非贵金属催化的碱性硫离子燃料电池放电特性
2012-12-05樊玉欠邵海波王建明张鉴清曹楚南
樊玉欠 邵海波 王建明 刘 倞 张鉴清 曹楚南
(浙江大学化学系,杭州310027)
非贵金属催化的碱性硫离子燃料电池放电特性
樊玉欠 邵海波*王建明 刘 倞 张鉴清 曹楚南
(浙江大学化学系,杭州310027)
燃料的选择对于燃料电池电极催化剂的选择、燃料电池的成本及其商业化有着至关重要的影响.寻求电化学活性好、成本低,并且能够为非贵金属催化剂所催化的燃料是一项有前景的工作.硫离子的电化学活性及其低成本使之成为一个具有吸引力的选择.本文以碱性硫离子作为燃料构建了碱性硫离子燃料电池.在室温条件下,单体电池在以非贵金属为催化剂的条件下获得了12.3 mW·cm-2的功率密度,此时的电流密度达到了42.8 mA·cm-2;50 h寿命测试显示了碱性硫离子燃料电池良好的稳定性.此外,通过离子色谱分析,在放电产物中检测到了硫代硫酸盐、亚硫酸盐以及硫酸盐.深度氧化使得硫离子具有更高的放电容量.与之前研究的燃料相比,硫离子具有成本低、运输容易、电化学活性高并且能够被非贵金属催化剂催化的优点.
燃料电池;碱性硫离子;非贵金属催化剂;深度氧化;电化学
1 Introduction
Fuel cells operated at low temperature are widely recognized as promising objectives which could perform high efficiency in transforming chemical energy to electric energy. Among the three key factors(fuel,catalyst,and electrolyte)for designing a fuel cell,the choice of fuel is an important issue influencing the selection of catalysts,the cost and commercialization of fuel cells.1Thus searching for electrochemical-active and low-cost fuels which could be oxidized by non-precious catalysts is a significant and attractive objective.Although hydrogen is considered to be an ideal fuel,the transportation and storage of hydrogen and the consumption of precious metalcoated catalysts are still critical problems for its applications.1-4Recently,various hydrogen-carriers,such as methanol,ethanol,hydrazine,borohydride,are adopted and their electrochemical performances are reported,either in order to ease the storage and transportation of fuels,or to lower the cost of catalysts.5-11Unfortunately,due to the low electrochemical activities of methanol or other hydrocarbons,platinum and its alloys are still considered to be the most effective catalysts,whereas other non-precious metal catalysts show low activities.5-8,12On the other hand,although borohydride and hydrazine exhibit high electrochemical activities even on non-precious catalysts,9,10,13,14the high cost for their production restricts their applications for fuel cells.
In the past decades,alkaline sulfide electrolyte has been reported as a power source in some electrochemical devices.15-18For example,sulfide in alkaline solution could be used in some metal-sulfur batteries,15,16rechargeable batteries,17and photoelectrochemical solar cells.18The native electrochemical activity and low cost of sulfide make it an attractive candidate for fuel cells.Besides,the electrochemical activity could be greatly enhanced by using low-cost catalysts such as carbon materials or cobalt salt.19Compared with the fuels metioned above,sulfide has merits of low-cost,easy transportation and storage, high electrochemical activity,and it could be catalyzed by non-precious catalysts.However,until now to the best of our knowledge,only several patents have referred alkaline sulfide as a fuel,and few research reports have paid attention to the usage of alkaline sulfide in a fuel cell system.20,21Herein based on all of the advantages,alkaline sulfide was proposed as a novel fuel to assemble alkaline sulfide fuel cells,and the electrochemical performance of the cells was investigated.
2 Experimental
2.1 Assembling single alkaline sulfide fuel cells
2.1.1 Anodes
All the solutions used were prepared by analytical grade reagents and deionzed water.Carbon spherules were used as carbon source which were prepared by dewatering-carbonization steps using sucrose as precursor.22For typical process,aqueous sucrose solution of 0.2 mol·L-1was firstly injected in stainless steel autoclave,and then hydrothermal treatment was proceeded at 190°C for 5 h.After rinsed by deionized water and ethanol,the obtained black power was further carbonized at 1000°C in a tube furnace in argon(99.999%)atmosphere.Micrographs of carbon spherules were taken with a scanning electron microscope(SEM,Hitachi:S4800,Fig.1a).
