5-异烟酰胺基异酞酸构筑的钕化合物的合成、晶体结构和荧光性质
2013-08-20陈满生许金生邓奕芳张春华邝代治
陈满生 许金生 邓奕芳 张春华 邝代治 聂 雪
(功能金属有机材料湖南省普通高等学校重点实验室,衡阳师范学院化学与材料科学系,衡阳 421008)
0 Introduction
Coordination polymers using carboxylic acids as ligands have been widely studied in the past decades[1-3]because of carboxylates′ diverse coordination styles as well as the carboxylic acids based coordination polymers′ rigid structures. In recent years, much attention has been drawn on the construction of lanthanide coordination polymers due to their unique optical, magnetic and catalytic applications[4-8]. As we known, lanthanide ions tend to coordinate with Odonor ligands in high and flexible coordination numbers (CNs), which can usually vary from eight to twelve. However, many factors can influence the synthesis of lanthanide coordination polymers, such as the character of ligand, the coordination geometry of lanthanide ions, and the reaction conditions.
Of all these factors, the choice of ligands is mostly important for constructing the structures[9-10].According to the above considerations,we have recently designed and synthesized a novel ligand: 5-(isonicotinamido)isophthalic acid namely, (H2INAIP)[11-15]. In order to further investigate the influence of organic ligands on the coordiantion architectures and related properties, reactions of with 5-(isonicotinamido)isophthalic acid as well as lanthanide (Nd) were carried out. Herein, we report the syntheses, crystal structures and properties of the coordination polymers, namely[Nd(HINAIP)(INAIP)(H2O)2]n·nH2O (1).
1 Experimental
1.1 Materials and instruments
The regents were used as commercial sources without further purification. Elemental analyses were performed on a Perkin-Elmer 240C elemental analyzer.The IR spectra were recorded on Bruker Vector22 FTIR spectrophotometer using KBr discs. Thermogravimetric analyses (TGA) were performed on a TGA V5.1A Dupont 2100 instrument heating from room temperature to 700 ℃under N2with a heating rate of 20 ℃·min-1. Luminescence spectra and lifetime were recorded at 77 K on an Edinburgh FLS920 phosphorimeter. UV-Vis spectra were recorded at room temperature on a Shimadzu UV-160A spectrophotometer in barium sulfate based paint.
1.2 Synthesis of the complex 1
The compound was hydrothermal synthesized under autogenous pressure. A mixture of Nd(NO3)3·6H2O (0.221 g, 0.5 mmol), 5-(isonicotinamido)isophthalic (0.287 g, 1.0 mmol), NaOH (0.021 g, 0.5 mmol)and H2O/DMF (9 mL, 2∶1) was heated in a 16 mL capacity Teflon-lined reaction vessel at 140 ℃for 3 d, the reaction mixture was cooled to room temperature over a period of 24 h. The product was collected by filtration, washed with H2O and air-dried,Yields 34% . Molecular formula is C28H23NdN4O13.Elemental Analysis, Calcd.(%): C, 43.76; H, 2.99; N,7.29. Found (%): C, 43.79; H, 2.95; N, 7.32. Main IR bands (cm-1): 3 393 (s), 1 695 (s), 1 606 (m), 1 556(m), 1 528 (m), 1 499 (w), 1 425 (m), 1 383 (s), 1 324(m), 1 068 (m), 790 (m), 674 (w), 594 (w).
1.3 X-ray crystallography
The X-ray diffraction measurement for 1 were performed on the Bruker Smart Apex ⅡCCD diffractometer with graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)at 293(2)K.The data were integrated by using the SAINT program[16], which also did the intensity corrections for Lorentz and polarization effect. An empirical absorption correction was applied using the SADABS program[17]. The structures were solved by direct methods using the program SHELXS-97 and all the non-hydrogen atoms were refined anisotropically on F2by the full-matrix least-squares technique using the SHELXL-97 crystallographic software package[18-19]. Crystal data and structure refinement parameters are listed in Table 1. The selected bond lengths and bond angles are given in Table 2.
CCDC: 958122.
