4-氯苯氧乙酸及 4,4′-bipy=4,4′-联吡啶构筑的层状锌配合物的合成、晶体结构
2013-09-15季宁宁张亚男石智强何国芳
季宁宁 张亚男 石智强 何国芳
(泰山学院化学化工学院,泰安 271021)
There has been great interest in the field of design and synthesis of coordination polymeric complexes,in particular,the carboxyl complexes,owing to their fascinating molecular topologies along with potential applications as functional materials[1-12].In the past few years,much emphasis was laid on using the rigid aromatic carboxylic acids as ligands[13-15]to synthesis the carboxyl complexes.But flexible aromatic carboxylic acids ligands have not been studied much maybe their varied geometries and conformations make it difficult to control the expected structures[15].P-chlorophenoxyacetic acid as a flexible ligand owing to the presence of the-OCH2-spacer has been extensively studied and well understood for mono-,dinuclear complexes[16].In order to obtain further information about the complex construction with flexible aromatic carboxylic acid,we synthesized a complex[Zn(PCPA)2(4,4′-bipy)(H2O)]n.We report here the synthesis,crystal structure and thermal stability.
1 Experimental
1.1 Materials and physical measurements
All reagents and solvents were used as purchased without further purification.IR spectra were recorded on a Nicolet 6700 spectrometer in the 4 000~400 cm-1region using the KBr pellet technique.The UV spectra wasobtained on a Heliosa UV spectrometer in the200~400 nm range at room temperature.Crystal structure determination was carried out on a Bruker Smart APEX Ⅱ CCD diffractometer.Elemental analyses of C,H and Nwere performed on a PE-2400(Ⅱ) apparatus.Thermogravimetric analysis (TG)data were collected on a METTLER TGA/DSC 1100 instrument under air atmosphere with a heating rate of 10 ℃·min-1.PXRD measurements were performed on a Bruker D8 Advance X-ray diffractometer using Cu Kαradiation(0.154 18 nm),in which the X-ray tube was operated at 40 kV and 30 mA.
1.2 Synthesis of[Zn(PCPA)2(4,4′-bipy)(H 2O)]n
A mixture of Zn(OAc)2·2H2O(0.110 g,0.5 mmol),PCPA (0.187 g,1.0 mmol),4,4′-bipy(0.156 g,1.0 mmol),Et3N (1.0 mmol,0.14 mL)were added into a mixed solvent of 10 mL anhydrous methanol and 5 mL water and stirred for 1 h.Then the mixture was transferred and sealed into a 23 mL Teflon-lined stainless steel autoclave,which was heated at 120℃for 3 d and then cooled to room temperature.The colorless crystals were obtained in 36%yield(based on Zn).Anal.Calcd.for C26H22ZnCl2N2O7(%):C,51.12;H,3.63;N,4.59.Found(%):C,51.07;H,3.57;N,4.65.IR (KBr,ν/cm-1):3 406,1 630,1 490,1 474,1 314,1 236,1 072,826,756,508.
1.3 Crystal structure determination
The single crystals of the title complex with approximate dimensions of 0.30 mm×0.20 mm ×0.12 mm was placed on a Bruker Smart APEXⅡCCD diffractometer.The diffraction data were collected at 295 K usingagraphite-monochromatic Mo Kαradiation(λ =0.071 073 nm)with the φ-ω scan mode in the range of 1.93°≤θ≤25.05°.The correction for Lp factors was applied.The structure was solved by direct methods with SHELXS-97 program[17]and refined by full-matrixleast-squarestechniqueson F2with SHELXL-97[18].All non-hydrogen atoms were treated anisotropically.The organic hydrogen atoms were placed in calculated positions with fixed isotropic thermal parameters and included in structure factor calcula-tions in the final stage of full-matrix least-squares refinement.The water hydrogen atoms were located in the difference Fourier map and refined isotropically.The final R=0.034 5,wR=0.091 4(w=1/[σ(Fo)+(0.038 8P)2+0.983 5P],where P=(Fo2+2Fc2)/3),S=1.083,(Δ/σ)max=0.000,(Δρ)max=629 and(Δρ)min=-533 e·nm-3.The crystallographic data and structure refinements for the complex are summarized in Table 1.The selected bond lengths and bond angles of the title complex are listed in Table 2,and the hydrogen bond details in Table 3.
