南海柳珊瑚鳞海底柏化学成分的研究
2012-11-24黄丽丝张晓勇漆淑华
黄丽丝,贺 飞,王 萍,张晓勇,漆淑华*
1中国科学院南海海洋研究所,广州510301;2中国科学院研究生院,北京100049
南海柳珊瑚鳞海底柏化学成分的研究
黄丽丝1,2,贺 飞1,王 萍1,2,张晓勇1,漆淑华1*
1中国科学院南海海洋研究所,广州510301;2中国科学院研究生院,北京100049
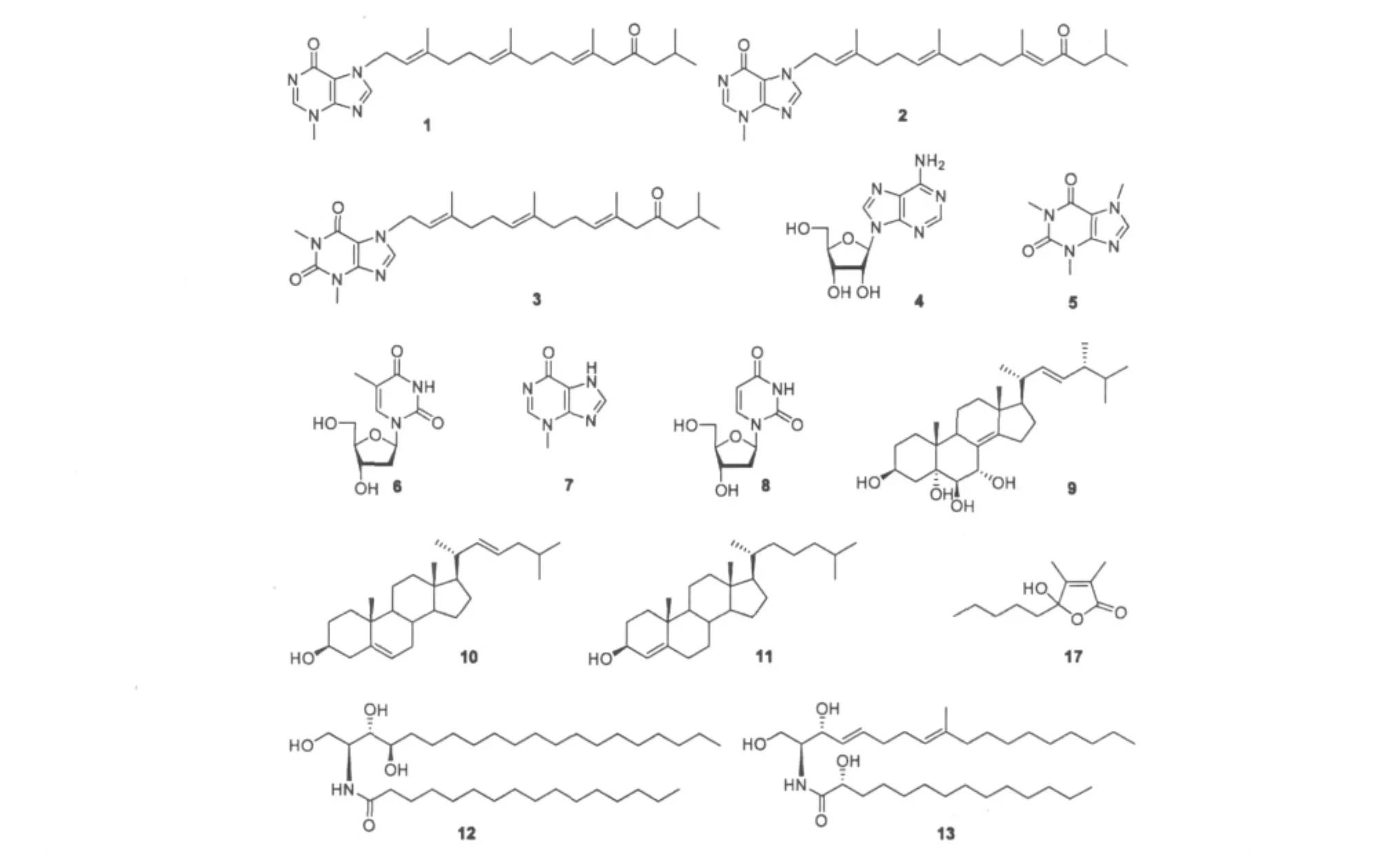
本文对南海柳珊瑚鳞海底柏Melitodes squarnata Nutting进行了化学成分的研究。通过用工业乙醇:二氯甲烷(2∶1)进行了提取,采用硅胶柱色谱、Sephadex LH-20凝胶色谱、高效液相半制备色谱和薄层制备等方法对鳞海底柏的化学成分进行了分离,并利用波谱分析技术和文献对照鉴定其结构。从鳞海底柏中分离得到了17个化合物,分别鉴定为:malonganenone E(1),malonganenone D(2),nuttingin A(3),腺嘌呤核苷(4),1,3,7-三甲基黄嘌呤(5),2'-脱氧胸腺嘧啶核苷(6),3-甲基-6-次黄嘌呤(7),2'-脱氧尿苷(8),(22E,24R)-ergosta-8(14),22-dien-3β,5α,6β,7α-tetrol(9),(22E)-胆甾-5,22-二烯-3β-醇(10),胆甾醇(11),(2S,3S,4R)-N-hexadecanoyl-2-amino-1,3,4-eicosanetriol(12),(2S,2'R,3R,4E,8E)-N-2'-Hydroxytetradecanoyl-2-amino-9-methyl-4,8-octadecaadiene-1,3-diol(13),鲨肝醇(14),十六烷基甘油醚(15),三油酸甘油酯(16)和4-hydroxy-2,3-dimethyl-2-nonen-4-olide(17)。
柳珊瑚;鳞海底柏;次生代谢产物
柳珊瑚(Gorgonian)俗称海扇、海鞭、海柳,广泛分布于世界热带、亚热带的各海域中。柳珊瑚属于肠腔动物门珊瑚虫纲八放珊瑚亚纲柳珊瑚目。鳞海底柏Melitodes squarnata Nutting属于柳珊瑚目中的海底柏科,有止咳止血,和味止泻,安神镇惊的功效,主治咳血,呕吐,腹泻,心神不安,怔忡烦乱,小儿惊风[1]。虽然国内外有关柳珊瑚化学成分的研究报道较多,但对于海底柏科属的研究报道却较少。日本科学家从赭色海底柏Melithaea ocracea中分离到4个甾体化合物:melithasterols A-D[2]。为阐明鳞海底柏的药用物质基础,我们对鳞海底柏的化学成分进行了研究。
1 仪器与材料
Brucker Avance 500型核磁共振波谱仪;高效液相色谱仪:岛津LC-20A,包括半制备柱ODS(250 mm×10 mm,5 μm),SPD-M20A检测器;Sephadex LH-20(Pharmacia Biotech Sweden);柱色谱用硅胶(100~200目,200~300目)和 TLC及 PTLC用GF254硅胶板为烟台江友硅胶开发有限公司生产,所用试剂均为分析纯产品。
南海柳珊瑚鳞海底柏(Melitodes squarnata Nut-ting)于2007年3月采自海南省三亚水域,由中国科学院南海海洋研究所李秀保博士和黄晖研究员鉴定。样品编号0802,标本保存于中国科学院南海海洋研究所广东省海洋药物重点实验室。
2 提取与分离
将采集的柳珊瑚鳞海底柏新鲜样品(湿重约10 kg)用工业乙醇-二氯甲烷(2∶1)浸泡三次,每次7天,提取液减压浓缩,得到膏状总提取物。膏状提取物加2倍体积蒸馏水混悬,依次用乙酸乙酯(4次)和正丁醇(4次)萃取,减压蒸馏,得乙酸乙酯部分35.17 g和正丁醇部分16.85 g。
