球形PVC-MnO2离子筛的制备及锂吸附性能
2012-11-09肖国萍童柯锋孙淑英于建国
肖国萍 童柯锋 孙淑英 于建国
球形PVC-MnO2离子筛的制备及锂吸附性能
肖国萍 童柯锋 孙淑英 于建国*
(华东理工大学,国家盐湖资源综合利用工程技术研究中心,上海 200237)
本实验室前期所制备的Li4Mn5O12超细粉末在卤水体系中对Li+具有较大的吸附容量和良好的选择性。但由于超细粉体的流动性和渗透性差,无法直接应用于固定床,需对粉末吸附材料进行成型造粒,以便于实际应用。本论文采用聚氯乙烯为粘结剂,制备出粒径约为2.0~3.5 mm的球形PVC-Li4Mn5O12,经盐酸处理后得到球形PVC-MnO2离子筛。并通过扫描电镜(SEM)、X射线衍射仪(XRD)、静态和动态连续锂吸附实验研究了球形离子筛形貌和锂离子吸附性能。结果表明,球形离子筛对Li+的吸附容量高达5.28 mmol·g-1,在混合溶液中对Li+具有良好的选择性,这对于在盐湖卤水或海水提锂具有重要的实用意义。
PVC-MnO2;离子筛;锂;吸附;成型
Interest in lithium element has been increasing because of wide applications in lithium batteries[1-3]and other fields[4].The requirement of lithium element has been increasing by 7%~11%per year.Brine is considered to be an important source of lithium element[5-6].Various methods have been studied for the recovery of lithium from brine/salt lake,such as adsorption[7-9],solvent extraction[10-13]and precipitation.Among the various recovery methods,the adsorption method is the most promising one for the separation system with a high ratio of Mg/Li from economic and environmental viewpoints.
Li-Mn-O ternary oxides were used as ion-sieve for lithium recovery from aqueous solution including brine and seawater[14-17],with extremely high selectivity for Li+and stability,since the Li-Mn-O framework could maintain the cubic spinel structure[18]during the Li+inserting and extracting process[19-20].A series of lithium manganese oxide adsorbent with different Li/Mn molar ratio, including LiMn2O4[21-22], Li1.33Mn1.67O4[19,23],Li1.6Mn1.6O4[24-27]were prepared via sol-gel method[28-32],solid-state reaction[33]and hydrothermal process[16].In our lab,Li4Mn5O12ultrafine powder was synthesized via a combination of hydrothermal reaction and a lowtemperature solid-phase calcinations process[14,34].It had the high adsorption capacity with the maximum Li+adsorption capacity as 6.62 mmol·g-1,and the high selectivity for Li+in solution(containing Li+,Na+,K+and Mg2+).
However,the lithium ion-sieve is stillnot employed on large scale,because ion-sieve is prepared in ultrafine powder.It is very difficult to use the ultrafine powder in a continuously dynamic operation due to the powder easily leading to flow out of the adsorber with the liquid.To overcome this practical problem,some scholars have tried to fix lithium ionsieve on different supporting materials like silica gel[35],cellulose-gel[36],polyacrylic hydrazide[37],non-woven fabric[38],polysulfone[38],and pitches[39].But none of these methods led to real success,because these preparation procedures are rather complicated and the loading amount of lithium ion-sieve in the composite material is very low[35].Furthermore,the binders tended to dissolve or hydrolyze[37]in aqueous solution.Finally,the combination of the supporting material skeleton and the lithium ion-sieve became loose during the adsorption and elution process[39].In the practical application,the separation material shaped is expected to be steady in the long-period adsorption-desorption cycles.Poly vinyl chloride (PVC)is very stable in alkaline or acid aqueous solution.Umeno[40]reported membrane type adsorbents using Li1.33Mn1.67O4powder embedded in PVC membrane.It can be operated under natural seawater flow.But the loading amount of powder is limited.And we have developed spherical PVCLiMn2O4composite material[41];it well maintained the property of the powder.But the capacity of λ-MnO2(H type of LiMn2O4)is very small and the Mn solution loss is great.So it has poor cycle ability.
