Cranial morphometric study of four giant flying squirrels (Petaurista) (Rodentia: Sciuridae) from China
2012-12-25LISongYUFaHongXueFei
LI Song, YU Fa-Hong, LÜ Xue-Fei
(1. State Key Laboratory of Genetic Resources and Evolution, Kunming Natural History Museum of Zoology, Kunming Institute of Zoology, the Chinese Academy of Sciences, Kunming 650223, China; 2. ICBR, University of Florida, Gainesville, FL 32610, USA; 3. Institute of Zoology, the Chinese Academy of Sciences, Beijing 100101, China; 4. Graduate School of the Chinese Academy of Sciences, Beijing 100049, China)
Cranial morphometric study of four giant flying squirrels (Petaurista) (Rodentia: Sciuridae) from China
LI Song1,*, YU Fa-Hong2, LÜ Xue-Fei3,4
(1. State Key Laboratory of Genetic Resources and Evolution, Kunming Natural History Museum of Zoology, Kunming Institute of Zoology, the Chinese Academy of Sciences, Kunming 650223, China; 2. ICBR, University of Florida, Gainesville, FL 32610, USA; 3. Institute of Zoology, the Chinese Academy of Sciences, Beijing 100101, China; 4. Graduate School of the Chinese Academy of Sciences, Beijing 100049, China)
The present study revisited the controversial taxonomic status ofPetaurista yunanensis,P. philippensis,P. hainana, andP. petauristaby using a considerably extended set of morphometrical characters (26 cranial variables from 60 adult specimen skulls). The results revealed no sexual dimorphism in any of the four species but confirmed significant craniometric differences among the four species in both the principal components analysis (PCA) and discriminant function analysis (DFA), with the greatest distinction observed betweenP. petauristaand otherPetauristaspecies. Both univariate and multivariate analysis indicated that the morphological differences betweenP. yunanensisandP. philippensiswere less than that betweenP. philippensisandP. hainana. The morphometric results were concordant in geographic patterns with mtDNA data from previous studies and indicated thatP. petaurista,P.hainana,P. philippensis, andP.yunanensiscould be recognized as valid species.
Petaurista;Cranial variables; Statistical analysis; Species
Giant flying squirrels (Petaurista) occur in lowlands and mountains up to 4 000 m in East and Southeast Asia from Pakistan and Kashmir. Currently, eight to ten forms are commonly accepted as validPetauristaspecies, with each divided into various forms or subspecies (Corbet & Hill, 1992; Wang, 2003; Thorington & Hoffmann, 2005). Because various species and subspecies with significant geographical variations are included within this genus, the taxonomy and the intra- and inter-specific phylogenetic relationships remain unclear and inconclusive. Several taxonomic studies and more than 18Petauristaforms, subspecies, or species have beendescribed on the basis of dental and cranial characteristics and external structures (Allen, 1940; Corbet & Hill, 1992; Ellerman, 1940; Hoffmann et al, 1993; Wang, 2003; Zhang et al, 1997).
Petauristaare widely distributed in China and more than ten distinct species are recognized (Corbet & Hill, 1992; Wang, 2003; Zhang et al, 1997). However, several of these species are referenced with very few specimens or based solely on skins with no corresponding skulls (Allen, 1940; Ellerman, 1940), and some are actually the synonyms or subspecies of either theP. petauristacomplex or theP. philippensiscomplex due to intraspecific geographic variations across their distributions in Asia.