Two types of electrodes were fabricated:carbon electrode (CE)and carbon electrode impregnated with cobalt salt(Co2+/ CE).For typical fabrication,15 mg carbon spherules were added into 800 μL ethanol solvent,and the bulk was placed under ultrasonic for 20 min.After that,60 μL Nafion®solution(5%, w)was injected in the bulk and ultrasonic for 10 min.Finally a carbon ink was obtained.After then,120 μL(about 2.4 mg carbon)of carbon ink was pipetted and spread on nickel foil(1 cm2)and dried at room temperature to obtain a CE.
In order to fabricate Co2+/CE,the CE was firstly immersed in 1 mol·L-1sodium sulfide electrolyte for 1 h in order to adsorb sulfide ions,then the electrode was washed by deionzed water to remove unadsorbed sulfide ions.After that the electrode was immersed in 1 mol·L-1cobalt sulfate solution for another hour.In this step,the cobalt ion reacted with adsorbed sulfide ions to form cobalt sulfide and precipitated on carbon spherules.Finally the electrode was washed by deionzed water to remove unadsorbed cobalt ions to obtain Co2+/CE.
2.1.2 Cathodes
A typical cathode was composed of catalyst layer,gas diffusion layer,and current collector.Catalyst layer was prepared by hot-pressing dough-like mixture of 5%(mass fraction)Pt/ Vulcan XC-72 carbon catalyst,acetylene black,polytetrafluoroethylene(PTFE)(in a 60:25:15 mass ratio)on a rolling machine.The gas diffusion layer was prepared using the same method with acetylene black,PTFE in a 1:1 mass ratio and sodium sulfate was used as pore-forming agent.Air cathodes were fabricated by hot-pressing catalyst layer and gas diffusion layer on nickel mesh(60#).23Cathodes were controlled with thickness about 0.4 mm and dried under vacuum at 80°C overnight before using.
2.1.3 Fuel cell assembly
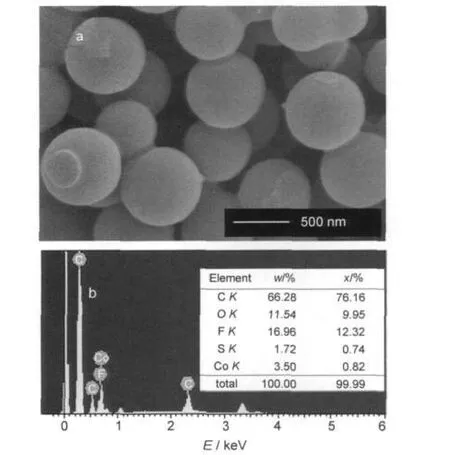
Fig.1 (a)SEM image of carbon spherules;(b)X-ray energy dispersive spectrum and elemental analysis of carbon spherules impregnated with cobalt salt
A simple fuel cell prototype was adopted in research,and the framework was made of polymethyl methacrylate(PMMA) and sealed by rubber gaskets.The half cell was assembled by using as-prepared CE or Co2+/CE as working electrode with a graphite rod as counter electrode and a saturated calomel electrode(SCE)as reference electrode.Single fuel cell was assembled by combining anode and cathode at both sides of cation exchange memberane(CEM,Nafion®117).24A well designed fuel cell prototype was described in Fig.2.The adopted alkaline sulfide fuel was an aqueous solution containing 1 mol·L-1Na2S and 1 mol·L-1KOH.Before using,it was purged with Ar (99.999%)for at least 1 h.For typical test,anode chamber was firstly purged with argon gas(99.999%)for at least 15 min, and then sulfide fuel was fed through it at a flow rate of 10 mL·min-1.Whereas in the cathode,KOH electrolyte with a flow rate of 0.5 mL·min-1was fed through cathodic chambers. Oxygen(99.9%)was introduced at a flow rate of 10 mL·min-1and the chamber pressure was maintained at atmosphere pressure.