In this paper,we developed spherical PVCLi4Mn5O12composite material with diameter of 2.0~3.5 mm by anti-solvent method,where using Li4Mn5O12ultrafine powder as ion-sieve precursor,poly vinyl chloride (PVC)as binder,N-methyl-2-pyrrolidone(NMP)as solvent.And then PVC-Li4Mn5O12was treated by hydrochloride acid to obtain the corresponding spherical PVC-MnO2ion-sieve.The prepared spherical ion-sieve was characterized by Scanning Electron Microscopy(SEM).And the adsorption and desorption behavior of the spherical PVC-MnO2ion-sieve was evaluated by the experimental measurements in the batch and fixed bed adsorber.The adsorption capacity and selectivity for Li+on the prepared spherical PVCMnO2ion-sieve were evaluated.
1 Experimental
1.1 Preparation and characterization of spherical PVC-MnO2ion-sieve
Li4Mn5O12ultrafine powder was synthesized via a combination of hydrothermal reaction and low-temperature solid-phase calcinations process in our lab,with the size about 20~140 nm in diameter and 0.8~4.0 μm in length[14,34].Synthetic route was as follows:the mixture solution of MnSO4(0.33 mol·L-1)and(NH4)2S2O8(0.33 mol·L-1)was added into a teflon-coated stainless autoclave;then the autoclave was sealed and heated at 423 K for 12 h,then cooled naturally to room temperature.The black precipitate was filtrated,washed completely with deionized water and dried at 393 K for 8 h to obtain β-MnO2(named as MO).The Li4Mn5O12precursor(named as LMO)was prepared by the wet impregnation with an aqueous solution of LiNO3(1 mol·L-1,nLi/nMn=1)into MO,then the mixture was heated to remove water by a rotary evaporator,dried at 393 K for 12 h and calcined at 673 K for 120 h in static air.
The spherical PVC-Li4Mn5O12was prepared by the anti-solvent method using poly vinyl chloride(PVC)as the binder(polymerization degree:1000±20,industrial grade,Shanghai Nuotai Chemical Co.,LTD).PVC was dissolved in N-methyl-2-pyrrolidone(NMP)(analytical reagent,Shanghai Chemical Co.,Shanghai,China)by stirring until PVC dissolved completely,then the Li4Mn5O12ultrafine powder was added into the solution and mixed uniformly.The slurry was dripped into deionized water and washed completely,and finally dried at 378 K for 12 h in static air to obtain spherical adsorbent with particle diameter of 2.0~3.5 mm.The spherical samples were prepared as following:66 mL of NMP,20.00 g of Li4Mn5O12and different content of PVC(7.00,6.00,4.87,3.50 and 3.00 g).Corresponding ionsieves are named as LMO-7.00,LMO-6.00,LMO-4.87,LMO-3.50,and LMO-3.00,respectively.A spherical PVC-MnO2(H typeofPVC-Li4Mn5O12)ion-sieve material can not be obtained when PVC adding amount is reduced to 3.00 g because of low viscosity.The spherical LMO samples were treated with a hydrochloric acid solution (1.0 mol·L-1,nH/nLi=2)(analytically reagent,Shanghai Chemical Co.,Shanghai,China)to extract lithium,resulting in a spherical PVCMnO2ion-sieve,remarked as SMO-7.00,SMO-6.00,SMO-4.87 and SMO-3.50,correspondingly.The powder MnO2ion-sieve (named as SMO)was obtained by extracting Li+from Li4Mn5O12precursor(LMO)powder with HCl solution.
The bulky phase of the powder samples(MO,LMO and SMO)was examined by X-ray diffraction(XRD)analysis using a Rigaku D/max 2550 X-ray diffractometer with Cu Kα radiation(λ=0.154 056 nm),operating at 40 kV,100 mA and scanning rate of 10°·min-1.The surface morphology and cross-section morphology were analyzed by Scanning Electron Microscopy (SEM,JSM-6360LV,JEOL,Japan).Samples for SEM were covered by gold to improve their electric conductivity before analysis.
1.2 Adsorption experiments in batch adsorber using spherical PVC-MnO2ion-sieve
The solution with pH=10.1 was adjusted by the buffer solution comprised of 0.1 mol·L-1NH4Cl and 0.1 mol·L-1NH3·H2O,the NH4Cl/NH3·H2O molar ratio being 0.25.
