Review on microorganisms of glacial snow and ice on the Tibetan Plateau
2013-07-03ZHANGShuhongHOUShuguiQINXiangYANPeiyingZHAOLongfeiLIANGFeng
ZHANG Shuhong,HOU Shugui,QIN Xiang,YAN Peiying,ZHAO Longfei,LIANG Feng
(1.Department of Life Science,Shangqiu Normal University,Shangqiu 476000,China;2.School of Geographic and Oceanographic Sciences,Nanjing University,Nanjing 210093,China;3.Cold and Arid Regions Environmental and Engineering Research Institute,Chinese Academy of Sciences,Lanzhou 730000,China;4.Biorefinery Engineering Lab of Henan Province,Shangqiu 476000,China)
0 Introduction
Glaciers are important to sustaining life on earth in that their movements carve out landscapes and deposit sediments supporting plant life,while annual melt waters feed perennial rivers sustaining riparian animals and mountain folk living downstream.Microorganisms are capable of rising into the air with airstream,then deposit onto the surface of glacial snow and ice with precipitation,and gradually deposited onto the ice cores[1].Microorganisms are deposited in accordance with the time series.Therefore ice cores record the changed characteristics of the number and structure of the microbial flora transported by atmosphere during different deposition time.The analysis of various microorganisms from different periods as well as their relation with temperature and environment will help to explore the information of collaborative change between life on Earth and cold environments.Therefore,the study of microorganisms of glacial snow and ice can not only help to discover new gene resources,enrich the research about microbial diversity,biological gene evolution and even the origin of life,but also provide new avenues for the research on relationship of microbial diversity with climatic and environmental changes.The Tibetan Plateau —the planet’s largest store of ice after the Arctic and Antarctic—is located in the low-latitude,high altitude (an average of 4,150 km)region.Because of the regional difference of microorganism from glacial snow and ice,the research of microorganisms from glacial snow and ice in this region is very significant.However,The Tibetan Plateau is warming about three times the global average,with temperature increases of 0.3℃or more per decade measured for the past half-century[2].This paper focused on the advances of microorganism from glacial snow and ice on the Tibetan Plateau,which review some critical issues related to viable microorganisms in cold terrestrial environments with regard to future searches for microbial life and/or its biological signatures on extraterrestrial objects.
1 Phenotypic characteristics of recovered isolates
One of the features of recovered bacteria from Puruogangri ice core[3]was pigmented colony dominance,which was the physiological mechanism of adaptation to extremely low temperature and of resistance to the environmental stress.However,the research of Zhang et al.[4]indicated that a significant population of large and non-pigmented cells existed in ER ice core of Mt.Everest.The different results were probably due to complex survival strategies in the extreme environments and different strategies.Although both Zhang et al.[4]and Shen et al.[5]used culture method in the research of ER ice core,Zhang et al.[4]flitrated ice core samples onto membranes,then spread onto both R2A and PYGV media.Shen et al.[5]used R2A medium,direct plating and enrichment methods.Different results were also probably due to few samples in each study,not reflecting the whole microbial level in the ice core.
2 Factors influencing microbial abundance and diversity in one glacier
2.1 Dust and abundance
In Malan ice core,the number of total microorganisms and culturable microorganisms in different layers showed that it related with the content of dust in ice.It is suggested that the distribution of microorganisms in ice depends on the transportation of materials during glacier development[6-7].The close relationship was also reported in Muztagh Ata[8]and Geladaindong[9]ice core.Dust particles protected microorganisms from desiccation and UV radiation,thus influenced the amount as well as the physiological state of the microbes in ice[10-11].
Although the above researches showed the close relation between bacterial concentrations and insoluble particles,the authors did not perform a correlation between them.The analysis from their reported data of Malan ice core[7]showed a weak negative correlation between bacterial concentrations and insoluble particles (r=-0.022,p=0.967,n=6)[12].From the reported data about Puruogangri ice core[7],a weak negative correlation (r=-0.031,p=0.896,n=20)was also found[12]between bacterial concentration and Ca2+concentration (a good proxy of dust in ice cores from the Tibetan Plateau[13-14]).A weak negative correlation (r=-0.135,p=0.351)between bacterial and Ca2+concentrations was also reported in the ER ice core[12].It is speculated that there might be other factors,together with micro-particles,which determine bacterial concentrations in glacial ice.