乙酸乙酯部分装入正相硅胶柱(200~300目; V柱体积=1581 cm3;干法装柱),依次用氯仿,氯仿/丙酮系统(氯仿∶丙酮=9∶1,8∶2,7∶3,5∶5),氯仿/甲醇系统(氯仿∶甲醇=9∶1,8∶2,7∶3,5∶5),甲醇,梯度洗脱,每400 mL为一个流分,经硅胶TLC检查,合并相同流分,得17个组分(A—Q)。流分B经Sephadex LH-20(氯仿∶甲醇=1∶1,洗脱),得到4个流分(B-1—B-4)。B-3依次经RP-18(40%~100% MeOH/H2O,梯度洗脱),正相硅胶柱层析(石油醚∶乙酸乙酯=20∶1,10∶1,5∶1,2∶1,1∶1,乙酸乙酯,梯度洗脱),HPLC(65%甲醇水溶液,洗脱)得到化合物17(1.9 mg)。流分C经Sephadex LH-20(氯仿∶甲醇=1∶1,洗脱),得到2个流分(C-1和C-2)。C-2依次经RP-18(10%~100%MeOH/H2O,梯度洗脱),正相硅胶柱层析(石油醚∶丙酮 =10∶1,5∶1,2∶1,1∶1,丙酮,梯度洗脱),正相硅胶柱层析(石油醚∶丙酮 =10∶1,5∶1,2∶1,1∶1,丙酮,梯度洗脱),TLC (氯仿∶丙酮=10∶1)得到化合物10(5.9 mg)。流分D经正相硅胶柱层析(石油醚∶丙酮=8∶1,4∶1,2∶1,丙酮,梯度洗脱),得到6个流分(D-1—D-5)。D-4依次经Sephadex LH-20(氯仿∶甲醇=1∶1,洗脱),RP-18(30%-100%MeOH/H2O,洗脱),HPLC(28%甲醇水溶液,洗脱),得到化合物5(2.7 mg)。流分G经Sephadex LH-20(氯仿∶甲醇 =1∶1,洗脱),得到2个流分(G-1和G-2)。G-1析出晶体,洗涤得化合物12(10 mg)。G-2经正相硅胶柱层析(石油醚∶丙酮=4∶1,洗脱),得化合物15(6.9 mg)。流分I经正相硅胶柱层析(石油醚∶丙酮=15∶1,10∶1,5∶1,2∶1,1∶1,丙酮,洗脱),得到15个流分。I-3依次经Sephadex LH-20(氯仿∶甲醇=1∶1,洗脱),正相硅胶柱得化合物13(2.0 mg)、14(5.0 mg)。I-12依次经Sephadex LH-20(氯仿∶甲醇 =1∶1,洗脱),HPLC (50%甲醇水溶液,洗脱)制备,得化合物1(5.0 mg)、2(2.9 mg)、3(2.8 mg)。I-14依次经常压硅胶柱(石油醚:丙酮=6∶1-丙酮,梯度洗脱),Sephadex LH-20(氯仿∶甲醇=1∶1,洗脱),常压硅胶柱层析(石油醚∶丙酮=5∶1,洗脱),得化合物9(6.0 mg)。流分P经Sephadex LH-20(氯仿∶甲醇 =1∶1,洗脱),得到4个流分(P-1—P-4)。P-2析出晶体,洗涤得化合物16(5.2 mg)。
正丁醇部分装入中压正相硅胶柱,依次用氯仿,氯仿∶甲醇=95∶1,9∶1,8∶2,7∶3,6∶4,5∶5,甲醇,进行梯度洗脱,每500 mL为一个流分,经硅胶TLC检查,合并相同流分,得10个组分(a-j)。流分b经Sephadex LH-20(氯仿∶甲醇=1∶1,洗脱),得到4个流分(b-1—b-4)。b-4经硅胶柱层析(氯仿∶甲醇= 300∶1-7∶1,洗脱),得化合物11(2.5 mg)。流分d经Sephadex LH-20(氯仿∶甲醇=1∶1,洗脱),得到4个流分(d-1—d-4)。d-3经正相硅胶柱层析(氯仿∶甲醇=8∶1),得化合物6(2.5 mg)、7(2.0 mg)。流分e经Sephadex LH-20(氯仿∶甲醇=1∶1,洗脱),得到3个流分(e-1—e-3)。e-2析出晶体,洗涤得化合物4(5.8 mg)。e-3依次经正相硅胶柱(氯仿∶甲醇=6∶1,洗脱),PTLC(氯仿∶甲醇=3∶1),得化合物8 (6 mg)。
3 结构鉴定