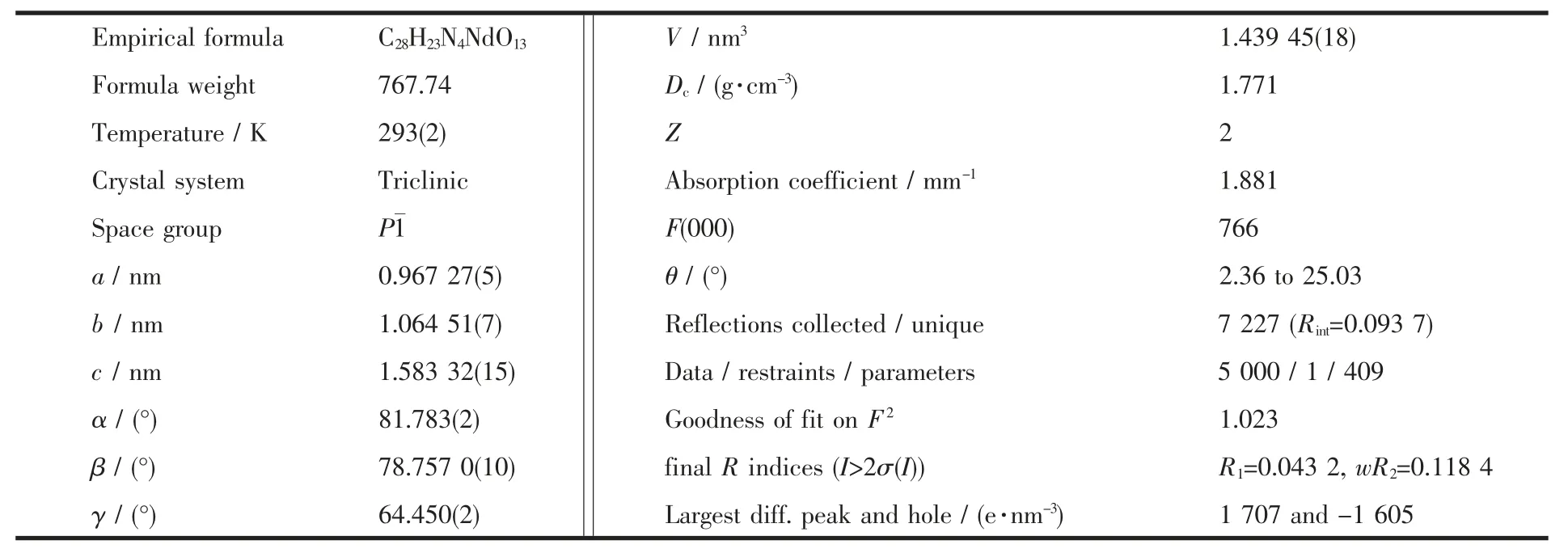
Table 1 Crystal data and structure parameters for complex 1
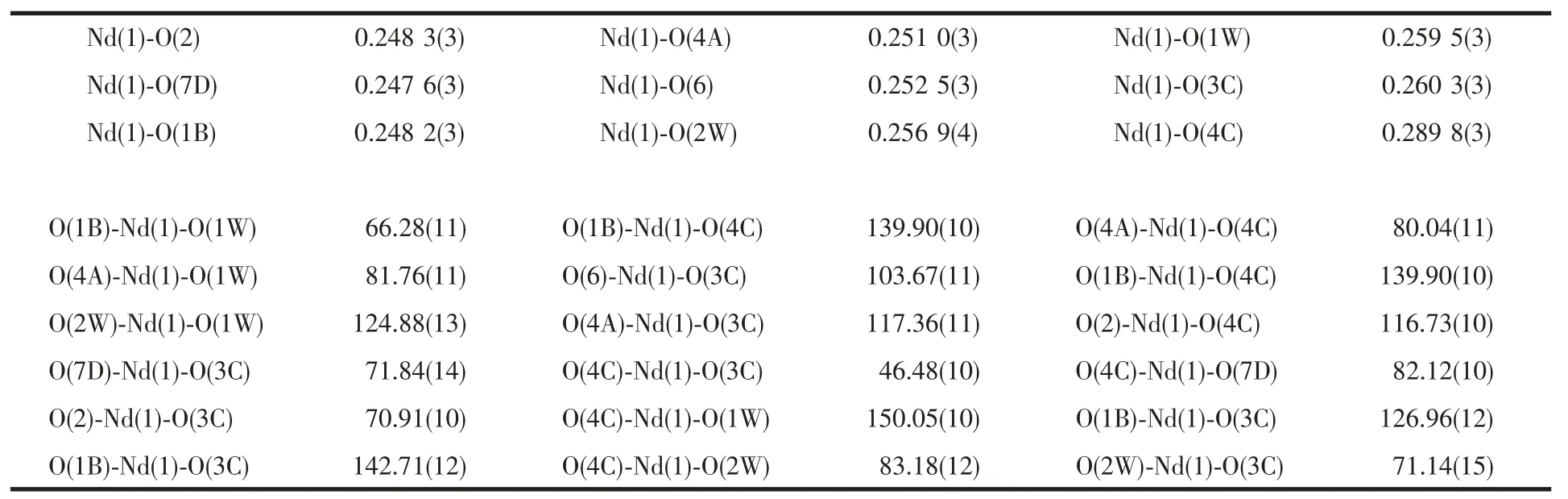
Table 2 Selected bond lengths (nm) and bond angle (°)
2 Results and discussion
2.1 Structure description
The results of structural analysis revealed that complex 1 has a 2D layer structure. It is noticed that in contrast to the complete deprotonation of carboxylate groups of H2INAIP to give INAIP2-, the ligand H2INAIP is only partial deprotonated in 1 to HINAIP-as confirmed by crystallographic and IR spectral data (a strong IR band from -COOH appeared at 1 695 cm-1,see experimental section).The asymmetric unit of 1 contains one Nd(Ⅲ)atom, one INAIP2-ligand,one HINAIP-ligand, two coordinated and one noncoordinated water molecules. As shownin Fig.1, the Nd(Ⅲ)ion is nine-coordinated and surrounded by five oxygen atoms from four INAIP2-ligands, two oxygen atoms from two HINAIP-ligands, and two terminal oxygen atoms from two aqua ligands to form a distorted tricapped trigonal prism. The coordination environment of Nd (Ⅲ)in complex 1 is different from that of {[Eu(L)(HL)]·2H2O}n[20], in which the Eu3+ions have eight coordination with distorted bicapped trigonal prismatic geometry. In 1, the Nd-O (carboxylate) bond distances range from 0.248 2(3) to 0.289 8(3) nm, and those of Nd-O(aqua)bonds are 0.256 9(4)and 0.259 5(3)nm. In complex 1, the HINAIP-anions adopt μ2-η1∶η1bridging coordination mode, while two carboxylate groups of each INAIP2-ligand have different coordination modes, one is μ2-η1∶η1bridging and the other one acts as μ2-η2∶η1-bridging coordination mode. Such connectivity repeats infinitely to give the 2D layer network as depicted in Fig.2.
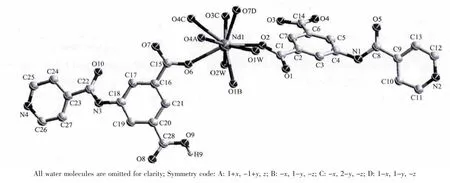
Fig.1 Molecular structure of complex 1
On the other hand, the uncoordinated isonicotinamido groups nitrogen atoms and carboxylate oxygen atoms provide the hydrogen-bonding donor and acceptor, respectively, resulting in the complex being extended into a 3D architecture through supramolecular interactions (Fig.2). The detailed data of hydrogen bonds for complex 1 are shown in Table 3.