The Li+adsorption kinetics were investigated by stirring (150 r·min-1)sphericalSMO samples(containing about 0.10 g MnO2)in 100.0 mL LiCl solution(pH=10.1)with uniform initial concentration of lithium ions(10.0 mmol·L-1)at 303 K.The exchange capacity of MnO2(Qt)was calculated according to equation (1),in which,Qtis the amount of metal ions adsorbed by MnO2at t time (mmol·g-1);Ct,concentration of Li+in solution at t time (mol·L-1);C0,initial concentration of Li+in solution (mol·L-1);V,solution volume(mL);W,weight of MnO2(g).
Qt=(C0-Ct)·V/W (1)
The Li+adsorption isotherms were carried out by stirring (150 r·min-1)spherical SMO samples(containing about 0.10 g MnO2)in 100 mL LiCl solution with differernt initial Li+concentration (1.94,4.07,6.25,7.93,9.75,11.96,16.58,20.13,23.99 mmol·L-1)for 96 h at 303 K.
The selectivity of the spherical PVC-MnO2ionsieve to Li+,Na+,K+,Ca2+and Mg2+ions in the mixture solution was investigated by the experimental measurements,where stirring 0.10 g MnO2containing in spherical PVC-MnO2in 10.0 mL solution (pH=10.1)containing Li+,Na+,K+,Ca2+and Mg2+of about 10.0 mmol·L-1for each one,respectively,for 96 h at 303 K.The concentrations of all metal ions in supernatant solution were determined in situ by Metrohm 861 Advanced Compact Ion Chromatograph (Metrohm Co.Ltd.,Switzerland)with Metrosep C4-100 column(1.7 mmol·L-1HNO3and 0.7 mmol·L-1dipicolinic acid as eluent).The exchange capacity of MnO2at equilibrium(Qe),distribution coefficient(Kd),separation factor(αLiMe)and concentration factor(CF)are calculated according to equation (2~5),in which,Qeis the amount of metal ions adsorbed per gram of MnO2(mmol·g-1);Ce,concentration of metal ions in solution at equilibrium(mol·L-1);C0,initial concentration of metal ions in solution(mol·L-1);V,solution volume(mL);W,weight of MnO2containing in PVC-MnO2(g).

pH titration curve was examined via Topp-Pepper method[42]by putting spherical SMO-4.87(containing about 0.10 g MnO2)into the conical flask with 10.0 mL 0.1 mol·L-1(LiCl+LiOH)mixture of different volume as shown in Table 1 and using 827 pH Lab (Metrohm Co.Ltd.,Switzerland)to analyze pH values in situ.
1.3 Breakthrough/elution experiments of lithium ion in fixed bed packed with spherical PVCMnO2ion-sieve
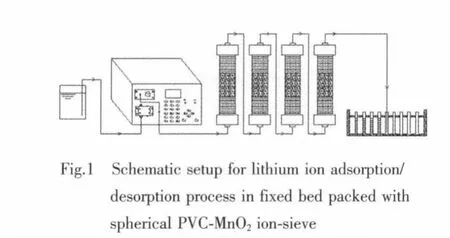
During dynamic adsorption process,the spherical SMO-4.87 ion-sieve were packed in a column(15),and then aqueous solution containing Li+was passed at a flow rate of 1.0 mL·min-1through the column from the bottom.After the column reached saturation,the column was washed with water and then eluted by 1.0 mol·L-1hydrochloric acid solution.The diagram of the flow sheet is shown as Fig.1.
In the fixed bed packed with SMO-4.87 sample,the adsorption/desorption experiments were carried out by inputting a pure Li+solution containing 10.0 mmol·L-1LiCl(pH=10.1)and mixture solution (pH=10.1)containing Li+,Na+,K+,Ca2+and Mg2+of about 10.0 mmol·L-1for each one,respectively.Details were as followings:solution containing 10.0 mmol·L-1LiCl(pH=10.1)was passed through the column (15 ×250 mm).The loading amount of SMO-4.87 is 6.88 g,containing 5.44 g MnO2.The operation conditions were as follows:loading height of the spherical ion-sieve SMO-4.87 was 16 cm;flow rate was 1.0 mL·min-1;liquid hourly space velocity(LHSV)was 2.12 h-1.After the adsorption process,the column was washed by water,then eluted by 1.0 mol·L-1HCl solution.