Corbet & Hill (1992) treatedP. albiventerin Pakistan and southwest China as the synonym ofP. petauristaand recognizedP.philippensisas a distinct species consisting of many forms formerly assigned toP. petaurista, including forms distributed in Taiwan (P. grandis), southwest Yunnan (P. yunanensis), and Hainan (P. hainana). After comparing the pelage and cranial characteristics ofP. petauristaandP. hainana, Huang et al (1995) consideredP. hainanato be a valid species, but Wang (2003) treatedP. hainanaas a subspecies ofP. yunanensis. Thorington & Hoffmann (2005) treated allPetauristaforms as eight valid species instead of nine as suggested by Corbet & Hill (1992), but they accepted the specific validity ofP. philippensisand the subspecies status ofP. yunanensisandP. hainana. Patterns of genetic variations observed in the complex ofP. philippensisbased on cytochromebgenes indicated thatP.hainana,P. albiventer, andP.yunanensiscould be distinct species (Yu et al, 2006). Some forms included inP. philippensiswarranted separate specific rank based on molecular data (Oshida et al, 2000a, b; Yu et al, 2006), but without further evidence from morphometric data, much remains to be done to ascertain conclusively these specific conclusions.
Most recent phylogenetic studies have focused on molecular data analysis, but tracing changes in morphological characters is also an important way to evaluate the distribution of the characters on which those taxonomic units are based. Morphometric data are important to understand biological phenomena and have been used to evaluate cranial, dental, and body measurements of many mammals (Muñoz-Muñoz & Perpinan, 2010; Slábová & Frynta, 2007; Zelditch et al, 2004). Quantitative analysis of intra- and inter-specific variations at the morphological level is useful for detecting patterns of geographic variations and delimiting intra- or inter-specific evolutionary units. To date, however, there are currently no published reports of quantitative analysis based on morphological characteristics that would allow the identification of the morphotypes in the complex ofP. philippensisandP. petaurista.
To discuss the taxonomic relationships ofP. philippensis,P. yunanensis,P. hainana, andP. petauristaand test previous taxonomic hypotheses, the present study conducted a comprehensive morphometric study on the above ChinesePetauristaspecies based on samples subsequently collected from southwest Yunnan and the Island of Hainan, China. Multivariate analyses were used to produce an overview of the associations between morphological variables and species patterns and discuss the taxonomic implications of these flying squirrels. Our morphometric study could be complementary to studies of variations of DNA sequences in flying squirrels.
1 Materials and Methods
1.1 Specimens and data collection
According to the taxonomic assignments of Allen (1940) and Zhang et al (1997), a total of 60 intact adult skull specimens ofP. petaurista, P. yunanensis, P. hainana,andP. philippensiswere examined for morphometric study (Append. I). These specimens are from the Kunming Institute of Zoology, Chinese Academy of Sciences (KIZ, CAS) (Kunming, China), the Institute of Zoology, Chinese Academy of Sciences (IOZ, CAS) (Beijing, China), and the Guangdong Entomological Institute (GDEI) (Guangzhou, China).
Twenty-six cranial variables taken with a digital caliper to the nearest 0.01 mm were used in the morphometric analysis as described by Musser (1979), Musser & Heaney (1992), Xia et al (2006), and Yang et al (2005), and depicted in Fig. 1 following Huang’s description (1995). The variables measured included: maximum length of skull (GLS), condylobasal length (CBL), basal length (BL), occipito-nasal length (ONL), palatal length (PL), length of palatal bridge (PBL), length of upper tooth row (LUTR), length of upper molars (LUM), maximum upper molars breadth (GUMB), rostral length (ROL) and breadth (ROB), auditory bulla length (ABL) and breadth (ABB), breadth of zygomatic plate (BZP), breadth of occipital condyles (BOO), height of occipital (HO), zygomatic breadth (ZOB), mastoid breadth (MTB), nasal length (NL) and breadth (BN), mandible length (ML), height of mandible (THM), length of lower diastema (LLD), length of lower molar row (LLMR), length of lower tooth row (LTR), and mandibular height (MH). In addition, the head and body length (HB), tail length (TL), hind foot length (HFL), and ear length (EL), which were compared to the original measurements labeled on the skins by the collectors.
1.2 Data analysis
Statistical analyses were performed using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). All variables were transformed into logarithms to eliminate the bias effect of large measurements in multivariate analysis (D’Elía & Pardiñas, 2004). Statistical differences were considered significant atP<0.05.