CCDC:850113.
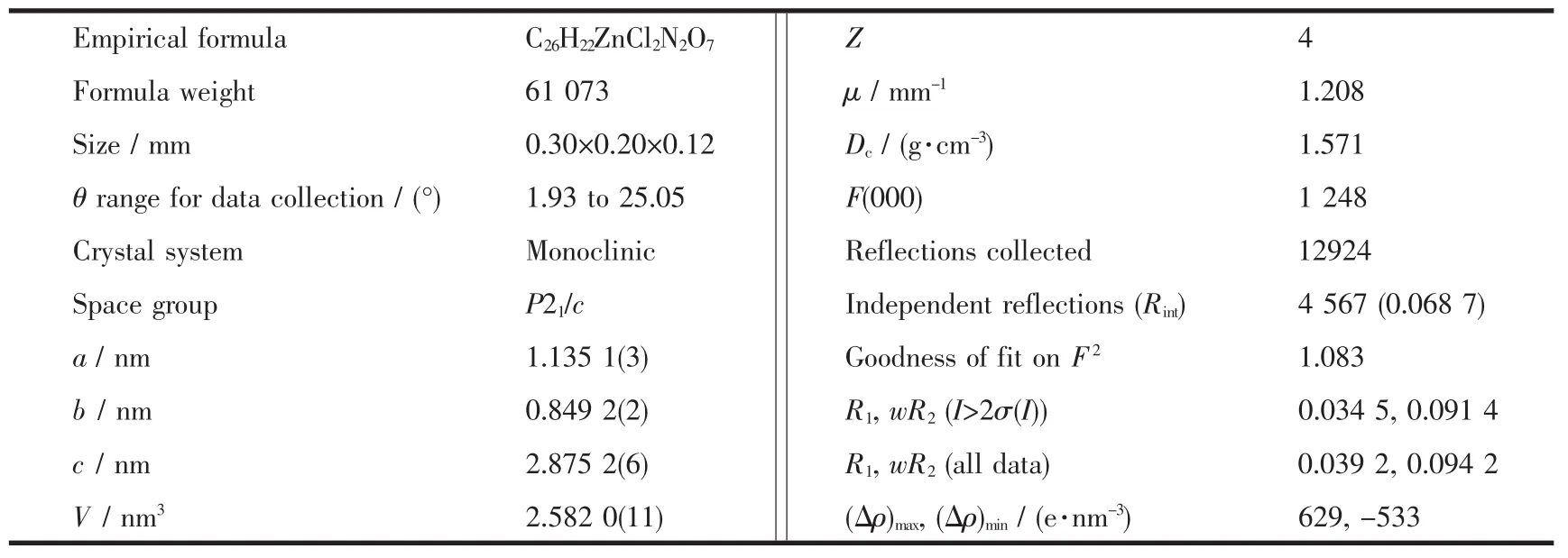
Table 1 Crystal data and structure refinements of the title complex

Table 2 Selected bond lengths(nm)and angles(°)for the title complex

Table 3 Hydrogen bond length and angles for the title complex
2 Results and discussion
2.1 IR spectra
The IR spectra of the title complex [Zn(PCPA)2(4,4′-bipy)(H2O)]nclearly shows both the presence of the PCPA and coordinated 4,4′-bipy.The strong peaks at 1 630 and 1 474 cm-1are attributed toνas(COO-)and νs(COO-)respectively.The value ofΔ(νas(COO-)-νs(COO-))is 156 cm-1,which is larger than 100 cm-1but smaller than 200 cm-1,indicating that part PCPA adopting chelating coordination mode[19]of the carboxyl group to the metal atom,which are finally confirmed by X-ray diffraction analysis.The adsorption at 1 490,826 and 615 cm-1are the characteristic adsorption peak of 4,4′-bipy.In addition to the above,the wide adsorption peak at about 3 406 cm-1is characteristic ofν(O-H)group in H2O.