2.2 Electrochemical measurements
Polarization curves of half cells and cell voltage,power density versus current density curves of single cells were measured on potentiostat with type of CHI-660A(Chenhua Instruments,Shanghai).Typical polarization curves of half cells were recorded by scanning the cell voltage from open circuit potential(OCP)positively to about-0.4 V(vs SCE)at a scan rate of 0.5 mV·s-1.Cell voltage,power density versus current density curves were measured by scanning the cell voltage from open circuit voltage(OCV)down to 0.15 V at a scan rate of 0.5 mV·s-1.Life test of single fuel cell was performed on a battery testing systerm of BT-2000,Arbin Instrument.During a typical test,the power density was determined by measuring voltages generated by fuel cell when it was loaded with a constant resistance.All electrochemical measurements in this study were conducted at 25°C,101 MPa.
2.3 Product analysis
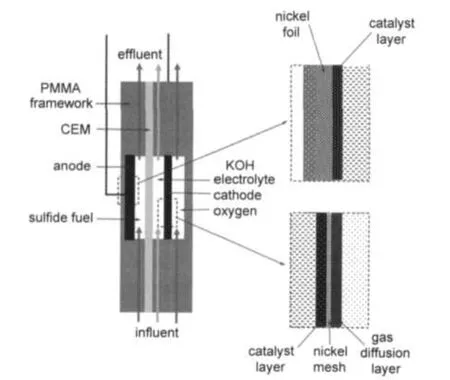
Fig.2 Schematic diagram of alkaline sulfide fuel cell
The discharge products of the single cells were analyzed by ion chromatography on a Dionex Model ICS-2000 system equipped with an AS11 separation column and an AG11 guard column(all supplied by Dionex).A certain volume(40 mL)of anode fuel was adopted as cycled fuel in this section.Single cell was discharged with a constant resistance until voltage fell down to 0.15 V.The obtained products were analyzed by ion chromatography after dilution of 2000 times with ultrapure water(18 MΩ·cm,purged withAr for at least 30 min).KOH solution was used as the eluent.
3 Results and discussion
3.1 Characterization of anode catalysts
Micrographs of carbon spherules with monodispersed particle size with diameter around 500-700 nm are shown in Fig.1a.X-ray energy dispersive spectrum and elemental analysis of carbon spherules impregnated with cobalt salt is shown in Fig.1b,which indicate that about 3.50%(mass fraction)cobalt ion has been impregnated.In order to investigate the electrochemical activity of alkaline sulfide fuel,polarization curves are examined on half cells with the as-fabricated CE and Co2+/CE as working electrodes.The results shown in Fig.3 indicate that both the two half cells have negative open circuit potential,-0.74 V(vs SCE)for CE,and-0.83 V(vs SCE)for Co2+/CE.When discharging,the two half cells have stable discharge plateaus at potential windows of-0.7 to-0.45 V,-0.8 to-0.5 V,respectively.No sulfur or other precipitation is observed on the working electrodes.Besides,the polarization curves also illustrate that Co2+/CE gives much lower overpotential than CE during the electrochemical oxidation process, which may be attributed to the catalysis of cobalt salt.These indicate that alkaline sulfide has stable electrochemical properties and could be potentially used as a fuel in fuel cells.
3.2 Electrochemical measurement of single cells
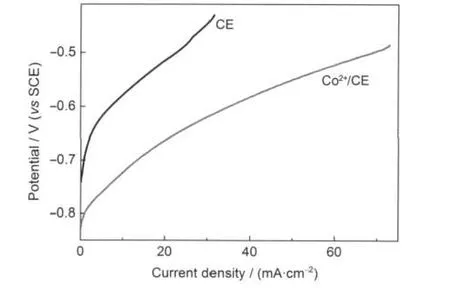
Fig.3 Polarization curves on half cellsPolarization curves were obtained by scanning potential from OCP to-0.4 V at a scan rate of 0.5 mV·s-1.The half cell was assembled by using as-prepared CE or Co2+/CE as working electrode with a graphite rod as counter electrode and a saturated calomel electrode(SCE)as reference electrode.The adopted alkalinesulfide fuel was an aqueous solution containing 1 mol·L-1Na2S and 1 mol·L-1KOH.
