Force measurement between mica surfaces in electrolyte solutions
2013-09-17ZhaoGutianGuoWeichuanTanQiyanQiuYinghuaKanYajingChenYunfeiNiZhonghua
Zhao Gutian Guo Weichuan Tan Qiyan Qiu Yinghua Kan Yajing Chen Yunfei,2 Ni Zhonghua
(1Jiangsu Key Laboratory for Design and Manufacture of Micro-Nano Biomedical Instruments,Southeast University, Nanjing 211189, China)
(2Key Laboratory of MEMS of Ministry of Education, Southeast University, Nanjing 210096, China)
T he DLVO theory indicates that there are two independent types of forces that govern the long-range interaction between similar colloidal particles immersed in aqueous solutions, i.e., the attractive van der Waals force and the repulsive double-layer force,which have been extensively studied for many times[1-3].The doublelayer force is derived from the overlapping of the electrical double-layer which can be affected by many factors,such as the concentration of cation and the adsorption capacity of cation to the negatively charged mica surface.Unlike the double-layer force,the van der Waals potential is largely insensitive to the variations in electrolyte concentration and pH,which is considered as fixed in a first approximation[1-2,4].Furthermore, the van der Waals attraction must always exceed the double-layer repulsion at a sufficiently short distance due to its power law interaction(W∝ - 1/Dn)[3], while the double-layer interaction energy remains finite or increases slowly asD→0.The balance between the van der Waals attraction and the double-layer repulsion determines the stability of colloidal dispersions[5].However, one cannot say that all the experimental phenomena are readily predicted by the DLVO theory because this theory fails when the surface separation is less than about 5 nm[1,6-11].As the most common solutes in the natural environment,monovalent and divalent ions are thought to play a key role in biological phenomena[12-14], it is necessary to investigate the properties of monovalent and divalent cations which are confined in two mica surfaces just like the confinement in a cell membrane.
The measurement of DLVO forces and other types of micro-forces is always a focus in collide science,and SFA can be used to measure the physical forces,including the van der Waals and the electrostatic forces between surfaces at the nanoscale and molecular levelin situand in real time[15-19].After years of improvements by Israelachvili and his co-workers,there are many versions of SFA available in the area,such as SFA MkⅠ,SFA MkⅡ, SFA Mk Ⅲ, SFA 2000[20].The SFA technique can be used in many fields, such as chemical, physical, biological, and geometrical properties.In this work, a brief introduction of the experimental system and process is presented and experimental results are compared with the DLVO theory.Results show that the DLVO forces are consistent with theoretical values in long range and they also have complex factors such as hydrated ion size,force barrier and critical concentration when the hydration force dominates in the short range.
1 Methods and Experimental Conditions
In this paper,the normal forces between molecularly smooth mica surfaces in monovalent and divalent ions solutions are measured on SFA 2000.Fig.1 shows the basic structure of SFA 2000 in our laboratory.
As shown in Fig.2, a multiple beam interference(MBI)fringe[20]is used to determine the distance between the surfaces and also the shape of the surfaces[3-4,21].For a typical SFA experiment,a pair of fresh mica surfaces are used as the surface substrates(about 1 to 3 μm),of which on the backside a thin layer silver film(about 55 nm)is coated for providing a good interfering pattern between the reflecting surfaces.The surfaces are glued onto a cylindrical-shaped glass disk and mounted in a cross-cylindrical configuration,which simulates a sphere-on-flat geometry.When white light is normally directed to the surfaces,it reflects back and forth between the silver layers.The transmitted light near the closest contactpointbetween the surfacescreates Newton's rings,which can be seen through a microscope objective.If the light passes through a spectrometer,these wavelengths are split up and appear as an array of fringes in the spectrogram.These fringes are called fringes of equal chromatic order(FECO)[22].
As the substrate of these experiments,the mica surface is widely used for the simple interpretation of surface force measurements due to its unique properties and the mica-water interface is considered to be an ideal system for the study of surface forces which have also been presented in many colloidal systems.Cleaved sheets of muscovite mica display a molecularly smooth planar surface which has a high negative lattice charge due to the periodic replacement of Si atoms by Al[14].The resultant charge of about 2.1 ×1014negative electronic charges per square centimeter area is exactly balanced in the crystal by surface K+ions on the mica surface[8].In these experiments,the forces between two molecularly smooth mica surfaces are measured over a range of concentrations in aqueous K+, Na+, and Mg2+chloride solutions.
The room temperature is 23 to 27℃measured with a Hg thermometer.Mica is ruby muscovite, grade 1, supplied by S&J Trading Inc.(NY),and the high-purity reagent(99.999%KCl, 99.999%NaCl, 99.99%MgCl2)is provided by Sigma-Aldrich.The water has a resistivity of typically 18.25 MΩ·cm and very low bubble persistence after several processes.The pH value of the water lies in the range of 5.4 to 5.8(due to dissolved CO2)after deaeration before filling the apparatus.Fig.3 shows the process of the SFA experiment in our laboratory.