2.2 Crystal structural
X-ray diffraction study demonstrates that the complex crystallizes in the monoclinic system with space group P21/c.The coordination environment around the Zn centers is shown in Fig.1.The Zn(Ⅱ)centers lie in a slightly disordered octahedral environment with the axial positions occupied by one water hydrogen atom and one carboxylate oxygen atom from one PCPA ligand,and the equatorial positions occupied by two carboxylate oxygen atoms of two different PBCA ligands and two nitrogen atoms from two 4,4′-bipy ligands.Here,the sum of the angles subtended at the Zn(Ⅱ)atom in the equatorial plane is 359.96°(close to 360°),so that the atoms N(1),N(2)D,O(1),O(4)and Zn(1)are almost in the same plane.The equatorial equation is 0.074 4x+8.431 2y-3.262 2z=1.559 0.The deviation of the atoms N(1),N(2)D,O(1),O(4)and Zn(1)from equatorial trigonal plane is 0.003 31,0.001 52,0.003 35,0.000 29 and-0.005 44 nm,respectively.The average deviation is-0.000 001 nm.Futhermore,the angles O(1W)-Zn(1)-O(2)Cin the axial place is 179.76(6)°which is almost close to the idea value of 180°.So conclusion could be drawn that the central Zn(Ⅱ)atom adopts a slightly distorted octahedral environment.The Zn-O bond lengths are ranging from 0.208 62(16)to 0.212 34(15)nm,which are slightly longer than the reported related Zn complex[20].The C-O bond