In this study, all related data were subjected to oneway ANOVA for calculating mean±SD.T-test was used to assess the sexual dimorphism between male and female groups by comparing the group means of cranial measurements. Multiple comparisons between taxa were made for all 26 cranial measurements to evaluate variations between samples. Multivariate analyses, including principal components analysis (PCA) and discriminant function analysis (DFA), were carried out to evaluate the degree of similarity and dissimilarity in cranial structures between the putative species and to determine how the taxa were related when all cranial characters measured are considered simultaneously.
The PCA is based upon the variance-covariance matrix of the log-transformed variables. The eigenvector scores describing the relative significance of each variable to the principal components were used to compare the cranial morphological similarities and differences. The PCA scatter-plot visually represented the variation among different individuals of the samples. The DFA was performed to investigate the integrity of the pre-defined groups and to predict group membership of specimens with the linear models of variables. Based on the derived discriminant functions, each individual was allocated to the group with nearest centroid, and the proportion of individuals allocated to each group was calculated.
2 Results
Mean±SDof 4 external and 26 cranial variables for the four taxa are presented in Tab. 1.
2.1 Univariate analysis
Univariate comparison revealed that the means of all variables were significantly different and, in general, tended to become progressively larger fromP. petaurista,P. hainana,P. yunanensis,toP. philippensis.Thet-tests of Equality of Group Means on 54 (30 males, 24 females) out of 60 specimens indicated there was no sexual dimorphism in the 26 cranial variables in the fourPetauristagroups (Tab. 2). Quantitative pairwise comparisons of all cranial variables between taxa indicated thatP. yunanensiswas morphologically similar toP. philippensis, with 11 cranial measurements showing no significant difference (P>0.05) (Tab. 3). Also, five cranial variables were not significantly different betweenP. yunanensisandP. hainana.
2.2 Multivariate analysis
