Eupenicillium sp.E-UN41产活性物质的分离与结构分析
2012-11-23杨煌建张祝兰任林英唐文力
杨煌建,张祝兰,任林英,唐文力,郑 卫
福建省微生物研究所福建省新药(微生物)筛选重点实验室,福州350007
Eupenicillium sp.E-UN41产活性物质的分离与结构分析
杨煌建,张祝兰*,任林英,唐文力,郑 卫*
福建省微生物研究所福建省新药(微生物)筛选重点实验室,福州350007
在筛选微生物来源代谢产物的过程中,分离得到一株正青霉Eupenicillium sp.E-UN41,发酵液经离子交换树脂和葡聚糖凝胶等方法分离得到化合物UN41A。通过核磁共振波谱和X-单晶衍射进行该化合物的分子构型和晶体结构分析,确定其为4-氨甲酰基-1-β-D-呋喃核糖-5-羟基咪唑,与咪唑立宾同质。晶体属斜方晶系,空间群P212121,晶胞参数a=7.4897(7),b=11.3801(15),c=14.0090(13)Å,V=1194.0(2)Å3,Z=4,Dc =1.553g/m3,F(000)=592。晶体结构分析表明,分子中存在咪唑环共平面。
正青霉;分离纯化;晶体结构;咪唑立宾
Introduction
Microfungi are a rich source of chemical diversity[1,2]. They are the source of more than 50%of metabolites in the pharmaceutical industry in either form of native or derivatives[3,4].In our screening course for new antibiotics from microfungi,a strain designated as Eupenicillium sp.based on its morphological characters was isolated from a soil sample collected in the region of Yunnan province,China.In the further investigation of its secondary metabolite,the major metabolite was predicted to be nucleoside analog by means of HPLC-UV-MS analysis.Moreover,it exhibited appreciable activity against Candida albicans.Herein,this paper reported the isolation,structural elucidation and single crystal of the metabolite from the strain Eupenicillium sp.E-UN41.
Experimental
General experimental procedures
UV spectra was obtained in absolute distilled water on a Ultrospec 2000 spectrophotometer.NMR analysis (1H,13C,HSQC and HMBC)was recorded in DMSO-d6on a Mercury Plus 400 NMR spectrometer.ESI-MS and HREI-MS data were taken on a Applied Bio-systems Mariner system 5130 Mass Selective Detector.IR spectra was obtained with a NICOLET NEXUS 470 FTIR spectrophotometer.RP-HPLC was conducted on a Shimadzu 20A with PDA detector and Shimadzu series with LC20AD pump.Single-crystal structures were measured on RIGAKU-MERCURY-CCD with Mo-K αradiation.The Sephadex G10(Pharmacia)column used water as solvent.Hydroxy type of strong basic anion exchange resin(HZ202)and gel type exchange resin(HZ008)were purchased from huazhen Co.Ltd,shanghai.
Strain culture,extraction and isolation
The strain of Eupenicillium sp.E-UN41.was inoculated in the seed medium(starch 1.5 g/L,glucose 1.0 g/L,soybean 0.2 g/L,malt sugar 0.6 g/L,yeast extract 0.3 g/L,MgSO4·7H2O 0.1 g/L,adjusted to pH 7.0)at 26℃,250 rpm for 48 h.Then,the 5.0 mL pre-cultures were transferred to the fermentation medium(starch 3.0 g/L,soybean 3.5 g/L,MgSO4·7H2O 0.1 g/L,CaCO30.2 g/L,adjusted to pH 7.0)and was cultured at 28℃,250 rpm for 7 days.
An embodiment of isolation and purification of procedures for active compound UN41A was as follows:The broths filtrate(pH 6.0)was adjusted to pH 9.0 and passed through a hydroxyl type of strong basic anion exchange resin(HZ202),to absorb the compound.The resin was eluted with 2%aqueous acetic,and the eluate was concentrated in vacuo to obtain an oily residue. Then,the oily residue was dissolved in a small amount of water and passed through gel type exchange resin (HZ008)thereby obtaining said powder.Further purification can be carried out by Sephadex G10 column. Preparation of the single crystal
The prism crystal of re-crystallized in the mixture solution of ethanol and water(2:1)at constant temperature(25℃).