length of carboxyl group in the two PCPA ligands are obvious different,(C(1)-O(1)0.125 2(3)nm and C(1)-O(2)0.124 0(3)nm)nearly identical while(C(9)-O(4)(0.125 3(3)nm)and C(9)-O(5)(0.122 9(4)nm)obvious different with the value ofΔ=0.0024 nm indicating that the PCPA liagands adopting monodentate and bidentate two coordination modes[18-19].
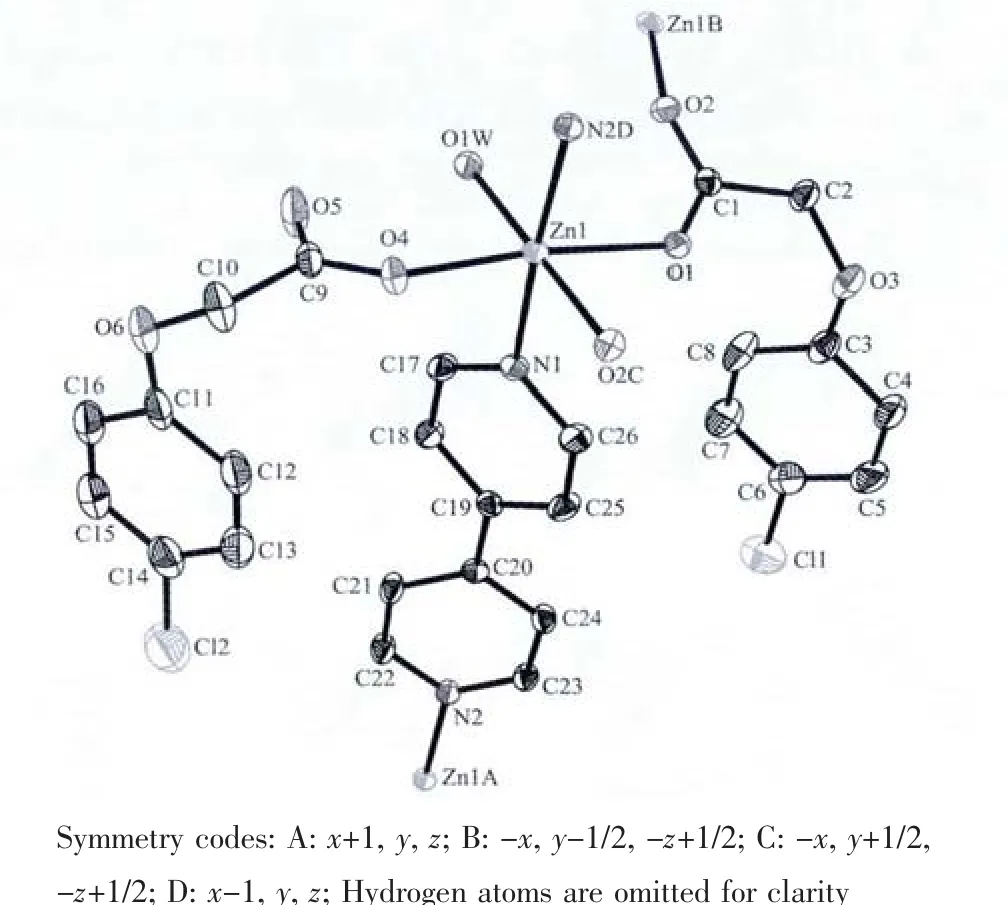
Fig.1 ORTEPview of the coordination environment of Zn(Ⅱ)of the title complex showing 30%thermal probability ellipsoids

Fig.2 (a)2D layer structure of the title complex;(b)Ladder-like motif constructed by adjacent Zn atoms
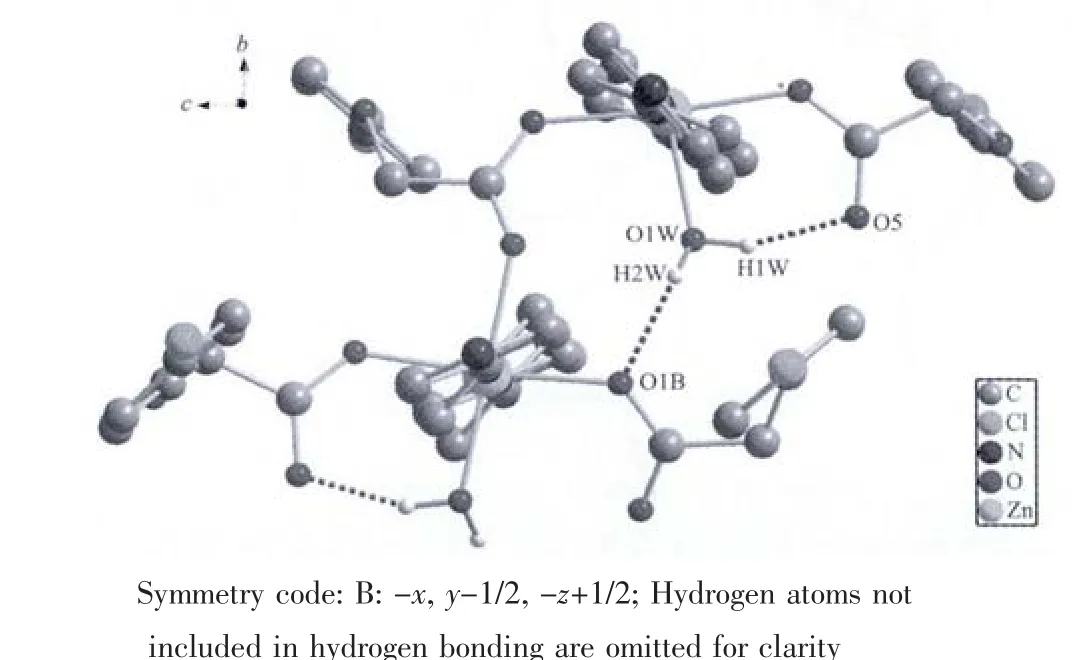
Fig.3 O-H…O hydrogen bonds in the 2D layer structure of the title complex
As shown in Fig.2a,the bidentate carboxylate groups of PCPA anions and 4,4′-bipy ligands bridge the Zn(Ⅱ)atoms to generate a two-dimensional(2D)layer structure.It is worth noting that a square grid coordination polymer is constructed by adjacent Zn atoms with dimensions of 1.135 13(5)nm×0.515 47(10)nm and the adjacent square grids are represented and formed ladder-like motif (Fig.2b).In the 2D layer structure,the intermolecular hydrogen bonds O(1W)-H(2W)…O(1)B(0.279 1(2)nm,155.0°,symmetry code:-x,y-1/2,-z+1/2)and intramolecular hydrogen bonds O(1W)-H(1W)…O(5)(0.258 2(2)nm,155.4°further stabilize the crystal structure(Fig.3).