In PCA, the eigenvalues for the first three principal components were 20.62, 1.64 and 0.85, respectively, accounting for 88.91% of the total variance (Tab. 4). Most characteristics with high positive loadings on the first principal component suggested that this component (79.32% of the total variance) represented size variation within the samples. All specimens on the first principal component were clustered as three groups,P. petaurista,P. hainana, and the group ofP. yunanensisandP. philippensiswith considerable overlaps. The second principal component (6.32% of the total variance) was strongly correlated with ROB, ABL, ABB, and BZP (loadings>0.50), and the third principal component (3.27% of the total variance) was correlated primarilywith HO (loadings>0.50) (Tab. 4). The first two principal components separated all specimens as four distinct groups (Fig. 2).
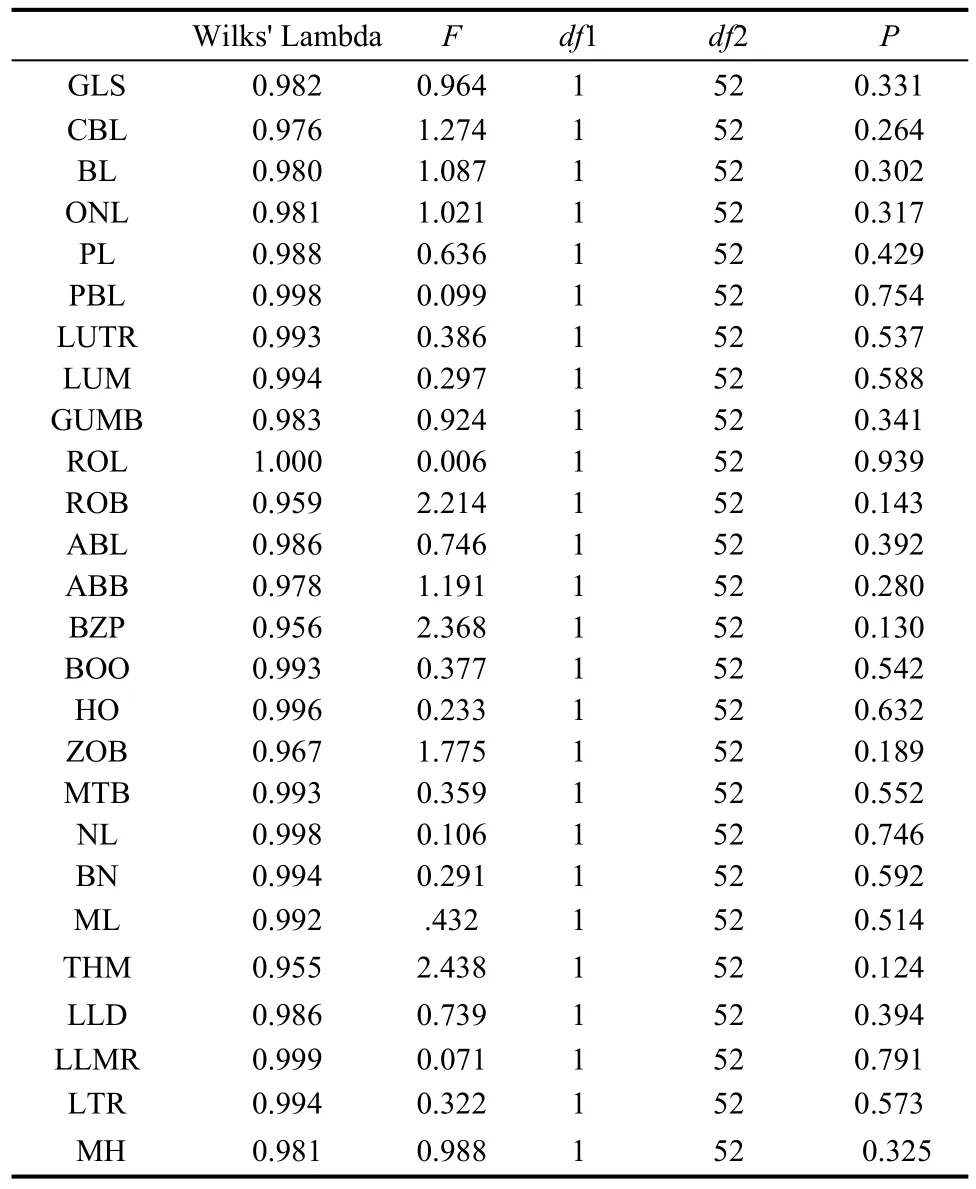
Tab. 2 t-tests of Equality of Group Means for male and female (variable codes are given in the text and Fig.1)
The DFA identified the major patterns of morphological divergence in the crania among the fourPetauristagroups. The variation pattern reflected by the first two discriminant functions was consistent with the morphological variations observed in the PCA, and all samples were clearly clustered as four distinguishable groups based on the 1stand 2nddiscriminant functions (Fig. 3). In specimen reclassification by DFA, all individuals were properly assigned to their original groups on the basis of the studied measurements. Fig. 4 is the geographic distributions of allPetauristasamples used in this study.
3 Discussion
One contentious issue regarding the ChinesePetauristais the taxonomic status ofP. yunanensis,P. philippensis,P. hainana, and the populations ofP. petauristain China, which have long been controversial (Corbet & Hill, 1992; Ellerman, 1940; Ellerman & Morrison-Scott, 1950; Hoffmann et al, 1993; Huang et al,1995; Oshida et al, 2000a, 2000b; Thorington & Hoffmann, 2005; Wang, 2003; Yu et al, 2006). By using a considerably extended set of morphometrical characters (26 cranial variables) and applying multivariate morphometric analyses, results of the present study confirmed the significant craniological differences inP. petaurista,P. hainana,P. yunanensis, andP. philippensis, withP. petauristahaving the most pronounced morphological variations, particularly in metrical components of cranial and body size.