Crystallographic data collection and structure determination
A colorless crystal with dimensions 0.8×0.12×0.4 mm was used for data collection.Unit cell parameters and 3661 intensities were measured on RIGAKU-MERCURY-CCD with a graphite monochromatic Mo K α radiation(λ=0.71073)at 293(2)K by ω/2θ scanning.From a total of 2639 reflections corrected in the 3.08≤θ≤27.47°,2639 were independent with Rint =0.0130,of which 2579 observed reflections with I>2σ(I)were used in the structure determination. The positions and anisotropic thermal parameters of all nonhydrogen atoms were refined on F2by full-matrix least-squares techniques with SHELXTL program package.The final refinement converged at reached to R1= 0.0334,wR =0.0946(w =1/[σ2(FO2) + (0.0590P)2+0.1583 P],where P =(FO2+2 Fc2)/3)(for 2639 unique reflections),(Δ/σ)max =0.000,S=1.102,(Δρ)max=0.401 and(Δρ) min=-0.153 e/Å3.
Results and discussion
The crude extract of Eupenicillium sp.E-UN41 was fractionated and purified by ion exchange resin and column chromatography to give the component UN41A as white powder.
Compound UN41A was white powder with m.p.of 202-205℃,and easily soluble in water,hardly soluble in ethanol,insoluble in most common organic solvents. The UV spectra showed bands at 248 and 278nm.Typical IR absorptions(KBr tablet)at 3334.7,3146,1632,1538,1444,1299,1207,1056,982,681 cm-1,demonstrated the presence of hydroxyl,conjugated carbonyl,and carbamoyl groups,respectively.The molecular weight was 259.2813 corresponding to the molecular formula for C9H13N3O6(calcd.259.0804),indicating 5 degrees of unsaturation.ESI-MS(+)spectrum exhibited a molecular ion peak at m/z 260.1[M+ H]+,m/z 128.2[M+H-ribofuanose]+and m/z 111.3[M+H-ribofuanose-NH2]+.The1H,13C NMR and DEPT spectra(Table 1)indicated the presence of five methine protons,which one was at position 2 in the figure 1(δH8.30,δC124.7 ppm),two were oxymethine protons(δH4.03,δC70.1;δH5.50,δC87.0 ppm),two were alkyl methine protons bearing oxygen (δH3.86,δC85.4;δH4.37,δC73.1 ppm),one methylene protons bearing oxygen(δH3.48,δH3.60,δC61.2 ppm),and three quaternary carbonyl carbons (δC99.0,155.1,161.7 ppm)including one carbonyl carbon(δC161.7 ppm).Two1H signals at δH6.75,δH7.04 ppm belong to aminocarbonyl in the1H NMR. Thus,the structure of UN41A was deduced to be 4-carbamoyl-1-β-D-ribofuranosyl imidazolium-5-olate by NMR spectra and the physico-chemical properties.All these data were very similar to mizoribine(Fig.1)re-ported in reference[5,6].

Table 1 1H and13C NMR data of UN41A(DMSO-d6,δH and δCin ppm,J in Hz)

Fig.1 Chemical structure of UN41A
In order to elucidate the three-dimensional structures of the molecule,we undertook an X-ray crystallographic analysis of its crystals(C9H13N3O6·H2O,F.W.277. 22).The crystals belonged to the orthorhombic P212121space group with a=7.4897(7),b =11.3801 (15),c=14.0090(13)Å,V=1194.0(2)Å3,Z =4,Dc=1.553g/m3,F(000)=592.Selected bond lengths and bond angles were listed in Table2,3.
The molecular structure thus determined,shown in Fig. 2,approved that UN41A was designated as 4-carbamoyl-1-β-D-ribofuranosyl imidazolium-5-olate.It was assumed that a zwitterion structure was in the aglycone (4(5)-carbamoyl imidazolium-5(4)-olate).The proton originally bonded to the O(2)atom can be regarded as being transferred to the N(3)atom.The O(2) atom lies on the imidazole ring plane,while the atoms, C(1),O(1),N(1),and C(5),deviated from the plane.One of the amino hydrogen atoms participates in the intramolecular hydrogen bond between N(1)and O (2).The ring puckering of the ribose moiety was described as C(7)-endo.The C(7)atom deviated from the mean plane of the remaining ring atoms toward the same side as C(9).