化合物1 分子式 C26H38N4O2,白色粉末。1H NMR(CDCl3,500 MHz)δ:8.02(s,H-2),7.70(s,H-8),3.83(s,H-10),5.09(d,J=7.0 Hz,H-1'),5.47(t,J=7.0 Hz,H-2'),2.10(m,H-4'),2.12(m,H-5'),5.08(m,H-6'),1.93(m,H-8'),2.05(m,H-9'),2.50(t,J=8.0 Hz,H-10'),6.01(s,H-11'),2.27(d,J=7.0 Hz,H-14'),2.00(m,H-15'),0.90 (d,J=6.5 Hz,H-16',17'),1.85(s,H-18'),1.79 (s,H-19'),1.57(d,J=4.0 Hz,H-20');13C NMR (CDCl3,125 MHz)δ:147.3(C-2),147.3(C-4),115.0(C-5),162.5(C-6),140.1(C-8),34.8(C-10),44.4(C-1'),117.7(C-2'),143.2(C-3'),39.4 (C-4'),26.1(C-5'),123.5(C-6'),135.2(C-7'),40.7(C-8'),25.7(C-9'),33.4(C-10'),158.2(C-11'),124.0(C-12'),200.6(C-13'),53.5(C-14'),25.1(C-15'),22.6(C-16',17'),19.2(C-18'),15.9 (C-19'),16.5(C-20')。以上波谱数据与文献[3]对照基本一致,故鉴定化合物为malonganenone E。
化合物2 分子式 C26H38N4O2,白色粉末。1H NMR(CDCl3,500 MHz)δ:8.02(s,H-2),7.68(s,H-8),3.83(s,H-10),5.08(d,J=7.5 Hz,H-1'),5.47 (t,J=7.5 Hz,H-2'),2.10(m,H-4',5'),5.06(m,H-6'),2.00(m,H-8',9'),5.20(t,J=6.5 Hz,H-10'),2.99(s,H-12'),2.26(d,J=7.0 Hz,H-14'),2.08(m,H-15'),0.90(d,J=6.5 Hz,H-16',17'),1.59(s,H-18'),1.57(s,H-19'),1.78(s,H-20');13C NMR(CDCl3,125 MHz)δ:147.2(C-2),147.3 (C-4),115.0(C-5),162.8(C-6),140.1(C-8),34.8 (C-10),44.4(C-1'),117.6(C-2'),143.4(C-3'),39.7(C-4'),26.1(C-5'),123.6(C-6'),135.7(C-7'),39.4(C-8'),26.5(C-9'),130.9(C-10'),128.9 (C-11'),53.5(C-12'),200.6(C-13'),50.2(C-14'),25.4(C-15'),22.6(C-16',17'),19.1(C-18'),15.9(C-19'),16.5(C-20')。以上波谱数据与文献[3]对照基本一致,故鉴定化合物为malonganenone D。
化合物3 分子式 C27H41N4O3,白色粉末。1H NMR(CDCl3,500 MHz)δ:7.68(s,H-8),3.65(s,H-10),3.83(s,H-11),5.09(d,J=7.5 Hz,H-1'),5.48 (t,J=7.5 Hz,H-2'),2.05(m,H-4'),2.15(m,H-5'),5.06(t,J=6.5 Hz,H-6'),2.10(m,H-8'),2.15 (m,H-9'),5.25(t,J=6.5 Hz,H-10'),3.08(s,H-12'),2.25(d,J=6.5 Hz,H-14'),2.18(m,H-15'),0.97(d,J=6.5 Hz,H-16',17'),1.71(s,H-18'),1.59(s,H-19'),1.79(s,H-20');13C NMR(CDCl3,125 