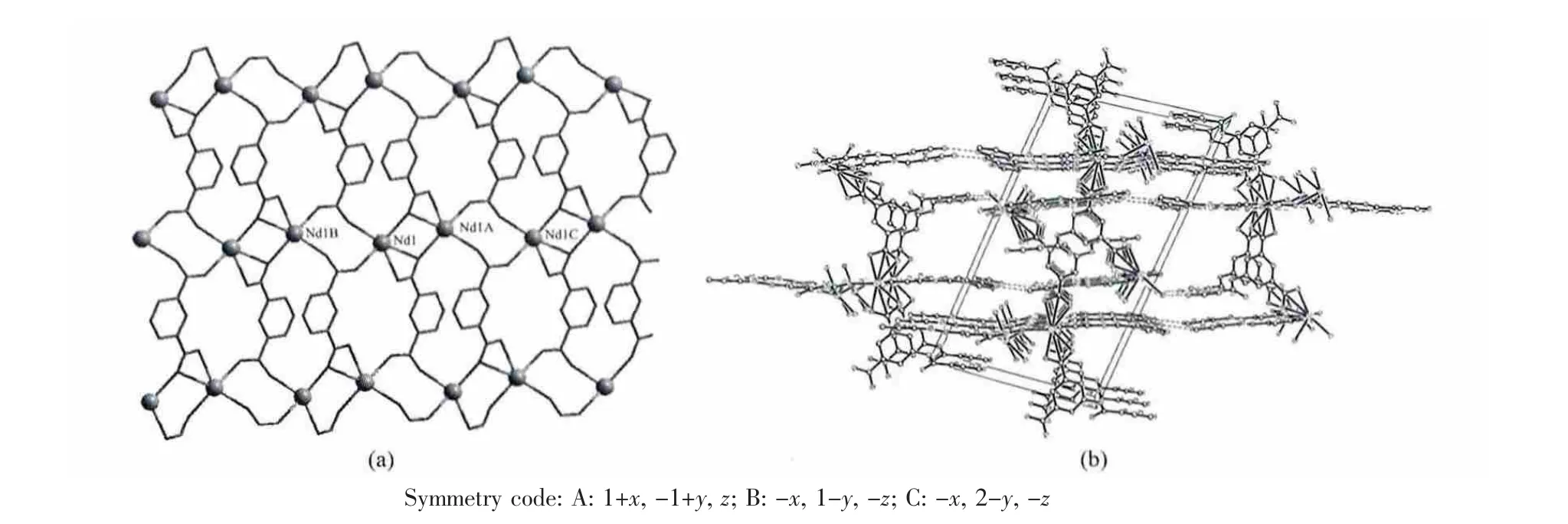
Fig.2 (a) 2D-Nd layer structure linked by INAIP2-and (b) 3D packing structure of 1
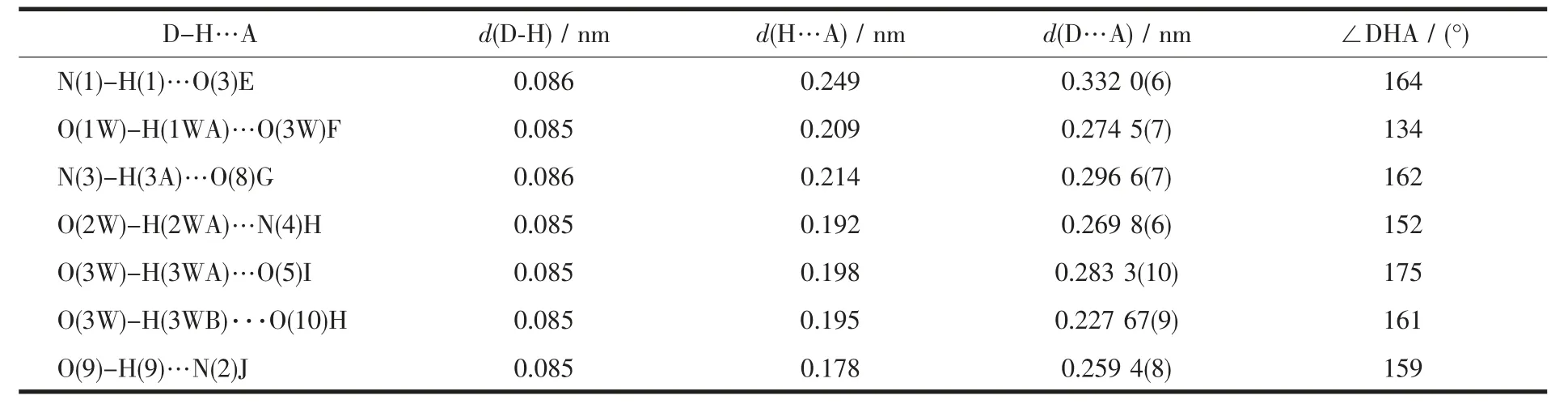
Table 3 Parameters of hydrogen bonds for the complex 1
2.2 IR and thermal gravimetric analyses
The infrared spectrum of the complex has been recorded and some important assignments are shown above. No strong IR band from -COOH appeared at nearly 1 700 cm-1(1 695 cm-1), indicating that the coordinated carboxylate groups are not all deprotonated,and the band at 3 415 cm-1, due to the ν(O-H) absorptions of water molecules. The characteristic bands at 1 606 and 1 556 cm-1should be assigned to the asymmetric and symmetric vibration of carboxylate groups of the ligand, respectively. These IR results are coincident with the crystallographic structural analyses. On the other hand, thermal gravimetric analyses (TGA) were performed to verify the thermal stability of the complexes. Complex 1 lost a weight of 7.11% was observed in the temperature range of 25~165 ℃, which corresponds to the release of two coordinated water molecules and one free water molecule(Calcd.7.03%),and then the residue decomposed upon further heating at about 305 ℃. Therefore, the results indicate that the complex has high stability (Fig.3).