The bonds N(3)-C(2),C(2)-C(3),and C(3)-N (2),of UN41A are slightly longer than those reported[7],suggesting that a valence bond structure 2 contributes considerably in addition to 1.The structure 2 also explained well that the exocyclic bond lengths of C (1)-C(2)and C(3)-O(2)are shorter than the typical single bond lengths expected for C(sp')-C(sp') and C(sp')-O,respectively,and the C(1)-O(1) length was longer than the C=O double bond length.It was noted that structures similar to 2 undoubtedly.

Fig.2 Three-dimensional crystalline structure of UN41A
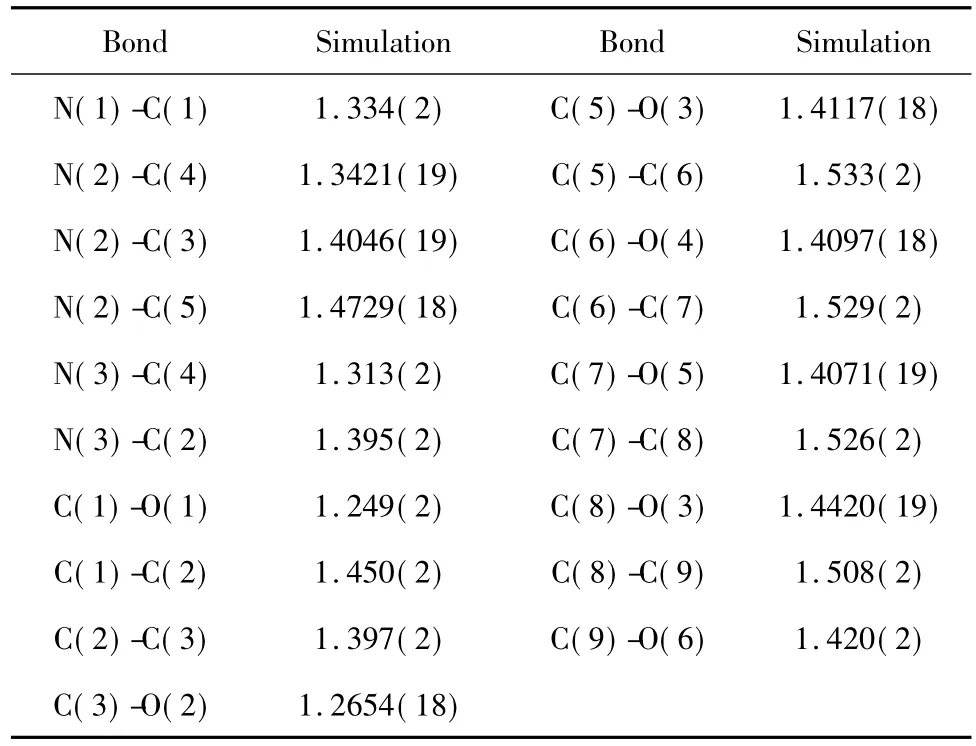
Table 1 The bond length data of UN41A crystal
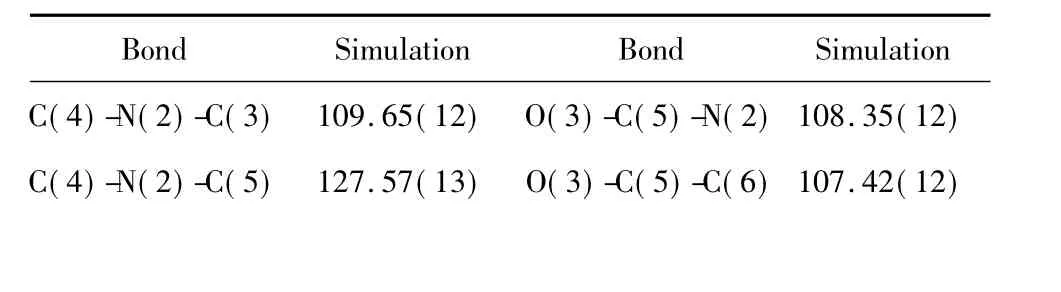
Table 3 The bond angle data of UN41A crystal

C(3)-N(2)-C(5) 122.54(12) N(2)-C(5)-C(6)113.05(11) C(4)-N(3)-C(2) 109.32(13) O(4)-C(6)-C(7)108.03(12) O(1)-C(1)-N(1) 122.95(15) O(4)-C(6)-C(5)110.14(12) O(1)-C(1)-C(2) 121.20(15) C(7)-C(6)-C(5)101.24(12) N(1)-C(1)-C(2) 115.78(14) O(5)-C(7)-C(8)114.52(13) N(3)-C(2)-C(3) 107.22(13) O(5)-C(7)-C(6)114.88(13) N(3)-C(2)-C(1) 123.80(14) C(8)-C(7)-C(6)101.62(12) C(3)-C(2)-C(1) 128.23(14) O(3)-C(8)-C(9)108.76(14) O(2)-C(3)-C(2) 132.35(14) O(3)-C(8)-C(7)104.72(11) O(2)-C(3)-N(2) 123.04(13) C(9)-C(8)-C(7)116.17(13) C(2)-C(3)-N(2) 104.59(13) O(6)-C(9)-C(8)112.88(14) N(3)-C(4)-N(2) 109.19(14) C(5)-O(3)-C(8)109.97(11)
In conclusion,the compound UN41A in the broth filtrate of Eupenicillium sp.E-UN41 was structurally elucidated as mizoribine,crystalline structure similar to 2. Mizoribin,also named Bredinin,was marketed in Japan,South Korea and China as an immunosuppressive agent for renal transplantation[8].Mizoribine produced less hepatotoxicity and bone marrow suppression than Azathioprine did,and the combination of mizoribine with Cyclosporin A efficiently prevented allograft rejection in humans.Mizoribine was also used for autoimmune diseases.The biological activities and the unique imidazole structure of the aglycone made it as an attractive target for further studies.
1 Frisvad JC,Thrane U,Filtenborg O,et al.Chemical Fungal Taxonomy.New York:Marcel Dekker,1998.289.
2 Mantle PG.Secondary Metabolites of Penicillium and Acremonium.New York:Plenum Press,1987.