MHz)δ:150.3(C-2),147.9(C-4),108.8(C-5),157.6(C-6),140.1(C-8),27.7(C-10),29.7(C-11),44.4(C-1'),117.6(C-2'),145.1(C-3'),39.4 (C-4'),26.1(C-5'),123.5(C-6'),135.9(C-7'),39.2(C-8'),39.7(C-9'),128.8(C-10'),136.1(C-11'),52.4(C-12'),203.9(C-13'),50.7(C-14'),24.3(C-15'),22.5(C-16',17'),16.1(C-18'),15.9 (C-19'),16.4(C-20')。以上波谱数据与文献[3]对照基本一致,故鉴定化合物为nuttingin A。
化合物4 分子式 C10H13N5O4,白色针晶。1H NMR(DMSO-d6,500 MHz)δ:8.34(s,H-2),8.13(s,H-8),7.34(s,NH2),5.88(d,J=6.0 Hz,H-1'),4.61(dd,J=11.0,6.0 Hz,H-2'),4.16(dd,J=6.5,3.5 Hz,H-3'),3.96(dd,J=6.5,3.5 Hz,H-4'),3.68,3.55(m,H-5');13C NMR(DMSO-d6,125 MHz) δ:156.0(C-5),152.2(C-2),148.9(C-4),139.8(C-8),119.2(C-5),87.8(C-1'),85.7(C-4'),73.3(C-2'),70.5(C-3'),61.5(C-5')。以上波谱数据与文献[4]对照基本一致,故鉴定化合物为腺嘌呤核苷。
化合物5 分子式C8H10N4O2,白色晶体。1H NMR(CDCl3,500 MHz)δ:3.41(s,1-CH3),3.58(s,3-CH3),3.99(s,7-CH3),7.50(s,H-8);13C NMR (CDCl3,125 MHz)δ:29.1(N1-Me),27.1(N3-Me),29.3(N7-Me),153.7(C-2),150.9(C-4),104.8(C-5),146.7(C-6),138.2(C-8)。以上波谱数据与文献[5]对照基本一致,故鉴定化合物为1,3,7-三甲基黄嘌呤。
化合物6 分子式 C10H14N2O5,白色晶体。1H NMR(DMSO-d6,500 MHz)δ:7.69(s,H-6),6.16(t,J=6.5 Hz,H-1'),2.06(m,H-2'),4.23(s,H-3'),3.75(m,H-4'),3.57(m,H-5'),1.76(s,CH3),5.23 (br s,3'-OH),5.02(br s,5'-OH);13C NMR(DMSO-d6,125 MHz)δ:150.3(C-2),163.6(C-4),109.2 (C-5),136.0(C-6),83.6(C-1'),39.7(C-2'),71.2 (C-3'),87.1(C-4'),61.2(C-5'),12.1(CH3)。以上波谱数据与文献[6]对照基本一致,故鉴定化合物为2'-脱氧胸腺嘧啶核苷。
化合物7 分子式 C6H6N4O,白色晶体。1H NMR(DMSO-d6,500 MHz)δ:3.88(s,CH3),7.79 (s,H-8),8.29(s,H-2);13C NMR(DMSO-d6,125 MHz)δ:35.7(CH3),120.1(C-5),143.6(C-2),150.3(C-4),152.2(C-8),154.8(C-6)。以上波谱数据与文献[7]对照基本一致,故鉴定化合物为3-甲基-6-次黄嘌呤。