2.2 Dust and diversity
In Puruogangri ice core,bacterial diversity,as estimated by Shannon indices (mean 2.91;SD 0.25;n=13)from denaturing gradient gel electrophoresis (DGGE)band pattern,had a positive correlation with Ca2+concentration (R=0.71;p<0.01),a good proxy of dust[15].The same result was got from ER ice core[12].The results indicated that dust concentrations might be important in determining bacterial diversity and bacterial diversity might be another suitable biological proxy for the reconstruction of past climatic and environmental changes preserved in glacial ice.
2.3 Temperature and abundance
Temperature represented by the variation δ18O value generally rose while the total concentrations of bacteria declined in Malan ice core[7].To the contrary,in Geladaindong ice core,bacterial abundance correlates positively with temperature[9].The temperature in Malan Glacier of continental type was lower than that in Geladaindong Glacier of continental-maritime type.Thus Geladaindong Glacier would provide better environment than Malan Glacier.Accordingly,better relationship between bacterial abundance and temperature could be found in maritime glacier[9].The positive relationship between temperature and microbial abundance was also reported in Yala Glacier,in which alga was studied in stead of bacteria[16].Price and Sowers[17]reported that the temperature dependence of metabolic rates of microbial communities in deep glacier ice was for survival of imprisoned communities,in which bacteria can repair macromolecular damage but are probably largely dormant.Segawa et al.[18]discovered that some bacteria in the surface snow of glaciers can grow during the warm season.
2.4 Altitudinal distribution of algal biomass and community
Algal biomass decreases with increasing altitude on Rikha-Samba Glacier in the western Nepali Himalayas[19].The same result was also from Yala and AX010 glaciers[20-21].In particular,the rate of decrease of algal biomass was larger in the upper snow environment than in the lower ice environment[20].Moreover,algal biomass at the same altitude was in a similar level at Yala and AX010 glaciers[21].Yoshimura et al.[20]analyzed the relationship between algal biomass and environmental conditions on Yala Glacier and suggested that the algal biomass was mainly limited by the amount of snow cover at each altitude during summer,because light intensity available for the algal growth mainly depends in thickness and frequency of snow cover on algal habitat.
Algal community also showed an altitudinal distribution on three glaciers of Rikha-Samba,Yala and AX010.In Yala glacier,Yoshimura et al.[20]Divided the glacier into 3 zones from the difference of algal community:the Lower Zone (5100-5200 m a.s.l.;stable ice-environment),with 7 species dominated by Cylindrocystis brbissonii;the Middle Zone (5200-5300 m a.s.l.;unstable transition area between ice-environment and snow-environment),with 11 species dominated by Mesotaenium berggrenii;and the Upper Zone (5300-5430 m a.s.l.;stable snow-environment),with 4 species dominated by Trochiscia sp..This vertical zonation of the algal community type may reflect the difference in summer climatic conditions with altitude.Species number and Simpson's diversity index was highest in the Middle Zone where environmental conditions frequently changed.The results seemed to agree with the intermediate disturbance hypothesis[20].In another Himalayas glacier of AX010,there are 2 types of snow algal community,corresponding to that of mass balance during the study period.Type 1 was the community lower than 5200 m a.s.l.that consists of 5 algal species dominated by C.brébissonii.Type 2 was the community at the highest sampling point that consists of 4 algal species dominated by Oscillatoriacean alga.Type 1 community was observed in the minus mass balance area (ablation zone)and Type 2 community was observed in the plus mass balance area (accumulation zone).The result suggests that the snow algal community was affected by the mass balance at that site[21].In Rikha-Samba Glacier,the algal community in 1996 and 1966 was Type 3,which included an alga of Oscillatriaceae cyanobacterium.This alga was an ice-environment specialist,observed in the middle to lower part of the glacial surface.Thus,the surface conditions of the drilling site in 1996 and 1986 might be similar to the ice surface in the middle to lower part of the glacier in 1998.The variation in algal community in the ice core suggests that altitudinal distribution of now algal community could shift up or down on the glacial surface from year to year[19].