Tab. 3 Multiple Comparisons between the four study species

Tab. 4 Factor loadings and percentage of variance explained for principal component analysis (variable codes are given in the text and Fig. 1)
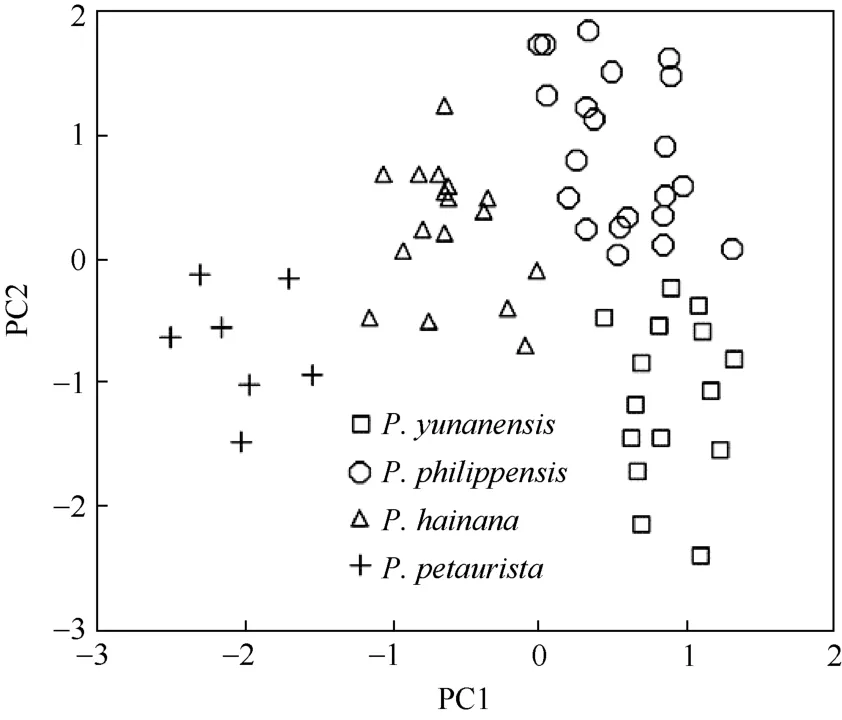
Fig. 2 Scatterplots of the samples in PCA space

Fig. 3 Plot of the samples of the four Petaurista species on discriminant canonical function 1 and 2

Fig. 4 Geographic distributions of samples used in the study
Pelage coloration had been applied for classification of flying squirrels and led to many taxonomical disagreements due to numerous color variations withinPetaurista, even between different sexes (Allen, 1940; Ellerman & Morrison-Scott, 1950; Oshida et al, 2004b). A series of color variations in pelage were observed among thePetauristaforms including both sexes (Allen, 1940; Oshida et al, 2004a), but the pairwise comparison of each of the 26 cranial variables revealed no sexual dimorphism in any of the four groups, implying that the divergence of coloration patterns in forms was due to environmental or genetic fluctuations over time.
Both univariate and multivariate analysis revealed that the skull morphometric characters used in this study were effective for discriminating the fourPetauristagroups.Our analyses demonstrated that the four morphotypes ofPetauristawere distinguished by a number of cranial characteristics. The morphometric variables, which caused the major distinction between those groups, were specifically located in the occipital, supraocular, and rostral regions, as well as in the prooticsquamosal length. Patterns of molecular sequence variations from previous studies (Oshida et al, 2000a; Yu et al, 2006) and the cranial morphological differences observed in this study indicate thatP.hainana,P. philippensis, andP.yunanensiscould be recognized as three distinct species. These differences were clear and reinforced the existence of three morphotypes of the complexP. philippensis; although the degree and form of the morphological differences might be related to their geographical variations. Even though molecular data suggested thatP. philippensisis closely related toP. hainanaand significantly distinct fromP.yunanensis(Yu et al, 2006), many characteristics beyond those related to external morphology and pelage coloration were observed to be held in common betweenP. yunanensisandP. philippensis. A good example is the similarity in the pattern of overall cranial structure in multiple comparison analysis. The degree of the morphological variations betweenP. yunanensisandP. philippensiswas less than that observed betweenP. philippensisandP. hainana, with eleven cranial measurements showing no significant difference (P>0.05) (Tab. 3).Petauristayunanensisoccurs from extreme southwestern Yunnan into Myanmar and Indochina and is extensively sympatric withP. philippensisin southwestern China (Wang, 2003; Zhang et al, 1997). The sharing of morphological characteristics betweenP. philippensisandP. yunanensisis related to their similar living conditions.