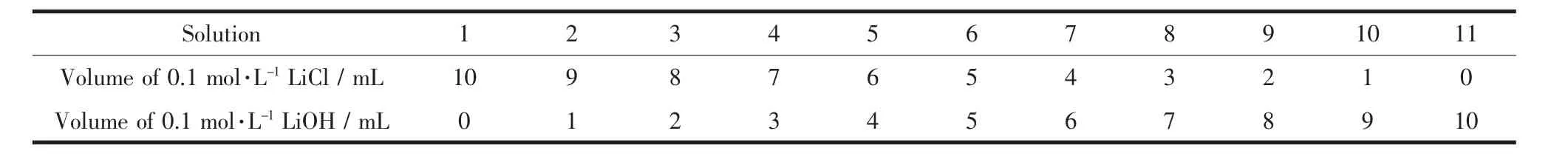
Table 1 Initial composition of solution for pH titration
A similar experiment was carried out by using a mixture solution(pH=10.1)containing Li+,Na+,K+,Ca2+and Mg2+of about 10.0 mmol·L-1for each one,respectively.The operation conditions were as follows:packed height of the spherical ion-sieve SMO-4.87 is 15 cm;flow rate was 1.0 mL·min-1;LHSV was 2.26 h-1.Then the column was washed by water,and eluted by 1.0 mol·L-1HCl solution.
2 Results and discussion
2.1 Material characterization
The powder XRD diffraction patterns of MO,LMO and SMO were presented in Fig.2.The reflections of MO can be readily indexed to pure β-MnO2phase[S.G.:P42/mnm (136),PDF No.24-0735]with lattice constant a=b=0.4405 nm,c=0.2869 nm.The LMO could be readily indexed to pure cubic phase Li4Mn5O12[space group:Fd3m (227),PDF No.46-0810]with lattice constant a=0.8165 nm,The SMO sample could be indexed to pure cubic phase λ-MnO2[space group:Fd3m(227),PDF No.44-0992]with lattice constant a=0.805 7 nm calculated according to the equation of 1/d2=(h2+k2+l2)/a2.It should be noticed that the XRD patterns of the LMO precursor and SMO ion-sieve were quite similar assembling the same cubic phase with lattice constants of 0.816 5 nm and 0.805 7 nm,respectively.The Li-Mn-O oxide was very stable during the Li+extraction process and the locations of manganese in the crystalstructure were wellmaintained,inducing a strong steric effectand thermodynamic potential for Li+.

The spherical PVC-Li4Mn5O12can not be prepared when PVC adding content was lower than 3.00 g in 66 mL NMP solution.Because the adding amount of PVC in NMP solution decides the cohesive force of PVC-NMP solution.When PVC dosage was too low,the powder of ion-sieve can easily leak out from the sphere during the adsorption-desorption process.When PVC adding content was large than 3.50 g in 66 mL NMP solution,the loss of the ultra fine powder in the sphere was less than 2.0%.Fig.3 showed the SEM images of SMO-7.00,SMO-6.00,SMO-4.87 and SMO-3.50,respectively.The ion-sieves after granulation were very regular sphere with diameter of 3.5 mm (as shown in Fig.3 (A)).The diameter of spherical ion-sieve was determined by the dropper size and the velocity of the slurry dripped into water.The outer surface of the sphere was very compact,and there were just only a few pores in the outer surface.The composite material maintains the porous structure through PVC network with a lot of large pores in the internal structure(as shown in Fig.3 (H)). This three-dimensional interpenetrating network can provide favorable conditions for ion mobility during the subsequent Li+adsorption and desorption process.By comparing the outer surface images B,C,D and E,it showed that the exposed dosage of MnO2in the outer surface was lower with the increasing of PVC adding content.But the cross-section images were not affected by PVC adding content.Comparing the outer surface and the crosssection of the spherical ion-sieve,it can be found that MnO2ion-sieve packed in the interior of the particle more loosely.

2.2 Li+adsorption kinetics on spherical PVC-MnO2 ion-sieve
Li+adsorption kinetics on spherical PVC-MnO2ion-sieve and ion-sieve powder (SMO samples)are respectively compared with a first-orderkinetics Lagergren equation(6).