Fig.1 Photograph of SFA 2000
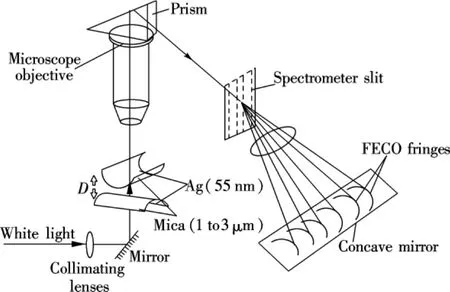
Fig.2 Schematic of SFA setup

Fig.3 Outline of the typical SFA experimental process
2 Results and Discussion
The forceFbetween two molecularly smooth curved surfaces(of radiusR)is measured using the method developed by Israelachvili and Adams[1].The valueF/Ris plotted in the graphs as a function of the surface separa-tion distanceD.The value is equal to 2πE,in whichEis the corresponding energy between the flat surfaces[2].The theoretical function of 1∶1 electrolyte solutions forF/Ris derived from the Poisson-Boltzmann(PB)equation which is shown as
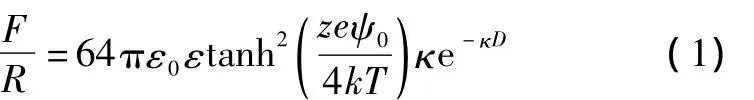
where
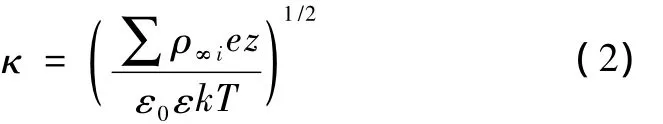
In Eq.(1),all the parameters are known except the surface potential ψ0when the experimental conditions are settled.There only exists a numerical solution for the PB equation in the case of 1∶2 electrolyte solutions or other types of non-symmetric electrolyte solutions.The characteristic length or thickness of the diffuse electric doublelayer,which describes the atmosphere near a charged surface,is known as the Deybe length,1/κ.The Deybe length is defined as

whereCis the concentration,mol/L;the unit of 1/κ is nm.
The results of the force measurements as a function of separation are given in NaCl,KCl and MgCl2solutions,respectively,as shown in Fig.4(a),Fig.4(b)and Fig.5.For ensuring experimental reproducibility,the mica sheets used in these experiments are all cleaved from the same original sheet.
The DLVO forces and the hydration force are shown in Fig.4(c).The measured double-layer repulsive force is well described by Eq.(1)at a constant surface potential.The dashed line indicates that the van der Waals force attraction causes the surfaces to jump into adhesive contact from the maximum at a short separation of about 3 nm.In Figs.4(a)and(b),it is apparent that additional short-range(about 3 nm)repulsive forces become dominant and prevent adhesive contact in a primary minimum above a certain concentration specific to each cation.In Na+solutions,hydration forces are observed at concentrations above about 10-2mol/L,while the K+solutions exhibit a hydration force in 10-3mol/L.The results suggest that the hydration force arises only when the cations are held to the mica surface in some specific way.According to the mass action model of mica surface charging[7],Na+and K+present hydration forces at different concentrations,which indicates that the hydrated energy of K+is greater than that of Na+.The distance at which the force barrier is broken by the van der Waals force indicates that the hydrated ion radius of Na+is greater than that of K+.However,most of the leading factors to the hydration forces are still not well understood from many experimental and theoretical studies.In Fig.4(d),the surface potential of the cation-adsorbed mica surface is obtained according to Figs.4(a)and(b)and Eq.(1).It is apparent that the surface potential of the mica surface does not decrease monotonically as the monovalent cation increases.
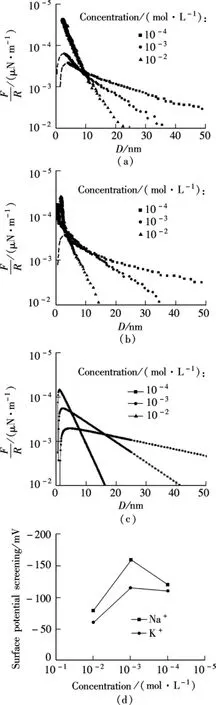
Fig.4 Force measurements between mica surfaces immersed in halogen electrolyte solutions and the theoretical value of monovalent ions.(a)Force measured in KCl solution;(b)Force measured in NaCl solution;(c)Theoretical value of 1:1 electrolyte solutions calculated by DLVO theory;(d)Apparent surface potential of mica in monovalent cation solutions
The results of force measurements for the pure water and the Mg2+chloride solutions are summarized in Fig.5.In these cases,the increase in the bulk cation concentration leads to the decrease in the magnitude of the surface potential and the Debye length is almost consistent with the numerical solution of the PB equation for 1∶2 electrolytes according to Eq.(2).