Further investigation of alkaline sulfide fuel was performed on single cells which were assembled by combining the as-fabricated CE and Co2+/CE with air cathodes.A well designed fuel cell prototype is illustrated in Fig.2.Cell voltage,power density as a function of current density curves are illurstrated in Fig.4.It can be seen that CE-catalyzed cell exhibits an open circuit voltage of 0.67 V.The maximum power density reaches 6.5 mW·cm-2with current density of 28.9 mA·cm-2,which indicates that sulfide has considerable electrochemical activity on low-cost carbon material.The result is delightful when compared with that of hydrogen or hydrocarbons,which is inactive on pure carbon materials.Besides,Co2+/CE-catalyzed cell gives a higher OCV of 0.73 V and yielded maximum power density of 12.3 mW·cm-2with current density of 42.8 mA·cm-2.Improved power output should be attributed to the catalysis of cobalt salt,which is in accordance with the results of half cell test.The results show that the electrochemical activity of sulfide could be enhanced by non-precious catalyst.Moreover, both the two single cells have stable discharge plateau at voltage range from 0.50 to 0.15 V.When being calculated by mass of anode catalysts,the maximum power densities are 2.9 and 5.1 W·g-1for CE and Co2+/CE-catalyzed cell,respectively.Although the maximum power density here is lower than that of PEMFCs or DMFCs which need precious metal catalysts in anodes,the anode catalysts for sulfide oxidation are much cheaper.
The durability of single cell is another important factor for evaluating the feasibility of applicating alkaline sulfide in a fuel cell.In order to investigate the durability of the as-assembled alkaline sulfide fuel cells,50-hour life tests were performed under constant load resistance of 10 Ω.The result is illustrated in Fig.5.It can be seen that during the test time of 50 h,both the two cells output electric energy stably.For CE-catalyzed single cell,the cell voltage is stable at(0.24±0.01)V with power density of 5.4 mW·cm-2(2.5 W·g-1),and for Co2+/ CE-catalyzed fuel cell,it keeps at(0.27±0.01)V with power density of 7.2 mW·cm-2(3.3 W·g-1).No obvious decrease was observed during the whole test time of 50 h.Besides,the electrodes were examined after 50-hour tests,and no sulfur or other precipitation was observed on the electrode.

Fig.4 Cell voltage and power density versus current density curves of single cellsThe adopted alkaline sulfide fuel was composed of 1 mol·L-1Na2S and 1 mol·L-1KOH.

Fig.5 Life tests of single cells with load resistance of 10 Ω
3.3 Analysis of discharge products of sulfide
As for discharge of alkaline sulfide fuel cells,sulfide ions in the anode are oxidized to higher valence state and donate electrons,then electrons transfer from anode to cathode through outside circuit;while in the cathode,oxgen molecules accept the electrons and are reduced to lower valence state.From the previous reports,25-27sulfide ions could be oxidized to thiosulfate,sulfite,and sulfate.Kinetics of oxidation of aqueous sulfide by oxygen was studied and the reaction pathway was illustrated.Herein in order to discover the electrochemical oxidation process of sulfide,the discharge products were analyzed by ion chromatography.Single cell using cycled sulfide fuel was discharged with a constant resistance until voltage fell down to 0.15 V.The obtained products were diluted by 2000 times with ultrapure water.The spectrograms are illustrated in Fig.6 and the analytical results are showed in Table 1.It can be seen from Fig.6 and Table 1 that considerable amount of sulfide ions were oxidized to thiosulfate,sulfite,and even sulfate in all the single cells,which means that sulfides have donated 4,6,and 8 electrons during discharge,respectively.Besides, Co2+/CE-catalyzed cell has higher catalytical efficiency than CE-catalyzed cell when they set with the same load,which demonstrates the catalysis of cobalt salt for oxidation of sulfide in single cell tests(Fig.2).For example,when loaded with resistance of 20 Ω,28.0%,4.24%,and 11.9%of sulfide were oxidized to thiosulfate,sulfite,and sulfate by Co2+/CE-catalyzed cell,respectively,while for CE-catalyzed cell,the reults were 26.2%,2.14%,and 7.67%,respectively.As shown from ion chromatography results,the alkaline sulfide fuel underwent deep oxidation during the fuel cell application.Deep oxidation of sulfide makes it a fuel with high capacity,which means that every sulfide ion could contribute eight electrons at the most. Moreover,it should be noted that during the discharge the color of fuel changed from transparent to orange-like,indicating that polysulfide was formed as intermediate product during test.However,the orange colored electrolyte is not stable and fades back to transparent after discharge within several hours.