3 Bull AT,Ward AC,Goodfellow M.Search and discovery strategies for biotechnology:the paradigm shift.Microbiol Mol Biol R,2000,64:573-606.
4 Bentley R.Mycophenolic acid:A one hundred year odyssey from antibiotic to immunosuppressant.Chem Rev.2000,100:3801-3825.
5 Gu RL,Tong WG,Pu J,et al.An improved total synthesis of Bredinin-a novel immunosuppressive agent.J Pharm Ind Chin,1986,17:33-35.
6 Fukukawa K,Sshuto A,Hirano T,et al.Synthesis of Bredinin from 1-β-D-ribofuranosyl-5-imidazolium-4-carboxamide by a photo-reaction.Chem Pham Bull,1986,34:3653-3657.
7 Karle J,Karle IL.The symbolic addition procedure for phase determination for centrosymmetric and noncentrosymmetric crystals.Acta Cryst,1966,21:849-859.
8 Yokota,S.Mizoribine:mode of action and effects in clinical use.Pediatr Int,2002,44:196-198.
Feburary 8,2012;Accepted March 28,2012
This research was supported by the funds of national major programs on new drug creation during the 11th Five-Year Plan Period(2010ZX09401-403)and Science and Technology Planning Project of Fujian Province(2009R10003-3)
Isolation and Structure Elucidation of an Active Metabolite from Eupenicillium sp.E-UN41
YANG Huang-jian,ZHANG Zhu-lan*,REN Lin-ying,TANG Wen-li,ZHENG Wei*
Fujian Provincial Key Laboratory of Screening for Novel Microbial Products,Fujian Institute of Microbiology,Fuzhou 350007
Eupenicillium sp.E-UN41 was obtained in the course of screening for bioactive microbial products,the compound UN41A was isolated by column chromatography of ion exchange resin and Sephadex G10,and purified by crystallization.The configuration and stereochemistry structure of UN41A were determined by NMR spectra and single crystal X-ray diffraction.UN41A was proved to be 4-carbamoyl-1-B-D-ribofuranosyl imidazolium-5-olate,the same structure as the immunosuppressant,mizoribine.The crystal of UN41A belonged to the orthorhombic P212121space group with a= 7.4897(7),b=11.3801(15),c=14.0090(13)Å,V=1194.0(2)Å3,Z=4,Dc=1.553g/m3,F(000)= 592.The crystal of UN41A revealed that there was a imidazole ring plane.
Eupenicillium;isolation and purification;crystal structure;Mizoribine
1001-6880(2012)09-1172-04
*Corresponding authors Tel:86-591-83470740;E-mail:jessylan9963@ sina.com;wzheng@pubz.fz.fj.cn
Q939.97;R979.5
A