Fig.5 Force measurement between mica surfaces
3 Conclusion
The experimental results are roughly consistent with theoretical predications,indicating that both the SFA experimental system and the experimental process are reliable.Based on the experimental results,we can obtain the profiles of the DLVO force,the hydration force,the adhesive contact and the force barrier in various concentrations.The hydration size can be concluded as Mg2+>Na+>K+.The surface potential in a lower concentration MgCl2solution decreases monotonically compared with that in pure water,which is different from that in K+and Na+solutions.This suggests that the screening effect of divalent cations to the surface potential of mica is more significant than monovalent cations.
[1]Israelachvili J N,Tandon R K,White L R,et al.Direct measurement of forces between Peo adsorbed on mica surfaces in aqueous-electrolyte[J].Abstracts of Papers of the American Chemical Society,1979(4):159-164.
[2]Israelachvili J N.Intermolecular and surface forces[M].Santa Barbara,CA,USA:Elsevier,2009:107-140.
[3]Tabor D,Winterto Rh.Direct measurement of normal and retarded Van Der Waals forces[J].Proceedings of the Royal Society of London.Series A:Mathematical and Physical Sciences,1969,312(1511):435-437.
[4]Israelachvili J N,Tabor D.Measurement of Vanderwaals dispersion forces in range 1.5 to 130 Nm [J].Proceedings of the Royal Society of London.Series A:Mathematical and Physical Sciences,1972,331(1584):19-25.
[5]Attard P.Recent advances in the electric double layer in colloid science[J].Current Opinion in Colloid&Inter-face Science,2001,6(4):366-371.
[6]Pashley R M.Hydration forces between mica surfaces in aqueous-electrolyte solutions [J].J Colloid Interf Sci,1981,80(1):153-162.
[7]Pashley R M.Dlvo and hydration forces between mica surfaces in Li+,Na+,K+,and Cs+electrolyte-solutions:a correlation of double-layer and hydration forces with surface cation-exchange properties[J].J Colloid Interf Sci,1981,83(2):531-546.
[8]Pashley R M,Israelachvili J N.Dlvo and hydration forces between mica surfaces in Mg2+,Ca2+,Sr2+,and Ba2+chloride solutions[J].J Colloid Interf Sci,1984,97(2):446-455.
[9]Pashley R M.Forces between mica surfaces in La3+and Cr3+electrolyte-solutions [J].J Colloid Interf Sci,1984,102(1):23-35.
[10]Grosberg A Y,Nguyen T T,Shklovskii B I.Colloquium:the physics of charge inversion in chemical and biological systems[J].Rev Mod Phys,2002,74(2):329-345.
[11]Levin Y.Electrostatic correlations:from plasma to biology[J].Rep Prog Phys,2002,65(11):1577-1632.
[12]Browning J L,Nelson D L,Hansma H G.Ca2+influx across excitable membrane of behavioral mutants of paramecium [J].Nature,1976,259(5543):491-494.
[13]Pietrement O,Pastre D,Fusil P,et al.Adsorption of DNA to mica mediated by divalent counterions:a theoretical and experimental study [J].Biophys J,2003,85(4):2507-2518.
[14]Hansma H G.Possible origin of life between mica sheets[J].J Theor Biol,2010,266(1):175-188.
[15]Borukhov I,Andelman D,Orland H.Steric effects in electrolytes:a modified Poisson-Boltzmann equation[J].Phys Rev Lett,1997,79(3):435-438.
[16]Bloomfield V A,Rouzina I.Use of Poisson-Boltzmann equation to analyze ion binding to DNA [J].Energetics of Biological Macromolecules,1998,295(2):364-378.
[17]Perel V I,Shklovskii B I.Screening of a macroion by multivalent ions:a new boundary condition for the Poisson-Boltzmann equation and charge inversion[J].Physica A,1999,274(3/4):446-453.
[18]Ben-Yaakov D,Andelman D.Revisiting the Poisson-Boltzmann theory:charge surfaces,multivalent ions and inter-plate forces[J].Physica A,2010,389(15):2956-2961.
[19]McCormack D,Carnie S L,Chan D Y C.Calculations of electric double-layer force and interaction free-energy between dissimilar surfaces [J].J Colloid Interf Sci,1995,169(1):177-196.
[20]Israelachvili J N,Min Y,Akbulut M,et al.Recent advances in the surface forces apparatus(SFA)technique[J].Rep Prog Phys,2010,73(3):1-5.
[21]Israelachvili J N,Tabor D.Measurement of van der Waals dispersion forces in the range 1.4 to 130 nm [J].Nature Phys Sci,1972,236(68):106-110.
[22]Israelachvili J N.Thin-film studies using multiple-beam interferometry[J].J Colloid Interf Sci,1973,44(2):259-272.
杂志排行
Journal of Southeast University(English Edition)的其它文章
- A personalized trustworthy service selection method
- L1-norm minimization for quaternion signals
- Feature combination via importance-inhibition analysis
- W-band sharp-rejection bandpass filter with notch cavities
- Factors affecting headway regularity on bus routes
- Optimization of RDF link traversal based query execution