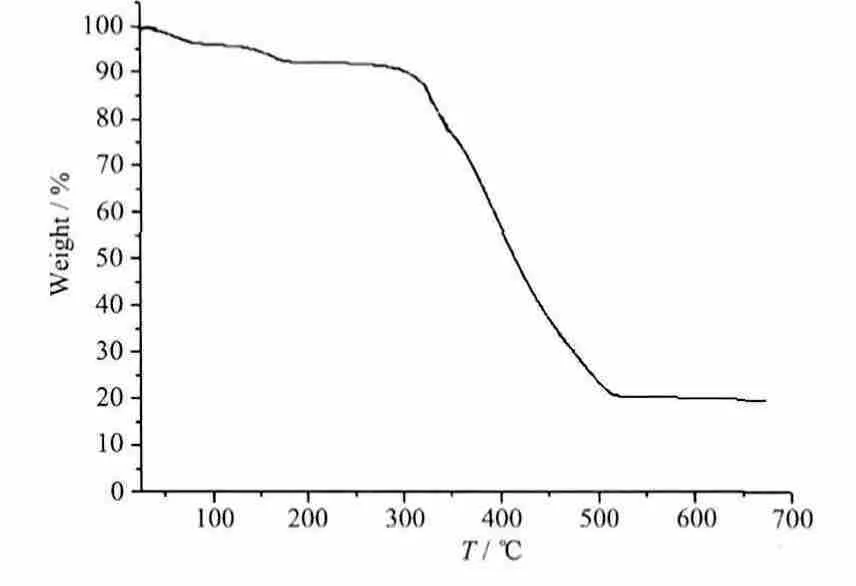
Fig.3 TGA curve of complex 1
2.3 Luminescent property and UV-Vis spectra
The fluorescence spectrum with solid sample for the complex was determined in near-infrared (NIR)region with the excitation of UV-rays at 77 K, the emission spectra was shown in Fig.4. The emission bands of 1 at around 890 nm are tentatively assigned to be4F3/2→4I9/2, at around 1 062 nm to be4F3/2→4I11/2and at 1 330 nm to be4F3/2→4I13/2transitions (Fig.4).Some crystal-field fine structures can be observed,which indicated that the Nd3+ions occupied the welldefined crystallographic sites in the compound.Among the three emission bands of the Nd complex,the transition band at 1 062 nm has the strongest emission intensity, which is potentially applicable for laser emission. On the other hand, the UV-Vis absorption spectra of complex 1 were investigated in the solid state. As shown in Fig.5, it could be found that the B band of INAIP2-ligand which is attributed to the π-π* transition in 235 nm. The most intense absorption at 265 nm could be assigned to the K band between the INAIP2-ligands and the central Nd3+ions[21].