In which,kadswas adsorption rate constant(s-1);t wascontacttime (h).Asshown in Fig.4,the adsorption rate of SMO-4.87 was fast till the contact time of 12 h,then the adsorption rate increased slowly and the process was close to the equilibrium after 48 h with the maximum adsorption capacity of about 4.33 mmol·g-1,which is less than that of the powder ionsieve(5.62 mmol·g-1)[14,34].The value of the adsorption rate constant was calculated to be 1.18×10-5s-1,decreased one order compared with the powder(3.29×10-4s-1)[14].Compared with other samples,adsorption capacityofSMO-7.00wassmall.Themaximum adsorption capacity was just 1.70 mmol·g-1,decreased to 30.25%compared with the powder ion-sieve[14,34].The resultsshow thatthe saturation adsorption capacity and adsorption rate were seriously affected by the granulation process.In the solution system,the mass transfer process between solid adsorbent and liquid adsorbate includes the following four continues steps: convection-diffusion, membrane-diffusion,particle-diffusion and ion-exchange or chemical adsorption.As to the Li+adsorption process,the particle-diffusion step is the rate-controlstep[43],therefore the larger particle size would aggravate the particle-diffusion effect.Moreover,PVC could block the structure pore and decrease the hydrophilicity of lithium ion-sieve, which leads to decrease of adsorption capacity and adsorption rate.This negative effect has been found in foam-type lithium adsorbent using pitches as binder,which capacity was about 2.21 mmol·g-1much less than that of powder(3.00 mmol·g-1)[39].The effect was easily seen during the granulation process,and further research to reduce the negative effect has been in progress.

2.3 Adsorption isotherm of lithium ion on spherical PVC-MnO2ion-sieve
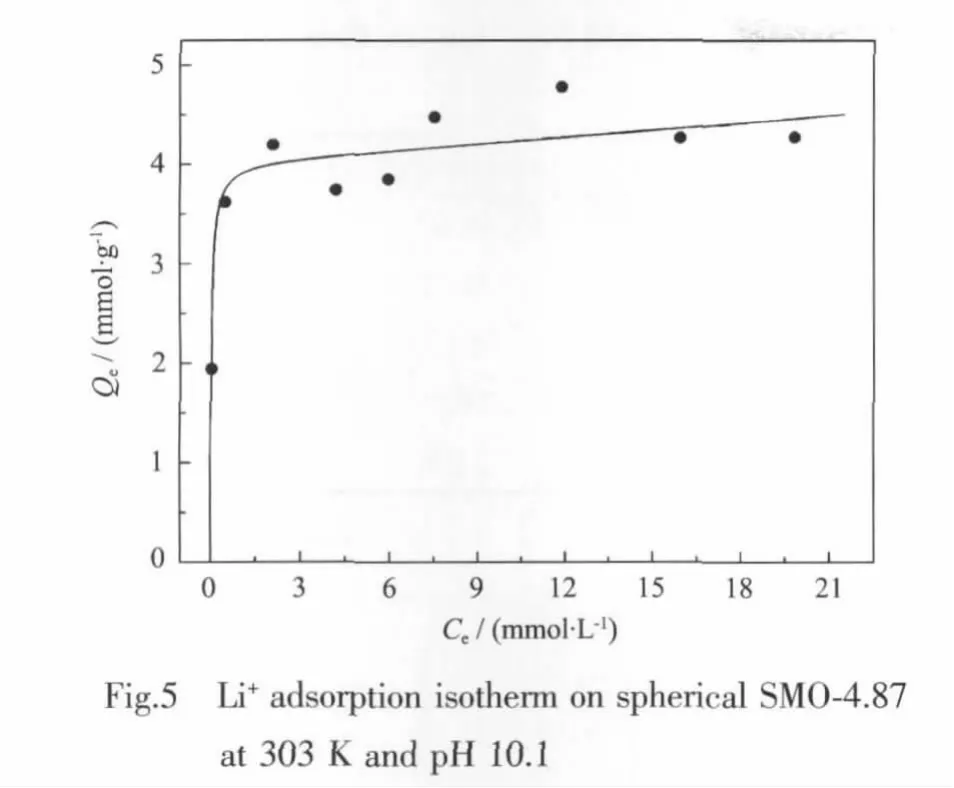
The Li+adsorption isotherm on spherical SMO-4.87 was shown in Fig.5.The equilibrium adsorption capacity (Qe)increased apparently before the Li+equilibrium concentration (Ce)reaching to 0.51 mmol·L-1,then increased slowly,with the maximum Li+equilibrium adsorption quantity to be 4.50 mmol·g-1.The spherical-type ion-sieve had higher Li+adsorption capacity when Li+concentration was just only 0.04 mmol·L-1,so this material is promising for the lithium recovery from aqueous solution with trace Li+.