3 Distribution of bacterial number,diversity and community along ice core depth
3.1 Bacterial number along ice core depth
The earliest report about microbial distribution along ice core depth was from Zhang et al.[22].He analyzed 23 samples at different layers in Malan ice core.The result showed that the number of total bacteria and the number of viable bacteria varied with a similar trend along the vertical distribution[22].The same result was got from Puruogangri ice core.No relationship was perceived between the total cell counts and increasing depth,which probably implied that bacteria were deposited into glacier episodically[3].Different result was from Zhang et al.[12].In that report,there was no consistent,monotonous decrease with increasing age of the ER ice core,but with a high fluctuation during 1712 –1911 AD,a period consistent with industrial revolution when boom of the human population and activities caused flourishing microbe in the wide environments of soil,air,marine,etc.Another different result was also from ER ice core.The abundances of cultivable bacteria and pigment-producing isolates varied synchronously along depth:higher abundance in the middle and lower at the top and bottom.It indicated that the middle part of the ice core was hospitable for the microbial survival[5].
3.2 Bacterial diversity along ice core depth
Culturable bacterial diversity was the highest in the middle part of the ER ice core.Two and three genera were obtained at the top and bottom part of the ice core,but five genera were recovered at the middle (10-15 m).The possible reason for such changing regularity may be that the climate in the surface layer was very severe;the microorganisms were in the state of deep physiological dormancy and could not be easily recovered.The middle part of the ice core was relatively hospitable,and the sub-sections in this depth were not so old;Furthermore,microorganisms in this part have enough available organic matter.So,the microorganisms in this layer were more active and more species could be recovered[5].However,the Shannon-Weaver index (H’)from DGGE banding pattern was not related to the sample depth in the Puruogangri ice core.Moreover,periodic fluctuation of H’was observed.This probably reflected a cycle of climatic changes.With DGGE and Shannon index analysis,differences between the samples along the length were higher,as indicated by the low similarity indices (0 – 43%)[15].However,different result was from ER ice core[12].Dendrograms generated by comparisons of the banding patterns from the samples indicated low discrepancy between samples along the ice core depth,as shown by the high similarity indices (53.83% – 99.15%).Clustering analysis demonstrated the percentage similarity between bacterial assemblages in the Puruogangri ice core.The closer sections of ice core showed higher resemblance in bacterial DNA structure[15].In ER ice core,similar result was also obtained.Patterns obtained from closer ice core depth generally shared more bands than patterns from more distant depth[12].The above two results may be attributed to the fact that the variation of climate and corresponding environment was a gradual process[15].
3.3 Bacterial species and community along ice core depth
Some bacterial population repeatedly occurred at different ice layers.In Puruogangri ice core,Cryobacterium sp.was abundant in ice layers of 6.14 m,31.53 m,71.13 m,and 81.07 m[3].The phylum Cryobacterium also originated from a continuous ice layer 8.30 to 9.09 m in Muztagh Ata[8].The Cryobacterium isolates from the Muztagh Ata ice core grew well at temperatures below 25℃,making them psychrophiles,which was favorable for their survival in the ice core[8].In Malan ice core,Brachybacterium sp.has been repeatedly isolated at different ice core depth such as 0.2 m,12.4 m and 28.0 m,and other deeper layers.Arthrobacter sp.has been detected at 28.0 m,34.8 m,54.3 m and 63.8 m[7].In ER ice core,Bacillus sp.was predominant at both 0-5 m and 6-10 m.Staphylococcus sp.was predominant at both 10-15 m and 15-20 m.And at the depths of 20-23 m,Bacillus sp.and Staphylococcus sp.took the same ratio of 40%[5].The above result was focused on bacterial genus distribution along ice core depth.The following is about the layered distribution of bacterial community.