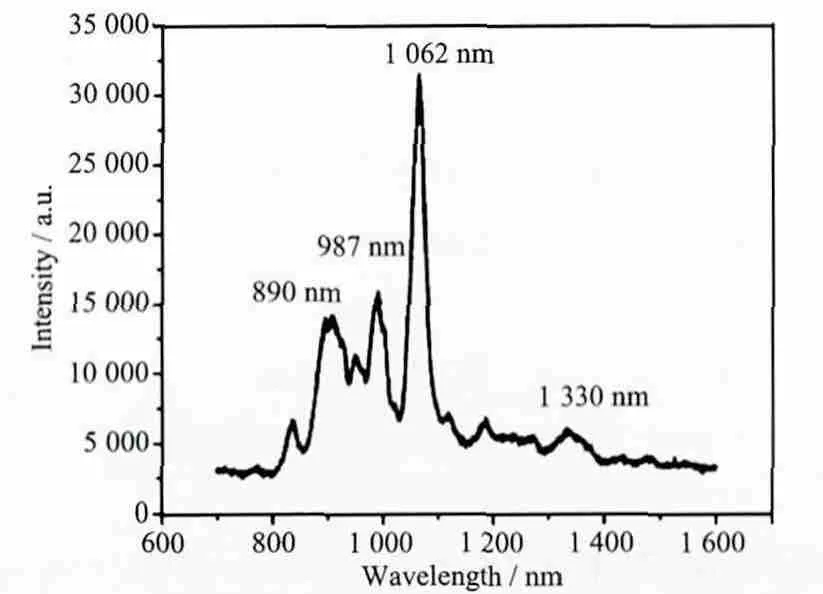
Fig.4 Emission spectum of the complex with the excitation at 315 nm at 77 K
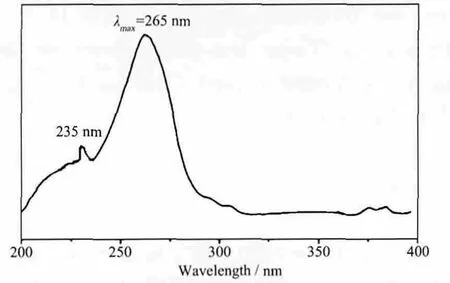
Fig.5 UV-Vis absorption spectroscopy of 1 in solid state
[1] James S L. Chem. Soc. Rev., 2003,32:276-288
[2] Kitagawa S,Kitaura R,Noro S. Angew.Chem. Int. Ed., 2004,43:2334-2375
[3] Zhang J P, Lin Y Y, Huang X C, et al. Chem. Commun.,2005:1258-1260
[4] Moulton B,Zaworotko M J.Chem.Rev.,2001,101:1629-1658
[5] Carlucci L, Ciani G, Proserpio D M. Coord. Chem. Rev.,2003,246:247-289
[6] Chen X Y, Zhao B, Shi W, et al. Chem. Mater., 2005,17:2866-2874
[7] Férey G. Chem. Soc. Rev., 2008,37:191-214
[8] Zhang X M, Hao Z M, Zhang W X, et al. Angew. Chem. Int.Ed., 2007,46:3456-3459
[9] Wan Y, Zhang L, Jin L, et al. Inorg. Chem., 2003,42:4985-4994
[10]Eddaoudi M, Kim J, Wachter J B, et al. J. Am. Chem. Soc.,2001,123:4368-4369
[11]Chen M S, Deng Y F, Zhang C H, et al. Inorg. Chem.Commun., 2011,14:944-947
[12]Chen M S, Chen M, Okamura T, et al. Microporous and Mesoporous Materials, 2011,139:25-30
[13]Chen M S, Chen M, Takamizawa S, et al. Chem. Commun.,2011,47:3787-3789
[14]Chen M S, Fan J, Okamura T, et al. Inorg. Chim. Acta,2011,366:268-274
[15]Chen M S, Bai Z S, Su Z, et al. Inorg. Chem. Commun.,2009,12:530-533
[16]SAINT,Cersion 6.02a;Bruker AXS Inc.:Madison,W1,2002.
[17]Sheldrick G M. SADABS, Program for Bruker Area Detector Absorption Correction; University of Göttingen, Göttingen,Germany, 1997.
[18]Sheldrick G M. SHELXS-97, Program for Crystal Structure Solution; University of Göttingen, Göttingen, Germany,1997.
[19]Sheldrick G M. SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen, Göttingen, Germany,1997.
[20]DENG Yi-Fang(邓奕芳), CHEN Man-Sheng(陈满生),ZHANG Chun-Hua(张 春 华), et al. Chinese J. Inorg. Chem.(Wuji Huaxue Xuebao), 2011,27:1654-1658
[21]JIANG Li-Hui(蒋 历 辉), MA De-Yun(马 德 运). Chinese J.Inorg. Chem.(Wuji Huaxue Xuebao), 2013,29:883-888