2.3 Thermal analysis and powder X-ray diffraction
The thermal analysis curve for the title complex is shown in Fig.4.The first weight loss of 3.08%(calcd.2.95%)between 86 and 112℃corresponds to the release of coordinated water molecules in the complex.The further decomposition occurred in the range of 223~431 ℃,which may be attributed to the elimination of 4,4′-bipy and PCPA.The remaining productsmay be ZnO(Obsd.13.02%,Calcd.13.33%).
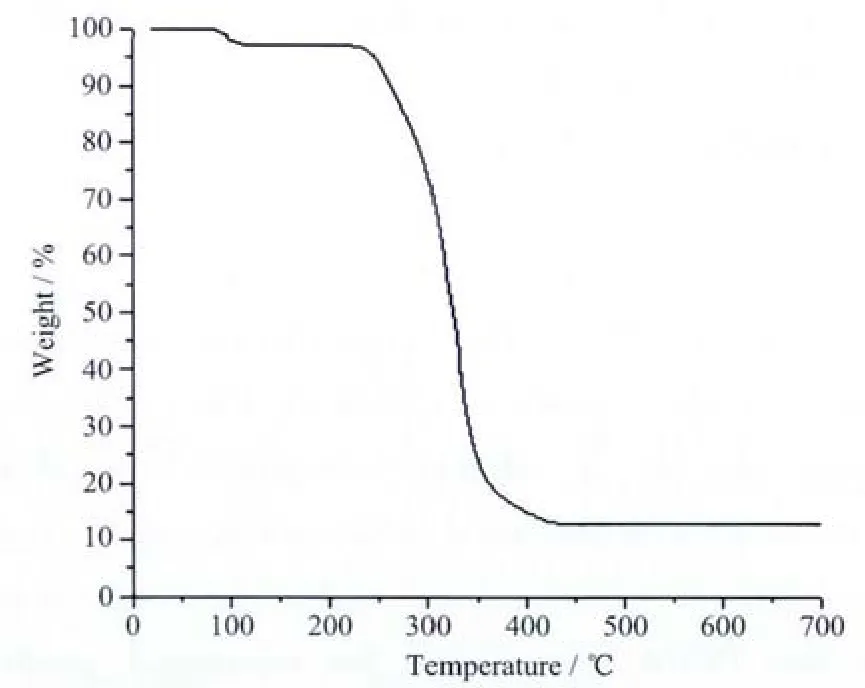
Fig.4 Thermal analysis curve of the title complex
In order to check the phase purity of the title complex,the powder X-ray diffraction(XRPD)pattern was recorded at room temperature.As shown in Fig.5,the peak positions of simulated and experimental pattern is in good agreement with each other,demonstrating the phase purity of the product.