Table 2 Lithium adsorption kinetics and simulation according to Lagergren equation

2.4 pH effect on lithium ion adsorption capacity of spherical PVC-MnO2ion-sieve
It is known that pH value of solution can significantly influence Li+adsorption capacity on ionsieve.The pH effect was studied by batch tests with changing the initial pH value of solution from 7 to 13.Fig.6 illustrated that the ion-sieve had two kinds of exchange groups,and this is verified with Wang[44].The change of pH value could be caused by the reaction of H+-Li+ion-exchange occurring with the uptake of lithium.And thenumberofreplaceableprotons responsible for adsorption reactions can be estimated by the difference of pH value.Fig.7 showed pH effect on lithium ion adsorption capacity of SMO-4.87.The apparentcapacity agreed with the Li+uptake determined from the decrease of the Li+concentration in the supernatant,suggesting thatthe metalion adsorption is similar to ion-exchange reaction.The Li+uptake increased as the initial solution pH increased.And the adsorption capacity reached 5.28 mmol·g-1at pH 12.35,which is 65.3%of the theoretical adsorption capacity estimated from the chemical formula.

2.5 Selectivity of spherical PVC-MnO2ion-sieve to Li+,Na+,K+,Ca2+and Mg2+ions in the mixture solution
Usually,there are many positive ions,such as Li+,Na+,K+,Ca2+and Mg2+in the brine.It is significant that the spherical PVC-MnO2ion-sieve should have a high selectivity to Li+in the brine in order to recovery Li+from the brine where the concentration of lithium ion is lower than the other ions.Table 3 showed the selectivity of Li+compared with uptake behaviors of other coexisting ions in brine including Na+,K+,Ca2+and Mg2+.In this table,C0is initial concentration of metal ions in solution (mol·L-1);Ceis concentration of metal ions in solution at equilibrium (mol·L-1);Qeis the amount of metal ions adsorbed per gram of MnO2at equilibrium (mmol·g-1);Kdisthe distribution coefficient,CF is the concentration factor;αLiMeis the separation factor.
The equilibrium distribution coefficients (Kd)of these metal ions were in the order of Li+>Mg2+>Ca2+>Na+>K+,indicating high selectivity for Li+among all the coexisting ions,similar to the ion selectivity of the manganese oxides powder[14,17].The distribution coefficient for Li+was 13 522.48 mL·g-1,that is in the same order with the powder (16 770.63 mL·g-1)[15],indicating this granulation method didn′t affect the lithium selective property of ion-sieve.Because PVC is inert material,has no adsorption ability to metal ions.The relatively high selectivity for Li+can well beexplained by the ion-sieve effect of the spinel lattice with a three-dimensional(1×3)tunnel suitable in size for fixing lithium ions in cubic phase MnO2nanocrystal obtained from Li4Mn5O12precursor.Spherical ion-sieve had higher selectively for lithium ions than magnesium ions,in spite of the similar ion radius of Li+and Mg2+.Since hydration energy of magnesium ions is about four times[45]higher than that of lithium ions,they may require higher energy in order to be dehydrated to enter the micro pores of lithium ion-sieve.

Table 3 Selectivity of spherical SMO-4.87 ion-sieve to Li+,Na+,K+,Ca2+and Mg2+ions in their mixture solution at 303 K and pH=10.1,V=10.0 mL,W(MnO2)=0.10 g
2.6 Breakthrough/elution behaviors of lithium ion in fixed bed packed with spherical PVC-MnO2 ion-sieve


The breakthrough curves and elution curves of lithium ion from the fixed bed packed with SMO-4.87 were shown in Fig.8 and Fig.9,where the feedstock was inputted with 10.0 mmol·L-1LiCl solution (pH=10.1),and the adsorbed Li+in the column was eluted by 1.0 mol·L-1HCl solution.The adsorption capacities of SMO-4.87 evaluated from the breakthrough curves were 4.55,4.91 and 4.99 mmol·g-1during the first,the second and the third adsorption process.Dynamic adsorption capacity was larger than static adsorption capacity,because the fresh aqueous solution was continuously injected into the fixed bed adsorber.The three breakthrough curves are overlapped with each other.During the three elution process,the Li+maximum content in the elution solution is 472.23,371.10 and 536.29 mmol·L-1,respectively.So the maximum concentration factor of lithium ion is 47.22,37.10 and 53.63,respectively.The elution contents of lithium evaluated from the elution curves,are 4.84,5.01 and 4.88 mmol·g-1,respectively.The elution content was consistent with the adsorption capacity.So spherical lithium ion-sieves after adsorption process can be eluted totally,and have good cycle stability.