Fig.6 Products analysis by ion chromatography(a)Co2+/CE-catalyzed fuel cell,discharged with load of 20 Ω;(b)CE-catalyzed cell,discharged with load of 20 Ω;(c)Co2+/CE-catalyzed fuel cell with load of 10 Ω;(d)CE-catalyzed cell with load of 10 Ω;(e)standard samples were analyzed with peaks:(x)sulfite(0.01 mg·L-1),(y)sulfate(0.01 mg·L-1),and (z)thiosulfate(0.01 mg·L-1).The products were tested after dilution of 2000 times.During a typical test,the hydroxyl concentration was firstly kept at 16 mmol·L-1,and then adjusted to 50 mmol·L-1,and then adjusted back to 16 mmol·L-1to the end.The injection volume was 25 μL with a fluid flow rate of 1 mL·min-1.
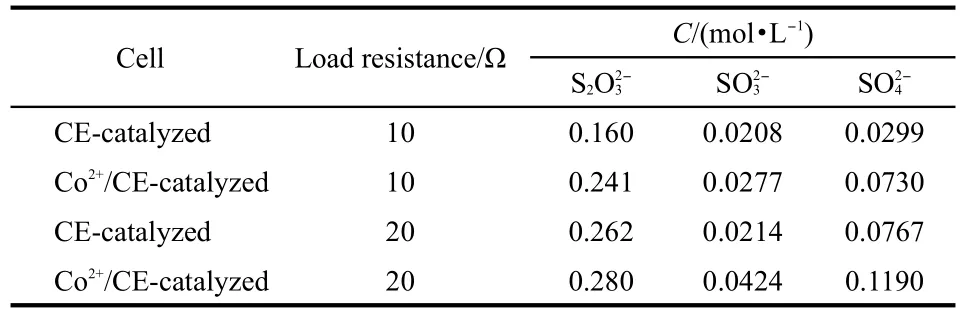
Table 1 Product analyses by ion chromatography
4 Conclusions
Alkaline sulfide fuel cells have been assembled by using alkaline sulfide fuel and non-precious anode catalysts,and single cell test shows that it could produce electric energy at room temperature with good durability.Our present work shows that alkaline sulfides have merits of low-cost and high electrochemical-activity,and could be catalyzed by non-precious catalysts. At room temperature,sulfide-fueled single cell records a maximum power density of 12.3 mW·cm-2with current density of 42.8 mA·cm-2by adopting non-precious anode catalysts.The life test indicates that alkaline sulfide fuel cells exhibit good durability.In this work,carbon and cobalt salt are used as catalysts.Although detailed cost of anode catalysts was not calculated,their principal elements are carbon and cobalt salts,and no expensive precursors or processing steps are required.The overall cost is much lower than that of Pt-based catalysts used in anodes of proton exchange membrane fuel cells(PEMFCs) or direct methanol fule cells(DMFCs).Besides,through ion chromatography analysis,considerable amount of thiosulfate, sulfite,and sulfate are detected.Deep oxidation of sulfide makes it a fuel with high capacity.In order to further improve the efficiency of the fuel cells,optimization of catalysts,cell assembly,and operation condition are required.
(1) Steele,B.C.H.;Heinzel,A.Nature 2001,414,345.
(2) Elam,C.C.;Padró,C.E.G.;Sandrock,G.;Luzzi,A.;Lindblad, P.;Hagen,E.F.Int.J.Hydrog.Energy 2003,28,601.
(3) Jain,I.P.Int.J.Hydrog.Energy 2009,34,7368.
(4) Holladay,J.D.;Hu,J.;King,D.L.;Wang,Y.Catal.Today 2009,139,244.
(5) Liu,H.;Song,C.;Zhang,L.;Zhang,J.;Wang,H.;Wilkinson, D.P.J.Power Sources 2006,155,95.
(6) Wasmus,S.;Küver,A.J.Electroanal.Chem.1999,461,14.
(7) Zhou,W.;Zhou,Z.;Song,S.;Li,W.;Sun,G.;Tsiakaras,P.; Xin,Q.Appl.Catal.B-Environ.2003,46,273.
(8)Antolini,E.J.Power Sources 2007,170,1.