There was a great difference in the proportion of the main phylogenetic groups along ice core depth.For instance,in Puruogangri ice core,there was only a small increase in Bacteroidetes and a significant decrease in Actinobacteria from P60 sample to the deeper P200 sample[3].In Muztagh Ata ice core,α-proteobacteria and Firmicutes were dominant in the spring ice layer of 1988,γ-proteobacteria,β-proteobacteria,Actinobacteria,and Bacteroidetes phyla were dominant in the ice layer from 1984 autumn to 1985 winter,γ-proteobacteria,βproteobacteria,Actinobacteria,Bacteroidetes,and Firmicutes were common in the 1972 spring-summer ice layer,and γ-proteobacteria,β-proteobacteria,and Actinobacteria dominated in the 1970 winter ice layer.Firmicutes was dominant in the spring seasons of both 1988 and 1972,but rarely occurred in the winter seasons of 1970 and 1985[23].In the ER ice core,there were two warm periods of Little Ice Age (LIA)from 32.11 to 37.89 m (1947–1958 AD)and 59.46 to 75.22 m (1752 –1847 AD),two cold periods of Little Ice Age (LIA)from 43.15 to 55.64 m (1868 –1932 AD)and 83.88 to 106.96 m (1054 –1686 AD)[12].Members of different phylogenetic groups were predominantly isolated in ice from the above 4 different core depths.Firmicutes,Actinobacteria and γ-Proteobacteria were most frequently distributed among the two warm and two cold periods.Whereas α-Proteobacteria,β-Proteobacteria and Deinococcus-Thermus distributed mostly during warm periods with high dust concentration.Moreover,α-Proteobacteria,β-Proteobacteria were recovered only from the shallow part of the ice core (32.47-63.99 m)[4].No similarity was found among the layered community distribution in the three ice cores.
4 Similarity and difference of glacial isolates distributed on the Tibetan Plateau
4.1 Abundance
Christner et al.[11]isolated bacteria entrapped in ice cores from different geographic locations and ice ages in Greenland,China,Peru and Antarctic.Ice samples from nonpolar,low latitude,high altitude glaciers in the Andes and Himalayas generally contained larger number of colony forming units and greater variety of bacterial species than that of polar ice samples,as predicted by their closer proximity to major biological ecosystems.However,the Tibetan Plateau is large.There are different environmental conditions in different regions (Fig.1),which might influence the abundance of glacial microorganisms.Concentration of culturable bacteria in the ER ice core is between 0 –7.0 CFU/ml[12],while that of other ice cores from the northern and central Tibetan Plateau(Guliya 7-180 CFU/ml[11],Malan 5-85 CFU/ml[22];Muztagh Ata 0-127 CFU/ml[8];Puruogangri 0-760 CFU/ml[3])show a much broad range.The above is the result of culturable bacteria of ice cores.The research of the abundance of total bacteria of ice cores is consistent to that of culturable bacteria.For instance,bacterial abundance in the ER ice core is 0.02×103-6.4×103cell/ml[12],while that in Guoqu is 3.2×103-830×103cell/ml[9],that in Puruogangri is 10×103-100×103cell/ml[3],that in Muztagh Ata is 103×103-199×103cell/ml[23].Therefore,from the ice core samples,the abundance of both culturable and total bacteria in the southern Tibetan Plateau is lower than that in the northern Tibetan Plateau (Fig.1).We also analyzed bacterial abundance in snow samples.Bacterial abundance in the southern glacial snow of ER,Zadang and Palong is 12×103cell/ml,540×103cell/ml and 11×103cell/ml,respectively[24].Whereas that of other glaciers from the northern Tibetan Plateau (Guoqu 450×103-87700×103cell/ml[24],Laohugou 80.0×103-139.8×103cell/ml[25])are higher than that of the former glaciers.Therefore,the result of snow samples is consistent to that of ice core samples.