Petaurista hainanawas considered a valid species based on both molecular and morphological data (Huang et al, 1995; Yu et al, 2006). Our morphometric results were concordant with mtDNA data of previous research (Oshida et al, 2000a; Yu et al, 2006) and demonstrated the significant differences betweenP. hainanaandP. yunnanensis/P. philippensis. In both PCA and DFA,P. hainanawas clearly separated from other three groups (Fig. 2, 3), with 21/26 cranial variables being significantly different (P<0.05) (Tab. 3).Petaurista hainanais confined to tropical forests on Hainan Island of China andP. philippensisandP.yunanensisare distributed in mountainous coniferous, dry deciduous and evergreen forests at different elevations in western Yunnan of China. The phenotypic divergence ofP. hainanain relation toP. philippensisandP. yunanensisis likely associated with their geographical distributions and living conditions and could be viewed as a reflection of adaptations to various ecological niches. The differences in skull morphology suggest thatP. hainanais neither the synonym ofP. philippensisnor a subspecies ofP. yunanensisorP. petaurista(Corbet & Hill, 1992; Thorington & Hoffmann, 2005; Wang, 2003),but a valid species in its own right.
The greatest distinction observed was betweenP. petauristaand otherPetauristaforms.Petaurista petauristadisplayed a relatively high level of diversity in skull morphology, with 22/26 cranial variables significantly different fromP. hainana,P. philippensis,andP. yunanensisatP<0.001 level (Tab. 3). Based on 26 morphological cranial variables, the specimens ofP. petauristaformed a distinct aggregate in both PCA and DFA (Fig. 2,3), consistent with the results of Oshida et al. (2000a) and Yu et al (2006). It is obvious thatP. petaurista,P. hainana,P. philippensis,andP. yunanensisare taxonomically distinct and distinct valid species.
Append I: specimens examined
Petaurista yunanensisn=15
Yingjiang, Yunnan: IOZ 25849(♂). Gongshan, Yunnan: KIZ 73442(♀), 73445(♂), 73744(♂), 73745(♀), 73823(♂), 830207(♀), 90039(♀), 90043(♀), 90051(♂), 90407. Tengchong, Yunnan: KIZ 76348(♀). Lianghe, Yunnan: KIZ 650236(♂), 650237(♂). Bijiang, Yunnan: KIZ 780102(♀).
Petaurista philippensisn=21
Xishuangbanna, Yunnan: IOZ 10457(♂), 10458(♂), 10460(♀), 15041(♀), 15042(♀),
15043(♂), 15044(♂), 61-003(♀). Luxi, Yunnan: IOZ 24009(♂), 24010(♀). Mile,
Yunnan: KIZ 84255(♂), 84258(♂), 84300(♂). Lvchun, Yunnan: KIZ 72104(♀).
Longling, Yunnan: KIZ 620028(♂). Cangyuan, Yunnan: KIZ 78053(♂). Pingbian,
Yunnan: KIZ 84117(♂), 84119(♂). Xinping, Yunnan: KIZ 77017(♀). Yunlong,
Yunnan: KIZ 200369(♀). Yunnan: KIZ 92005.
Petaurista hainanan=17
Hainan: GDEI 0403(♀), 0404(♂), 0499(♀), 0524(♂), 0611(♀), 0618(♂), 0621(♀),
0622(♂), 0623(♀), 0624(♂), 0625(♂), 0626(♂), 0703(♂), 0704(♂), 0705(♀),
0714(♀), 0717(♀).
Petaurista petauristan=7
Xiangzhou, Guangxi: IOZ 00433(♂). Northern Guangdong: GDEI 2279, 2284, 2286(♀), 2287, 2288(♂), 2289.
Allen GM. 1940. The mammals of China and Mongolia[M]. New York: The American Museum of Natural History, 729-745.