化合物8 分子式C9H12N2O5,白色晶体。1H NMR(DMSO-d6,500 MHz)δ:11.27(br s,H-3),5.62(d,J=8.2 Hz,H-5),7.85 1H,d,J=8.2 Hz,H-6),6.15(dd,J=6.5,7.2 Hz,H-1'),2.08(m,H-2'),4.23(m,H-3'),3.78(dd,J=7.1,3.8 Hz,H-4'),3.55(dd,J=12.1,3.8 Hz,H-5');13CNMR(DMSO-d6,125MHz)δ:150.4(C-2),163.1(C-4),101.7 (C-5),140.4(C-6),84.0(C-1'),40.0(C-2'),70.3 (C-3'),87.3(C-4'),61.1(C-5')。以上波谱数据与文献[8]对照基本一致,故鉴定化合物为2'-脱氧尿苷。
化合物9 分子式 C28H46O4,白色晶体。1H NMR(CDCl3,500 MHz)δ:3.97(m,H-3),2.10(dd,J=12.7,11.1 Hz,H-4),3.75(br s,H-6),3.39(br s,H-7),0.85(s,H-18),1.24(s,H-19),1.02(d,J= 6.5 Hz,H-21),5.16(dd,J=15.2,7.8 Hz,H-22),5.24(dd,J=15.2,5.9 Hz,H-23),0.90(d,J=6.5 Hz,H-26),0.92(d,J=6.5 Hz,H-27),0.93(d,J= 6.5 Hz,H-28);13C NMR(CDCl3,125 MHz)δ:17.0 (C-19),17.9(C-18),18.0(C-28),19.0(C-26),19.4(C-11),21.2(C-27),22.5(C-21),25.2(C-15),26.8(C-16),30.6(C-2),30.7(C-1),34.4(C-25),34,8(C-10),38.4(C-12),39.1(C-9,C-20),39.5(C-4),43.0(C-24),43.2(C-13),56.3(C-17),66.8(C-3),70.4(C-7),71.4(C-5),79.4(C-6),125.8(C-8),135.3(C-23),137.4(C-22),151,3(C-14)。以上波谱数据与文献[9]对照基本一致,故鉴定化合物为(22E,24R)-ergosta-8(14),22-dien-3β,5α,6β,7α-tetrol。
化合物10 分子式 C27H44O,白色晶体。1H NMR(CDCl3,500 MHz)δ:5.35(m,H-22),5.26(m,H-23),5.17(m,H-6),3.67(m,H-3),1.19(s,H-19),0.92(d,J=6.5 Hz,H-21),0.86(d,J=7.0 Hz,H-26),0.85(d,J=7.0 Hz,H-27),0.67(s,H-18);13C NMR(CDCl3,125 MHz)δ:37.2(C-1),31.6(C-2),71.8(C-3),42.3(C-4),140.8(C-5),121.7(C-6),31.9(C-7),31.9(C-8),50.1(C-9),36.5(C-10),21.1(C-11),39.8(C-12),42.3(C-13),56.8 (C-14),24.3(C-15),28.2(C-16),56.1(C-17),11.8(C-18),19.4(C-19),36.0(C-20),18.7(C-21),134.7(C-22),130.4(C-23),39.2(C-24),29.4 (C-25),24.3(C-26),24.4(C-27),以上波谱数据与文献[10]对照基本一致,故鉴定化合物为(22E)-胆甾-5,22-二烯-3β-醇。