4.2 Diversity
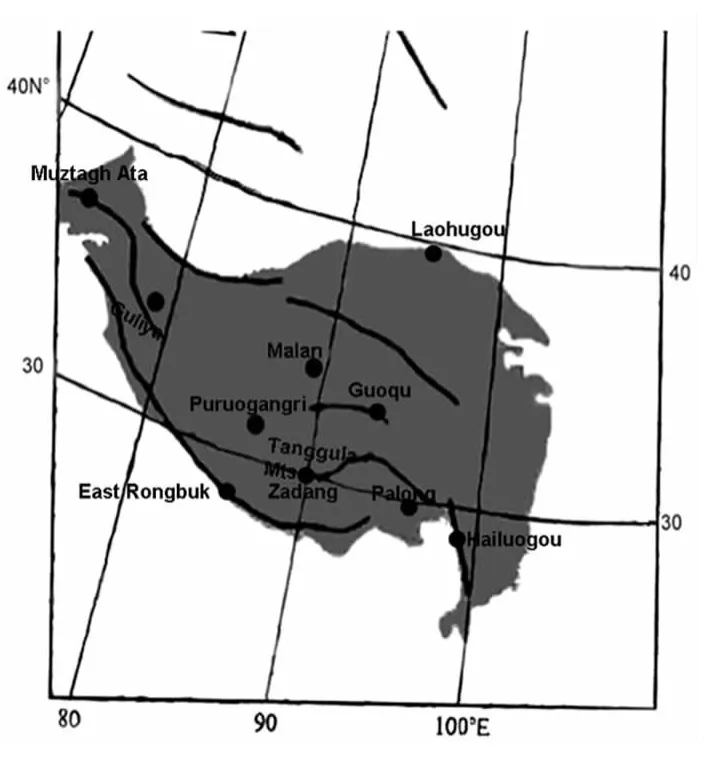
Fig.1 Ice core sites invested on the Tibetan Plateau
The earliest report was from Zhang et al.[22].He compared the flora of microbes in the ice samples of Malan with that of Arctic and Antarctica.Only prokaryotes were found in Malan ice core samples,and most of them were Bacillus sp.No filamentous fungi and alga were found,although they prevail in Greenland and Antarctica ice core[26-27].Under the cold,arid,high radiant (especially UVB radiation),lack of organic nutrition environment,only bacteria can survive and become predominant in Malan ice core[22].Succedently,most researches were focused on glacial bacteria,such as ER[4,24,28];Guoqu[24];Muztagh Ata[8];Malan[29-32];Puruogangri[3];Laohugou[28];Hailuogou[28];Palong[24];Zadang[24].The diversity of bacteria collected from snow pit samples of ER,Laohugou and Hailuogou glaciers on the Tibetan Plateau was investigated by DGGE and H’index.H’index in Hailuogou Glacier was the highest (mean=0.95,SD=0.06,n=3),that in Laohugou Glacier was the second (mean=0.79,SD=0.03,n=3)and that in ER Glacier was the last (mean=0.62,SD=0.25,n=3).The result was due to different concentration and size of microparticle,different altitude,different atmospheric circulations,and different temperatures of the three locations[25].Similar result was from Liu et al.[24].The Shannon H index through culture-independent molecular analysis of 16S rRNA gene clone library was 4.0 in Zadang,3.1 in Palong No.4,2.5 in Guoqu and 2.2 in East Rongbuk.Zadang Glacier at the north of the Tibetan Plateau with lower altitude preserved the most diverse bacteria than ER Glacier at the south of the Tibetan Plateau with higher altitude[24].The two researches indicated a complicated correlation between bacterial and their glacial living environments.
4.3 Community composition
Here we summarized the whole distribution of bacterial community from all regions invested on the Tibetan Plateau.
Among the diverse snow bacteria,the classes of Actinobacteria,Bacteroidetes,Proteobacteria showed widespread distribution among glaciers of the Tibetan Plateau.In the class of Actinobacteria,Brevibacterium occured in three glaciers of ER,Guoqu and Malan.Kocuria existed in three glaciers of ER,Muztagh Ata and Puruogangri.Arthrobacter was found in Guoqu,Muztagh Ata,Malan,and Puruogangri.Rhodococcus was common in Puruogangri,Laohugou and Palong (Table 1).
In the subclass of α-proteobacteria,Sphingomonas occurs in three glaciers of ER,Guoqu and Malan.Brevundimonas existed in four glaciers of Muztagh Ata,Puruogangri,Laohugou and Hailuogou Glacier.In the subclass of β-proteobacteria,Rhodoferax occurs in three glaciers of ER,Puruogangri,and Hailuogou.Polaromonas occured in two glaciers of ER and Guoqu.In the subclass of γ-proteobacteria,Pseudomonas was common in Guoqu,Muztagh Ata,Malan and Zadang glaciers.Actinobacteria existed in ER,Muztagh Ata and Puruogangri.Psychrobacter was found in Puruogangri and Zadang (Table 1).
In the class of Bacteroidetes,Flectobacillus were from ER,Malan,Puruogangri.Flavobacterium distributed widely in glaciers of ER,Muztagh Ata,Malan,Puruogangri,Laohugou,Zadang.Hymenobacter was from ER,Guoqu,Hailuogou,Zadang (Table 1).