Fig.5 Experimental and Simulated powder X-ray diffraction patterns of the title complex
2.4 UV spectra
The UV spectra of the title complex and the free ligands PCPA and 4,4′-bipy have been studied in CH3CN solvent at room temperature.As illustrated in Fig.6,the strong absorption peaks at approximate 214,239 nm for ligand PCPA,214,227 nm for ligand 4,4′-bipy and 236 nm for the title complex,respectively.Comparing the complex and the free ligands,we can see that the absorption spectra peak shape of the title complex is essentially the same as the PCPA,but the location of the peak exhibits a certain blueshift about 3 nm.So the absorption is mainly due to the n-π*transition of the ligand PCPA.
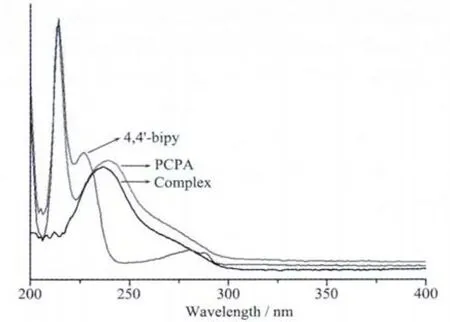
Fig.6 UV spectra of the title complex and ligands PCPA and 4,4′-bipy
Reference:
[1]Maspoch D,Ruiz-Molina D,Veciana J.Chem.Soc.Rev.,2007,36:770-818
[2]Nouar F,Eubank JF,Bousquet T,et al.J.Am.Chem.Soc.,2008,130:1833-1835
[3]Chun H P,Jung H J.Inorg.Chem.,2009,48:417-419
[4]Ono K,Yoshizawa M,Akita M,et al.J.Am.Chem.Soc.,2009,131:2782-2783
[5]XU Gui-Ji(徐贵基),PAN Zhao-Rui(潘兆瑞),ZHENG He-Gen(郑和根),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(9):1551-1556
[6]SHI Zhi-Qiang(石智强),JI Ning-Ning(季宁宁),ZHAO Xue(赵雪),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(2):251-256
[7]JI Ning-Ning(季宁宁),SHI Zhi-Qiang(石智强),ZHAO Ren-Gao(赵仁高).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(6):1025-1030
[8]LIU Tong-Fei(刘同飞),ZNI Guang-Hua(崔广华),JIAO Zni-Huan(焦翠欢),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(7):1417-1422
[9]SHI Zhi-Qiang(石智强),JI Ning-Ning(季宁宁),HE Guo-Fang(何国芳),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(8):1507-1512
[11]LIU Jia-Lu(刘家禄),ZHAO Guo-Liang(赵国良).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(8):2021-2026
[12]Shi Z Q,Ji N Ning,Zhao R G,et al.Struct.Chem.,2011,22:225-233
[13]Zhang SS,Niu S Y,Wang L,et al.Asian J.Chem.,2006,18:1885-1887
[14]Miyazawa M,Irie Y,Kashimoto K,et al.Inorg.Chem.Commun.,2009,12:336-339
[15]YANG Ying-Qun(杨颖群),LI Chang-Hong(李昶红),LI Wei(李薇),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(10):1890-1894
[16]Sun Y X,Wang Z,Zhang H H,et al.Inorg.Chim.Acta,2007,360:2565-2572
[17]Sheldrick G M.SHELXL 97,Program for the Solution of Crystal Structure,University of Göttingen,Germany,1997.
[18]Sheldrick G M.SHELXL 97,Program for the Refinement of Crystal Structure,University of Göttingen,Germany,1997.
[19]Nakamota K,Translated by HUANGDe-Ru(黄德如),WANG Ren-Qing(汪仁庆).Infrand and Raman Spectra of Inorganic and Coordination Compounds,3rd Ed.(无机和配位化合物的红外和拉曼光谱.3版).Beijing:Chemical Industry Press,1986.
[20]Wang Z,Zhang H H,Chen Y P,et al.J.Solid.State.Chem,2006,179:1536-1544