturation in male rhesus macaques.Physiology & Behavior, 58(2): 215-221.
Brunner E. 1997. Socioeconomic determinants of health: stress and the biology of inequality. BMJ, 314(7092): 1472.
Burch WM. 1982. Urine free-cortisol determination-a useful tool in the management of chronic hypoadrenal states. JAMA: the Journal of the American Medical Association, 247(14): 2002-2004.
Cattet M, Christison K, Caulkett NA, Stenhouse GB. 2003. Physiologic responses of grizzly bears to different methods of capture. Journal of Wildlife Diseases, 39(3): 649-654.
Cavogelli S A. 1999. Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta.Animal Behaviour, 57(4): 935-944.
Chamove AS, Bowman RE. 1976. Rank, rhesus social behavior, and stress. Folia Primatologica, 26(1): 57-66.
Chase ID, Tovey C, Spangler-Martin D, Manfredonia M. 2002.Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proceedings of the National Academy of Sciences, 99(8): 5744-5749.
Coe CL, Mendoza SP, Levine S. 1979. Social status constrains the stress response in the squirrel monkey. Physiology & Behavior, 23(4):633-638.
Cohen S, Line S, Manuck SB, Rabin BS, Heise ER, Kaplan JR. 1997.Chronic social stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. Psychosomatic Medicine,59(3): 213-221.
Constable S, Parslow A, Dutton G, Rogers T, Hogg C. 2006. Urinary cortisol sampling: a non‐ invasive technique for examining cortisol concentrations in the Weddell seal, Leptonychotes weddellii. Zoo Biology, 25(2): 137-144.
Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. 2006.
Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and Comparative Endocrinology, 147(3): 255-261.Dhabhar FS, Mcewen BS. 1999. Enhancing versus suppressive effects of stress hormones on skin immune function. Proceedings of the National Academy of Sciences, 96(3): 1059-1064.
Ekkel ED, Dieleman SJ, Schouten WGP, Portela A, Cornélissen G,Tielen MJ, Halberg F. 1996. The circadian rhythm of cortisol in the saliva of young pigs. Physiology & Behavior, 60(3): 985-989.
Feng X, Wang L, Yang S, Qin D, Wang J, Li C, Lü L, Ma Y, Hu X.2011. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proceedings of the National Academy of Sciences, 108(34): 14312-14317.
Gammell MP, De Vries H, Jennings DJ, Carlin CM, Hayden TJ. 2003.David's score: a more appropriate dominance ranking method than Clutton-Brock et al. 's index. Animal Behaviour, 66(3): 601-605.
Gatti R, Antonelli G, Prearo M, Spinella P, Cappellin E, De Palo EF.2009. Cortisol assays and diagnostic laboratory procedures in human biological fluids. Clinical Biochemistry, 42(12): 1205-1217.
Gow R, Thomson S, Rieder M, Van Unm S, Koren G. 2010. An assessment of cortisol analysis in hair and its clinical applications.Forensic Science International, 196(1-3): 32-37.
Gust DA, Gordon TP, Hambright MK, Wilson ME. 1993. Relationship between social factors and pituitary-adrenocortical activity in female rhesus monkeys (Macaca mulatta). Hormones and Behavior, 27(3):318-331.
Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR,McClure HM. 1991. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain, Behavior, and Immunity, 5(3): 296-307.
Kawachi I, Kennedy BP. 2006. The Health of Nations: Why Inequality is Harmful to Your Health. New York: New Press.
Keay JM, Singh J, Gaunt MC, Kaur T. 2006. Fecal glucocorticoids and their metabolites as indicators of stress in various mammalian species:a literature review. Journal of Zoo and Wildlife Medicine, 37(3): 234-244.
Keverne EB, Meller RE, Eberhart A. 1982. Dominance and subordination: concepts or physiological states. // Chiarelli AB,Corruccini RS. Advanced Views in Primate Biology. Berlin: Springer-Verlag: 81-94.
Li HQ, Zhang YH, Li BG. 2004. Review on dominance hierarchy of Non-human Primates. Acta Theriologica Sinica, 24(1): 53-60 (in Chinese).
Manogue KR, Leshner AI, Candland DK. 1975. Dominance status and adrenocortical reactivity to stress in squirrel monkeys (Saimiri sciureus). Primates, 16(4): 457-463.
Manuck SB, Marsland AL, Kaplan JR, Williams JK. 1995. The pathogenicity of behavior and its neuroendocrine mediation: An example from coronary artery disease. Psychosomatic Medicine, 57(3):275-283.
Mcewen BS, Lasley EN. 2004. The End of Stress as We Know It.Washington: Joseph Henry Press.
Mendoza SP, Mason WA. 1991. Breeding readiness in squirrel monkeys: female-primed females are triggered by males. Physiology &Behavior, 49(3): 471-479.
Mendoza SP, Coe CL, Lowe EL, Levine S. 1978. The physiological response to group formation in adult male squirrel monkeys.Psychoneuroendocrinology, 3(3-4): 221-229.
Millspaugh JJ, Washburn BE, Milanick MA, Beringer J, Hansen LP,MeyerTM. 2002. Non-invasive techniques for stress assessment in white-tailed deer. Wildlife Society Bulletin, 30(3): 899-907.
Mormède P, Andanson S, Aupérin B, Beerdad B, Guémenée D,Malmkvistf J, Mantecag X, Manteuffelh G, Prunetc P, van Reenend CG,Richarde S, Veissierb I. 2007. Exploration of the hypothalamicpituitary-adrenal function as a tool to evaluate animal welfare.Physiology & Behavior, 92(3): 317-339.