Table 1 Regional distribution of bacterial community from glacial snow and ice on the Tibetan Plateau by culture and culture-independent methods
In the class of Firmicutes,Bacillus was from ER,Guoqu,Malan,Puruogangri,Zadang (Table 1).
From Table 1,bacterial diversity in the ER Glacier was the highest.The results were from three reports,one about ice core and two about snow samples.It indicated that more researches gave higher bacterial diversity and more comprehensive investigation.
5 Seasonal variation of bacterial abundance and diversity
5.1 Seasonal variation with different patterns at two glaciers
Snow bacterial abundance and diversity at the Guoqu Glacier and the ER Glacier located in the central and southern Tibetan Plateau were investigated using a 16S rRNA gene clone library and flow cytometry approach.Bacterial abundance was observed to show seasonal variation,with different patterns,at the two glaciers.High bacterial abundance occurs during the monsoon season at the ER Glacier and during the non-monsoon season at the Guoqu Glacier.Seasonal variation in abundance is caused by the snow bacterial growth at the ER Glacier,but by bacterial input from the dust at the Guoqu Glacier[33].
Seasonal variation in bacterial diversity is more distinct at the Guoqu Glacier than at the ER Glacier.Bacteria found in the Guoqu snow samples from the monsoon season belong to 7 phyla and 15 genera,and 2 were unclassified.However,bacteria from the non-monsoon season belong to only 3 phyla and 3 genera,and 2 were unclassified.Snow bacteria at the ER Glacier also exhibit seasonal variations.But the bacterial difference between the two seasons is not as distinct as that in the Guoqu Glacier.Bacterial diversity at the two glaciers exhibits different responses to various environmental conditions.More bacteria at the Guoqu Glacier are connected with those from soil environments,while more bacteria affiliated with marine environments occur at the ER Glacier[33].
5.2 Seasonal variation with different patterns at one glacier with different samples
Four samples from ER ice core,Himalayas,were investigated for concentration of culturable bacteria to understand seasonal variations of culturable bacteria on the Tibetan Plateau.The average concentrations of culturable bacteria are 5.0 CFU mL-1,0.8 CFU mL-1,0.1 CFU mL-1and 0.7 CFU mL-1during the premonsoon (spring),monsoon (summner),postmonsoon (autumn)and winter seasons,respectively.The highest concentration of culturable bacteria was during the premonsoon season,due to the transportation of continental dust stirred up by the frequent dust storms during spring[34].Whereas,different result was from snow samples instead of ice core samples.Bacterial abundance analyzed by flow cytometry was the highest (2.3×104cells mL-1)in the summer snow,and the lowest (6.9×103cells mL-1)in the winter snow.The result was due to large amount of vapor from Indian Ocean transporting more bacteria and in situ growth in summer snow[35].The two reports with different results were probably in the case of different samples and different analyzing approaches.For the former one,the glacier surface usually appears as an apparent transition from snow to firn (the mixed snow–ice environment)to solid-ice/water-flow.Thus,microbial abundance and diversity changes in response to temperature,nutrient content at long time scale.For the latter one,culture-dependent method would identify a significantly smaller subset of the species because of the potential selection of bacteria competent to grow on solid media[36].Whereas flow cytometer was used to analyze microbial communities stained with the nucleic acid specific dye SYBR Green I.Therefore the latter method can analyze the total number of bacteria.
The two reports also showed the difference of bacterial diversity.In the ER ice core,the numbers of culturable bacteria with different ARDRA patterns from RFLP analysis were 10,15,1 and 2 for the glacial ice deposited during the premonsoon,monsoon,postmonsoon and winter seasons,respectively,suggesting that culturable bacteria deposited in ER ice core during monsoon season are more diverse than that deposited during the other seasons[34].However,with 16S rRNA gene clone library,bacterial diversity in ER glacial snow deposited in winter was the highest,and that in the snow layer deposited in summer was the lowest.Nine genera occurred in winter snow,14 genera in summer snow[35].The two results on seasonal bacterial diversity were not entirely different.More efforts with the same techniques are necessary to better understand the mechanisms as possible drivers of microbial population variability between glacial snow and glacial ice.