化合物11 分子式 C27H46O,白色针晶。1H NMR(CDCl3,500 MHz)δ:3.53(m,H-3),5.35(t,J =2.3 Hz,H-6),0.68(s,H-18),1.01(s,H-19),0.91(d,J=7.2 Hz,H-21),0.84(d,J=6.5 Hz,H-26),0.82(3H,d,J=6.5 Hz,H-27);13C NMR (CDCl3,125 MHz)δ:37.2(C-1),31.6(C-2),71.8 (C-3),42.3(C-4),140.8(C-5),121.7(C-6),31.9 (C-7),31.9(C-8),50.1(C-9),36.5(C-10),21.1 (C-11),39.8(C-12),42.3(C-13),56.8(C-14),24.3(C-15),28.2(C-16),56.1(C-17),11.8(C-18),19.4(C-19),36.1(C-20),18.7(C-21),36.2 (C-22),23.8(C-23),39.5(C-24),28.0(C-25),22.8(C-27)。以上波谱数据与文献[11]对照基本一致,故鉴定化合物为胆甾醇。
化合物12 分子式C36H73NO4,白色针晶。1H NMR(CDCl3,500 MHz)δ:8.06(br d),5.07~5.12 (m,H-2),4.48~4.50(dd H-1),4.38~4.41(t,H-3),4.27~4.30 1H,t,H-4),4.49(br s),2.44~2.48(t,H-2''),1.94~1.96(m,H-5),1.24[m,(CH2)11,H-1',2',1''',2''',3'''],0.83~0.87(t,H-3');13C NMR(CDCl3,125 MHz)δ:62.5(C-1),54.2(C-2),76.4(C-3),74.1(C-4),34.0(C-5),31.2(C-6),31.2[(CH2)11]33.2(C-1'),26.8(C-2'),14.3(C-3'),31.2[(CH2)9],174.5(C-1''),36.9(C-2''),27.6(C-3''),31.2(C-4''),33.4(C-1''')。以上波谱数据与文献[12]对照基本一致,故鉴定化合物为(2S,3S,4R)-N-hexadecanoyl-2-amino-1,3,4-eicosanetriol。
化合物13 分子式C33H63NO4,白色针晶。1H NMR(CDCl3,500 MHz)δ:0.87(t,J=7.0 Hz,H3-18,H3-14'),1.25—1.33(br s,H2-12—H2-17,H2-5'—H2-13'),1.36(m,H2-11),1.50(m,Hb-3'),1.58(br s,H3-19),1.60(m,Ha-3'),1.95(dd,J= 6.5 Hz,H2-10),2.06(m,H2-7),2.11(m,H2-6),2.21(m,OH-3,OH-2',OH-1),3.42(br s,Ha-1),3.71(m,H-2),3.90(d,J=11.5Hz,Hb-1),4.26(br s,H-3),5.35(m,H-8),5.53(1H,m,H-4),5.78(m,H-5),6.37(d,J=8.1 Hz,NH)。以上波谱数据与文献[13]对照基本一致,故鉴定化合物为(2S,2'R,3R,4E,8E)-N-2'-Hydroxytetradecanoyl-2-amino-9-methyl-4,8-octadecaadiene-1,3-diol。
化合物14 分子式 C21H44O3,白色针晶。1H NMR(CDCl3,500 MHz)δ:3.72(1H,dd,J=11.4,3.7 Hz,H-1a),3.65(1H,dd,J=11.7,5.1 Hz,H-1b),3.86(1H,m,H-2),3.49(4H,m,H-3,-1'),1.58 (2H,m,H-2'),1.26(30H,br s,H-3'~17'),0.88 (3H,t,J=7.0 Hz,H-18')。以上波谱数据与文献[14]对照基本一致,故鉴定化合物为鲨肝醇。