Corbet GB, Hill JE. 1992. The Mammals of the Indomalayan Region: A Systematic Review[M]. Oxford: Oxford University Press, 308-313.
D’Elía G, Pardiñas UFJ. 2004. Systematics of Argentinean, Paraguayan, and Uruguayan swamp rats of the genusScapteromys(Rodentia, Cricetidae, Sigmodontinae)[J].J. Mammal,85(5): 897-910.
Ellerman JR. 1940. The Families and Genera of Living Rodents. Volume I[M]. London: British Museum, 281-290.
Ellerman JR, Morrison-Scott TCS. 1950. Checklist of Palaearctic and Indian mammals, 1758 to 1946[M]. London: British Museum (Natural History), 460-465.
Hoffmann RS, Anderson CG, Thorington RW, Jr., Heaney LR. 1993. Family sciuridae[M]. //Wilson DE, Reeder DM. Mammal Species of the World: A Taxonomic and Geographic Reference. 2nded. Washington D.C.: Smithsonian Institution Press, 462-463.
Huang WJ, Chen YX, Wen YX. 1995. Rodentia of China[M]. Shanghai: Fudan University Press, 50-59. (in Chinese)
Muñoz-Muñoz F, Perpiñán D. 2010. Measurement error in morphometric studies: comparison between manual and computerized methods[J].Ann Zool Fennici,47: 46-56.
Musser GG. 1979. Results of the Archbold Expeditions. No.102. The species ofChiropodomys,arboreal mice of Indochina and the Malay Archipelago[J].Bull Am Nus Nat Hist,162: 377-445.
Musser GG, Heaney LR. 1992. Philippine Rodents: Definitions of Tarsomys and Limnomys plus a preliminary assessment of phylogenetic patterns among native Philippine murines (Murinae, Muridae)[J].Bull Am Nus Nat Hist, 211: 1-138.
Oshida T, Lin LK, Masuda R, Yoshida MC. 2000a. Phylogenetic relationships among Asian species ofPetaurista(Rodentia, Sciuridae), inferred from mitochondrial cytochromebgene sequences [J].Zool Sci, 17(1): 123-128.
Oshida T, Lin LK, Yanagawa H, Endo H, Masuda R. 2000b. Phylogenetic relationships among six flying squirrel genera, inferred from mitochondrial cytochromebgene sequences[J].Zool Sci,17(4): 485-489.
Oshida T, Shafique CM, Barkati S, Fujita Y, Lin LK, Masuda R. 2004a. A preliminary study on molecular phylogeny of giant flying squirrels, genusPetaurista(Rodentia, Sciuridae) based on mitochondrial cytochromebgene sequences[J].Russian Journal of Theriology,3: 15-24.
Oshida T, Shafique CM, Barkati S, Yasuda M, Hussein NA, Endo H, Yanagawa H, Masuda R. 2004b. Phylogenetic position of the small Kashmir flying squirrel,Hylopetes fimbriatus(=Eoglaucomys fimbriatus), in the subfamily Pteromyinae[J].Can J Zool,82(8): 1336-1342.
Slábová M, Frynta D. 2007. Morphometric variation in nearly unstudied populations of the most studied mammal: the noncommensal house mouse (Mus musculus domesticus) in the near East and Northern Africa[J].Zool Anz,246(2): 91-101.
Thorington RW Jr, Hoffmann RS. 2005. Family Sciuridae[M] // Wilson DE, Reeder DM. Mammal Species of the World: A Taxonomic and Geographic Reference. 3nded. Washington D.C.: The Johns Hopkins University Press, 770-772.