(9) Serov,A.;Kwak,C.Appl.Catal.B-Environ.2010,98,1.
(10) Ma,J.;Choudhury,N.A.;Sahai,Y.Renew.Sust.Energ.Rev. 2010,14,183.
(11) Demirci,U.B.J.Power Sources 2007,169,239.
(12) Serov,A.;Kwak,C.Appl.Catal.B-Environ.2009,90,313.
(13) Liu,B.H.;Li,Z.P.;Suda,S.J.Electrochem.Soc.2003,150, A398.
(14)Asazawa,K.;Yamada,K.;Tanaka,H.;Oka,A.;Taniguchi,M.; Kobayashi,T.Angew.Chem.Int.Edit.2007,46,8024.
(15) Peramunage,D.;Licht,S.Science 1993,261,1029.
(16) Bendikov,T.A.;Yarnitzky,C.;Licht,S.J.Phys.Chem.B 2002, 106,2989.
(17) Remick,R.J.;Ang,P.G.P.ElectricallyRechargeableAnionically Active Reduction-Oxidation Electrical Storage-Supply System. U.S.PatentAppl.4485154,1984.
(18) Licht,S.Nature 1987,300,148.
(19) Hodes,G.;Manassen,J.;Cahen,D.J.Electrochem.Soc.1980, 127,544.
(20) Bolmer,P.W.Electrochemical Oxidation of Hydrogen Sulfide. U.S.PatentAppl.3249522,1966.
(21) Zito,R.;Kunz,L.J.Method of Operating a Fuel Cell Using Sulfide Fuel.U.S.PatentAppl.3920474,1975.
(22) Wang,Q.;Li,H.;Chen,L.;Huang,X.Carbon 2001,39,2211.
(23) Bidault,F.;Brett,D.J.L.;Middleton,P.H.;Brandon,N.P. J.Power Sources 2009,187,39.
(24) Gülzow,E.;Schulze,M.;Gerke,U.J.Power Sources 2006, 156,1.
(25) Chen,K.Y.;Morris,J.C.Environ.Sci.Technol.1972,6,529.
(26) Kleinjan,W.E.;Keizer,A.;Janssen,A.J.H.Water Res.2005, 39,4093.
(27) Fischer,H.;Schulz-Ekloff,G.;Wohrle,D.Chem.Eng.Technol. 1997,20,462.
July 26,2011;Revised:November 3,2011;Published on Web:November 9,2011.
Discharge Performance of Alkaline Sulfide Fuel Cells Using Non-Precious Anode Catalysts
FAN Yu-Qian SHAO Hai-Bo*WANG Jian-Ming LIU Liang ZHANG Jian-Qing CAO Chu-Nan
(Department of Chemistry,Zhejiang University,Hangzhou 310027,P.R.China)
The choice of fuel is an important issue influencing the selection of catalyst,cost,and commercialization of fuel cells.Electrochemically-active and low-cost fuels that can be oxidized by non-precious catalysts are an attractive objective.The native electrochemical activity and low cost of sulfide make it a suitable candidate.Fuel cells using alkaline sulfide as a fuel were developed.At room temperature,a single cell containing non-precious anode catalysts achieves a maximum power density of 12.3 mW·cm-2with a current density of 42.8 mA·cm-2.Life tests show that alkaline sulfide fuel cells exhibit good durability.Ion chromatography detected considerable amounts of thiosulfate,sulfite,and sulfate.The deep oxidation and high capacity of sulfide make it an attractive fuel candidate.Compared with other fuels, sulfide has the advantages of being inexpensive,easy to transport,possesses high electrochemical activity,and can be catalyzed by non-precious catalysts.
Fuel cell;Alkaline sulfide;Low-cost catalyst;Deep oxidation;Electrochemistry
10.3866/PKU.WHXB20122890 www.whxb.pku.edu.cn
*Corresponding author.Email:shaohb@zju.edu.cn;Tel:+86-571-87951513.
The project was supported by the Zhejiang Provincial Natural Science Foundation of China(Y406192)and Zhejiang Provincial Top Key Discipline of New Materials and Process Engineering,China(20110928).
浙江省自然科学基金(Y406192)与浙江省新材料及加工工程省重中之重学科开放课题(20110928)资助项目
O646