化合物15 分子式 C21H44O4,白色蜡状固体。1H NMR(CDCl3,500 MHz)δ:4.72(d,J=5.1 Hz,2-OH),4.59(t,J=5.8 Hz,1-OH),3.85(m,H-2),3.67(m,H-3),3.44~3.54(m,H-1,1'),1.57 (m,H-2'),1.25(H-3'~15'),0.88(t,J=6.7 Hz,H-16')。以上波谱数据与文献[15]对照基本一致,故鉴定化合物为十六烷基甘油醚。
化合物16 分子式 C57H104O6,无色油状。1H NMR(CDCl3,500 MHz)δ:5.34(m,H-9',H-9'',H-10',H-10''),5.27(m,H-2),4.31(dd,J=11.7,6.0 Hz,H-1,H-3),4.16(dd,J=11.7,6.0 Hz,H-1,H-3),2.31 m,H-2',H-2''),0.88(t,J=2.5 Hz,H-18',H-18'')。以上波谱数据与文献[16]对照基本一致,故鉴定化合物为三油酸甘油酯。
化合物17 分子式 C11H18O3,白色针晶。1H NMR(CD3OD,500 MHz)δ:0.91(t,J=7.0 Hz,9-CH3),1.80(s,3-CH3),1.94(s,2-CH3),1.75(m,H-5),1,95(m,H-5),1.32-1.35(m,H-7),1.18(m,H-6),1.32(m,H-6),1.21(m,H-8),1.38(m,H-8);13C NMR(CD3OD,125 MHz)δ:174.5(C-1),160.4 (C-2),125.7(C-3),109.2(C-4),36.9(C-5),32.8 (C-7),23.8(C-6),23.5(C-8),14.2(C-9),10,8(2-Me),8.27(3-Me)。以上波谱数据与文献[17]对照基本一致,故鉴定化合物为4-hydroxy-2,3-dimethyl-2-nonen-4-olide。
1 Shao CL(邵长伦),Fu XM(傅秀梅),Wang CY(王长云),et al.Investigation on the status of coral reef resources and medicinal research in ChinaⅢ.Status of folk medicinal usage and medicinal research.Period Ocean Univ China(中国海洋大学学报,自科版),2009,39:691-698.
2 Kobayashi M,Kanda F.Isolation and structure of four novel oxygeneted sterols from a gorgonian coral Melithaea ocracea.J Chem Soc,Perkin Trans,1991,1:1177-1179.
3 Sorek H,Rudi A,Benayahu Y,et al.Nuttingins A-F and malonganenones D-H,tetraprenylated alkaloids from the Tanzanian gorgonian Euplexaura nuttingi.J Nat Prod,2007,70:1104-1109.
4 Wen YM(温燕梅),Qi SH(漆淑华),Zhang S(张偲).Studies on chemical constituents of gorgonian Junceella fragilis from South China Sea.J Trop Oceanol(热带海洋学报),2007,26:73-77.
5 Chen QG(陈清光),Zeng LM(曾陇梅),Su JY(苏镜娱).Study on the chemical constituents of Haliclona sp..Chin J Mar Drugs(中国海洋药物),1998,17:1-4.