Wang YX. 2003. A Complete Checklist of Mammal Species and Subspecies in China: A Taxonomic and Geographic Reference[M]. Beijing: China Forestry Publishing House, 155-159. (in Chinese)
Xia L, Yang QS, Ma Y, Feng ZJ, Zhou LZ. 2006. Guide to the measurement of mammal skull III: Rodentia and Lagomorpha[J].Chinese J Zool,41(5): 68 -71. (in Chinese)
Yang QS, Xia L, Ma Y, Feng ZJ, Quan GQ. 2005. A guide to the measurement of mammal skull I: Basic measurement[J].Chinese J Zool, 40(3): 50-56. (in Chinese)
Yu FR, Yu FH, Peng JF, Kilpatrick CW, McGuire PM, Wang YX, Lu SQ, Woods CA. 2006. Phylogeny and biogeography of thePetaurista philippensiscomplex (Rodentia: Sciuridae), inter- and intraspecific relationships inferred from molecular and morphometric analysis [J].Mol Phylogenet Evol, 38(3): 755-766.
Zelditch ML, Swiderski DL, Sheets HD, Fink WL. 2004. Geometric Morphometrics for Biologists: A Primer[M]. New York: Elsevier Academic Press.
Zhang YZ et al. 1997. Distribution of Mammalian Species in China[M]. Beijing, China: China Forestry Publishing House, 150-154. (in Chinese)
中国四种鼯鼠的头骨形态学
李 松1,*, 俞发宏2, 吕雪霏3,4
(1.中国科学院昆明动物研究所遗传资源与进化国家重点实验室,昆明动物博物馆,云南 昆明650223; 2.美国佛罗里达大学 生物技术研究中心,佛罗里达32610,美国; 3.中国科学院动物研究所,北京100101; 4.中国科学院研究生院,北京100049)
针对长期以来有关鼯鼠分类地位的争议, 该研究基于查看、测取60号鼯鼠成体头骨(每号头骨测取26个可量性状)共计1 560个数据, 运用多变量、单变量分析方法, 对鼯鼠属(Petaurista)中的P. yunanensis, P. philippensis, P. hainana以及P. petaurista头骨可测量数据进行了统计学分析, 以探讨上述4种鼯鼠的头骨形态差异以及P. yunanensis和P. hainana的分类地位。结果显示:(1)上述可测量头骨性状在该4种鼯鼠中不存在性二型现象; (2)上述4种鼯鼠在所测量的头骨性状上两两间均存在显著差异; (3)P. philippensis与P. hainana之间的头骨形态差异程度远大于P. yunanensis与P. philippensis之间的差异。该结果在宏观统计分析水平上为上述4种鼯鼠的种地位有效性提供了佐证, 与前人基于分子水平(mtDNA)的种地位有效性研究结果相似。
鼯鼠属; 头骨变量; 统计分析; 物种
959.837; Q954.54+2
A
0254-5853-(2012)02-0119-08
2011-07-25;接受日期:2011-11-20
10.3724/SP.J.1141.2012.02119
date: 2011-07-25; Accepted date: 2011-11-20
s: National Natural Science Fund of China (30970332); National Basic Research Program of China (973 Program) (2007CB411600); Natural Science Found of Yunnan Province (2007C099M); Special Subjects Funds of National Natural Science Fund of China (J0930004)
*Corresponding author (通信作者), E-mail: lis@mail.kiz.ac.cn
book=126,ebook=258
We thank Prof. ZJ FENG, Prof. Y MA, Prof. GQ QUAN and Prof. QS YANG from the Institute of Zoology, the Chinese Academy of Sciences, and Prof. HJ HU and Prof. P YANG from the Guangdong Entomological Institute (GDEI) for their generous help in checking specimens. We extend special thanks to Mr. Kevin Holland of the University of Florida for advice and help on the manuscript.
杂志排行
Zoological Research的其它文章
- Bacterial expression and purification of biologically active human TFF2
- 香鱼补体成分C9基因的克隆、序列分析及表达
- Smallest bitter taste receptor (T2Rs) gene repertoire in carnivores
- A new spider species of the genus Sudharmia from Sumatra, Indonesia (Araneae, Liocranidae)
- Complete mitogenome of the Lesser Purple Emperor Apatura ilia (Lepidoptera: Nymphalidae: Apaturinae) and comparison with other nymphalid butterflies
- Acute lesions of primary visual cortical areas in adult cats inactivate responses of neurons in higher visual cortices