6 Summary and outlook
With the methods of 16S rRNA and phylogenetic tree,the research about microorganism from glacial snow and ice on the Tibetan Plateau began late,but with high starting point.However,much more researches are still needed.Firstly,some unique measures need to be taken to microorganisms in “viable but non culturable”(VBNC)state.Moreover,the technique of Biolog can provide more information about the function of recovered microorganisms.Secondly researches about archaea and fungi are too little.More studies are still needed.Thirdly,microbial resources from glacier need to be excavated,e.g.radiation resistant bacteria,psychrophilic bacteria,bacteria producing low temperature enzyme.Fourthly,the relation of glacial microorganisms with biogeochemical cycle and mass balance will help us to know the ecological significance of glacial microorganisms during glacial retreat.Fifthly,research on samples from different altitude,different environmental condition of snow,ice and soil may reflect microbial distribution mechanism in different glacial environment.
[1]Wake CP,Dibb J E,Mayewski PA,et al.The chemical composition of aerosols over the eastern Himalayas and Tibetan Plateau during low dust periods[J].Atmos Environ ,1994,28:695 –704.
[2]Institute for Governance & Sustainable Development (IGSD)."Retreat of Tibetan Plateau Glaciers Caused by Global Warming Threatens Water Supply and Food Security." Retrieved October 11,2011 from the Institute for Governance & Sustainable Development[EB/OL].http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0CBwQFjAA&url=http%3A%2F%2Fwww.igsd.org%2Fdocuments%2FTibetanPlateauGlaciersNote_10August2010.pdf&ei=Y03RTt2kE4fc0QGzsKUW&usg=AFQjCNG9QKdJtT4NQ6B1vX8IHGmnyP1n7g.
[3]Zhang,X,Yao T,Xu S,et al.Phylogenetic and physiological diversity of microorganisms isolated from Puruogangri ice core[J].Microb Ecol,2008,55:476 –488.
[4]Zhang S,Hou S,Yang G,et al.Bacterial community in the East Rongbuk Glacier,Mt.Qomolangma (Everest)by culture and culture-independent methods[J].Microbiol Res,2010,165:336 –345.
[5]Shen L,Yao T,Xu B,et al.Variation of culturable bacteria along depth in the East Rongbuk ice core,Mt.Everest[J].Geoscience Frontiers,2012,3:327-334.
[6]Zhang X,Ma X,Yao T,et al.Diversity of 16S rRNA and environmental factor influencing microorganisms in Malan ice core[J].Chinese Sci Bull,2003,48:1146 –1151.
[7]Yao T,Xiang S,Zhang X,et al.Microorganisms in the Malan ice core and their relation to climatic and environmental changes[J].Global Biogeochem Cy 20,GB1004,2006,doi:10.1029/2004GB002424.
[8]Xiang S,Yao T,An L,et al.16S rRNA sequences and differences in bacteria isolated Muztagh Ata Glacier at increasing depths[J].Appl Environ Microbiol,2005,71:4619 –4627.
[9]Yao T,Liu Y,Kang S,et al.Bacteria variabilities in a Tibetan ice core and their relations with climate change[J].Global Biogeochem Cy 22,GB4017,2008,doi:10.1029/2007GB003140.
[10]Priscu JC,Adams EE,Lyons WB,et al.Geomicrobiology of subglacial ice above lake vostok,Antarctica[J].Science,1999,286:2141 –2144.
[11]Christner BC,Mosley-Thompson E,Thompson LG,et al.Recovery and identification of viable bacteria immured in glacial ice[J].Icarus,2000,144:479 –485.
[12]Zhang S,Hou S,Wu Y,et al.Bacterial diversity in Himalayan glacial ice and its relationship to dust[J].Biogeosciences,2008,5:1741 –1750.
[13]Sheng W,Yao T,Li YF.Variation of Ca2+within the Guliya ice core and its climatic significance[J].J Glaciol Geocryol,1999,21:19 –21.
[14]Wu G,Yao T,Thompson LG.Microparticle record in the Guilya ice core and its comparison with polar records since the last interglacial[J].Chinese Sci Bul,2004,49:607 –611.
[15]Zhang X,Yao T,An L,et al.Study on vertical profile of bacterial DNA structure in Puruogangri ice core by denaturing dradient del electrophoresis[J].Ann of Glaciol,2006,43:160 –166.