6 Peng Y(彭燕),Zheng JX(郑建仙),Huang RM(黄日明),et al.Study on the nitrogen compounds from Asterina sp..Chin Tradit Herb Drugs(中草药),2010,41:208-210.
7 Lichtenberg D,Bergmann F,Ringel I.Assignment of individual signals of aromatic protons in NMR-spectrum of 6-substituted purines.J Magn Reson,1972,6:600-604.
8 Zou ZR(邹峥嵘),Yi YH(易杨华),Yao XS(姚新生),et al.Studies on Chemical Constituents of Acaudina molpadioides Semper.Chin J Nat Med(中国天然药物),2004,2:348-350.
9 Ishizuka T,Yaoita Y,Kikuchi M.Sterol constituents from the fruit bodies of Grifola frondosa(Fr.)SF Gray.Chem Pharm Bull,1997,45:1756-1760.
10 Xu YY(徐圆缘),Li L(李玲),Yi YH(易杨华),et al.Studies on chemical constituents of Anthogorgia sp..Acad J Sec Mil Med Univ(第二军医大学学报),2010,31:421-424.
11 Liu D(刘东),Lin WH(林文瀚),Deng ZW(邓志威),et al.Steroids from marine sponge Spheciospongia sp.from the South China Sea.J Shenyang Pharm Univ(沈阳药科大学学报),2010,27:191-194.
12 Liao L(廖柳),Wang N(王楠),Liang Q(梁秋),et al.Three ceramides from gorgonian Echinogorgia sp..Chin Tradit Herb Drugs(中草药),2010,41:851-854.
13 Yaoita Y,Kohata R,Kakuda R,et al.Ceramide constituents from five mushrooms.Chem Pharm Bull,2002,50:681-684.
14 Yao ZR(姚宗仁),Zhong HM(钟惠民).Studies on the Chemical Constituents of Soft Coral Lobophytum sp..J Qindao Univ Scien Technol(青岛科技大学学报),2009,30:135-137.
15 Li YB(李友宾),Xiang Y(相宇),Huang WH(黄卫华),et al.Chemical constituents from Hirudo nipponica Whitman.Strait Pharm J(海峡药学),2009,21:75-77.
16 Shao LJ(邵立军),Wang JN(王建农).Chemical constituents in roots of Boehmeria nivea.Chin Tradi Herb Drugs(中药材),2010,40:683-686.
17 Hasegawa T,Yamada K,Shigemori H,et al.Isolation and identification of a growth inhibitor from blue light-illuminated cress seedlings.J Plant Growth Regul,2002,37:45-47.
Chemical Constituents of South China Sea Gorgonian Melitodes squarnata Nutting
HUANG Li-si1,2,HE Fei1,WANG Ping1,2,ZHANG Xiao-yong1,QI Shu-hua1*1Guangdong Key laboratory of Marine Materia Medica,South China Sea Institute of Oceanology,Chinese Academy of Sciences,Guangzhou 510301,China;2Graduate School of Chinese Academy of Sciences,Beijing 100049,China
Seventeen compounds were isolated by silica gel column,sephadex LH-20 and HPLC chromatograph,and their structures were identified to be malonganenone E(1),malonganenone D(2),nuttingin A(3),adenosine(4),1,3,7-trimethyl-xanthine(5),2'-deoxythymidine(6),3-methyl-6-hypoxanthine(7),2'-deoxyuridine(8),(22E,24R)-ergosta-8(14),22-dien-3β,5α,6β,7α-tetrol(9),(22E)-cholest-5,22-dien-3β-ol(10),cholesterol(11),(2S,3S,4R)-N-hexadecanoyl)-2-amino-1,3,4-eicosanetriol(12),(2S,2'R,3R,4E,8E)-N-2'-hydroxytetradecanoyl-2-amino-9-methyl-4,8-octadecaadiene-1,3-diol(13),batyl alcohol(14),cetyl glycerin ether(15),glycerol trioleate(16),and hydroxy-2,3-dimethyl-2-nonen-4-olide(17)on the basis of spectroscopic analysis and comparison with literatures..
gorgonian;Melitodes squarnata;second metabolites
1001-6880(2012)04-0432-06
2011-05-20 接受日期:2011-11-15
国家自然科学基金项目(20872151,40976090)
*通讯作者 Tel:86-20-89022112;E-mail:shuhuaqi@scsio.ac.cn
R284.2
A