Owen MA, Czekala NM, Swaisgood RR, Steinman K, Lindburg DG.2005. Seasonal and diurnal dynamics of glucocorticoids and behavior in giant pandas. Ursus, 16(2): 208-221.
Russell E, Koren G, Rieder M, Van Uum S. 2011. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37(5): 589-601.
Saltzman W, Schultz-Darken NJ, Scheffler G, Wegner FH, Abbott DH.1994. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiology & Behavior, 56(4): 801-810.
Saltzman W, Schultz-Darken NJ, Wegner FH, Wittwer DJ, Abbott DH.1998. Suppression of cortisol levels in subordinate female marmosets:reproductive and social contributions. Hormones and Behavior, 33(1):58-74.
Sapolsky RM. 1990. Adrenocortical function, social rank, and personality among wild baboons. Biological Psychiatry, 28(10): 862-878.
Sapolsky RM. 2004a. Social status and health in humans and other animals. Annual Review of Anthropology, 33: 393-418.
Sapolsky RM. 2004b. Why Zebras Don't Get Ulcers: The Acclaimed Guide to Stress, Stress-Related Diseases, and Coping-Now Revised and Updated. New York: Holt Paperbacks.
Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science, 308(5722): 648-652.
Sapolsky RM, Share L J. 1994. Rank‐ related differences in cardiovascular function among wild baboons: Role of sensitivity to glucocorticoids. American Journal of Primatology, 32(4): 261-275.
Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive,stimulatory, and preparative actions. Endocrine Reviews, 21(1): 55-89.Schilling A, Perret M. 1987. Chemical signals and reproductive capacity in a male prosimian primate (Microcebus murinus). Chemical Senses, 12(1): 143-157.
Shively CA. 1998. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biological Psychiatry, 44(9): 882-891.
Shively CA, Clarkson TB. 1994. Social status and coronary artery atherosclerosis in female monkeys. Arteriosclerosis, Thrombosis, and Vascular Biology, 14(5): 721-726.
Shively CA, Laber-Laird K, Anton RF. 1997. Behavior and physiology of social stress and depression in female cynomolgus monkeys.Biological Psychiatry, 41(8): 871-882.
Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J,Lanier T. 2005. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis). Biological Psychology,69(1): 67-84.
Siegrist J, Marmot M. 2004. Health inequalities and the psychosocial environment--two scientific challenges. Social Science & Medicine,58(8): 1463-1473.
Singh M, D'souza L, Singh M. 1992. Hierarchy, kinship and social interaction among Japanese monkeys (Macaca fuscata). Journal of Biosciences, 17(1): 15-27.
Singh M, Sharma A, Krishna B. 2003. Methodological considerations in measurement of dominance in primates. Current Science, 84(5): 709-713.
Snowdon CT, Savage A, Mcconnell PB. 1985. A breeding colony of cotton-top tamarins (Saguinus oedipus). Laboratory Animal Science,35(5): 477-480.
Steklis HD, Raleigh MJ, Kling AS, Tachiki K. 1986. Biochemical and hormonal correlates of dominance and social behavior in all male ‐groups of squirrel monkeys (Saimiri sciureus). American Journal of Primatology, 11(2): 133-145.
Van Schaik C, Van Noordwijk M, Van Bragt T, Blankenstein MA. 1991.A pilot study of the social correlates of levels of urinary cortisol,prolactin, and testosterone in wild long-tailed macaques (Macaca fascicularis). Primates, 32(3): 345-356.
Vining RF, Mcginley RA, Maksvytis JJ, Ho KY. 1983. Salivary cortisol:a better measure of adrenal cortical function than serum cortisol.Annals of Clinical Biochemistry, 20(P6): 329-335.
Von Der Ohe CG, Servheen C. 2002. Measuring stress in mammals using fecal glucocorticoids: opportunities and challenges. Wildlife Society Bulletin, 30(4): 1215-1225.
Wallner B, Möstl E, Dittami J, Prossinger H. 1999. Fecal glucocorticoids document stress in female Barbary macaques (Macaca sylvanus). General and Comparative Endocrinology, 113(1): 80-86.
Weingrill T, Gray DA, Barrett L, Henzi SP. 2004. Fecal cortisol levels in free-ranging female chacma baboons: relationship to dominance,reproductive state and environmental factors. Hormones and Behavior,45(4): 259-269.
Wennig R. 2000. Potential problems with the interpretation of hair analysis results. Forensic Science International, 107(1-3): 5-12.
Whitten PL, Stavisky R, Aureli F, Rusell E. 1999. Response of fecal cortisol to stress in captive chimpanzees (Pan troglodytes). American Journal of Primatology, 44(1): 57-69.
Wilkinson RG. 2001. Mind the Gap: Hierarchies, Health and Human Evolution. New Haven: Yale University Press.
Willard SL, Shively CA. 2011. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis). American Journal of Primatology, 74(6): 528-542.
Yodyingyuad U, De La Riva C, Abbott D, Herbert J, Keverne EB. 1985.Relationship between dominance hierarchy, cerebrospinal fluid levels of amine transmitter metabolites (5-hydroxyindole acetic acid and homovanillic acid) and plasma cortisol in monkeys. Neuroscience,16(4): 851-858.
Zhao HT, Zhang J, Zhu ZR, Li BG, Wang XW. 2011. The methods of research on the dominance of female in non-human primates. Acta Anthropologica Sinica, 30(4): 415-424 (in Chinese).