[16]Yoshimura Y,Kohshima S,Takeuchi N,et al.Snow algae in a Himalayan ice core:new environmental markers for ice-core analyses and their correlation with summer mass balance[J].Ann Glaciol,2006,43:148 –153.
[17]Price PB,Sowers T.Temperature dependence of metabolic rates for microbial growth,maintenance,and survival[J].Proc Natl Acad Sci USA,2004,101:4631 –4636.
[18]Segawa T,Miyamoto K,Ushida K,et al.Seasonal change in bacterial flora and biomass in mountain snow from the Tateyama Mountains,Japan,analyzed by 16S rRNA gene sequencing and real-time PCR[J].Appl Environ Microb,2005,71:123–130.
[19]Takeuchi N,Fujita K,Nakazawa F,et al.A snow algal community on the surface and in an ice core of Rikha-Samba Glacier in Western Nepali Himalayas[J].Bull Glaciol Res,2009,27:25 –35.
[20]Yoshimura Y,Kohshima S,Ohtani S.A community of snow algae on a Himalayan glacier:change of algal biomass and community structure with altitude[J].Arct Antarct Alp Res,1997,29:126 –137.
[21]Takeuchi N,Kohshima S,Fujita K.Snow algae community on a Himalayan glacier,Glacier AX010 East Nepal:relationship with summer mass balance[J].Bull Glacier Res,1998,16:43 –50.
[22]Zhang X,Yao T,Ma X,et al.Analysis of the characteristics of microorganisms packed in the ice core of Malan Glacier,Tibet,China[J].Sci China Ser D,2001,44 (Supp):369 –374.
[23]An L,Chen Y,Xiang S,et al.Differences in community composition of bacteria in four glaciers in western China[J].Biogeosciences,2010,7:1937 –1952.
[24]Liu Y,Yao T,Jiao N,et al.Bacterial diversity in the snow over Tibetan Plateau Glaciers[J].Extremophiles,2009,13:411–423.
[25]Zhang S,Yang G,Hou S,et al.Abundance and diversity of glacial bacteria on the Tibetan Plateau with environment[J].Geomicrobiol J,2010,27:649 –655.
[26]Abyzov SS.Microorganisms in the Antarctic ice[M].In:Friedmann,E I (ed)Antarctic Microbiology,New York,1993.265–295.
[27]Ma LJ,Catharine MC,Starmer WT,et al.Revival and characterization of fungi from ancient polar ice[J].Mycologist,1999,13:70 –73.
[28]Zhang S,Yang G,Wang Y,et al.Abundance and community of snow bacteria from three glaciers in the Tibetan Plateau[J].J Environ Sci (China),2010,22:1418 –1424.
[29]Zhang X,Diversity of microorganisms and DNA entrapped in glacier ice of Qinghai-Tibet Plateau and its relation with environment[D].Chinese Academy of Sciences.Lanzhou,China (in Chinese),2002.
[30]Ma X,Studies on detection,recovery,isolation and characterization of bacteria in low-temperature extreme environment[D].Lanzhou University.Lanzhou.China (in Chinese),2004.
[31]Xiang S,Yao T,An L,et al.Community change of bacteria in the Malan Ice Core and its relation to climate and environment[J].Chin Sci Bull,2004,49:1869 –1875.
[32]Xiang S,Yao T,An L,et al.Bacterial diversity in Malan ice core from the Tibetan Plateau[J].Folia Microbiol,2004,49:269 –75.
[33]Liu Y,Yao T,Jiao N,et al.Abundance and diversity of snow bacteria in two glaciers at the Tibetan Plateau[J].Front Earth Sci China,2009,3:80 –90.
[34]Zhang S,Hou S,Ma X,et al.Culturable bacteria in Himalayan ice in response to atmospheric circulation [J].Biogeosciences,2007,3:765 –778.
[35]Liu Y,Yao T,Kang S,et al.Seasonal variation of snow microbial community structure in the East Rongbuk Glacier,Mt.Everest[J].Chinese Sci Bull,2006,12:1476 –1486.
[36]Kisand V,Wikner J.Combining culture-dependent and-independent methodologies for estimation of richness of estuarine bacterioplankton consuming riverine dissolved organic matter[J].Appl Environ Microbiol,2003,69:3607 –3616.