Fig.10 and Fig.11 showed the breakthrough curves and elution curves,when inputting the a mixture solution of Li+,Na+,K+,Ca2+and Mg2+ions,about 10.0 mmol·L-1for each element,respectively.From Fig.10,it can be seen that the breakthrough behavior of Na+,K+and Mg2+rapidly reached the initial concentration after 200 mL fractions,whereas Li+just reached 3.2%of initial concentration after 200 mL fractions.The dynamic adsorption capacity for Li+,Na+,K+and Mg2+was 3.07,0.11,0.08 and 0.07 mmol·g-1,respectively.The Li+adsorption capacity in the mixed solution was lower than that in pure Li+solution,because the other metal ions in the solution has competition adsorption effect with Li+.From elution curves,we can see that the metal ions can be easily extracted from the spherical material.Na+,K+,Mg2+has been extracted totally when injecting 50 mL 1.0 mol·L-1HCl solution.Li+can be extracted totally when injected 100 mL 1.0 mol·L-1HCl solution.We want to further separate Li+from other ions through sub-receiving elution solution.But the results indicated that the elution rate of all the metal ions has no great difference.Sub-receiving elution can not further purify the Li+.The maximum Li+concentration in the eluent sample is 580 mmol·L-1,the maximum Li+enrichment factor is about 58 and that the molar ratio of Li/Mg,Li/Na,Li/K is 15,60 and 53,respectively.
3 Conclusions
With the Li4Mn5O12ultrafine powder synthesized by our lab and the developed PVC granulation process,the spherical PVC-Li4Mn5O12with particle diameter 2.0~3.5 mm was prepared by anti-solvent method.And it was treated with hydrochloric acid solution to obtain spherical PVC-MnO2ion sieve,which well maintains the excellent adsorption behavior of the ultrafine powder with a high Li+adsorption capacity of 4.50 mmol·g-1,and also it has a high Li+selectivity when recovering Li+from the solution containing Li+,Na+,K+,Mg2+.The experimental results from the pH titration curve in the solution (LiCl+LiOH)show that the Li+adsorption capacity increases with the pH value and reaches 5.28 mmol·g-1at pH 12.35.According to the experimental results carried out in a column packed with spherical PVC-MnO2ion-sieve,the Li+dynamic adsorption capacity can reach 3.07 mmol·g-1when recovering Li+from the simulated brine(containing Li+,Na+,K+and Mg2+,10.0 mmol·L-1for each ion),and Li+adsorbed on the spherical ion-sieves can be desorbed easily by 1.0 mol·L-1HCl solution.The Li+concentration in the simulated brine (containing Li+,Na+,K+and Mg2+,10.0 mmol·L-1for each ion)can be enriched up to 58 times in the eluent solution(580 mmol·L-1,the Li+maximum effluent concentration)when desorption Li+in the fixed bed using 1.0 mol·L-1HCl solution passthrough the column.Therefore,the spherical PVC-MnO2ion-sieve (H type of PVCLi4Mn5O12)is promising to be utilized in lithium recovery from brine or sea water.
[1]Armstrong A R,Bruce P G.Nature,1996,381(6582):499-500
[2]Scrosati B.Nature,2011,473(7348):448-449
[3]Tarascon J M,Armand M.Nature,2001,414(6861):359-367
[4]Murugan G S,Varma K B R,Takahashi Y,et al.Appl.Phys.Lett.,2001,78(25):4019-4021
[5]SONG Peng-Sheng(宋彭生),LI Wu(李武),SUN Bai(孙柏),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(5):801-815
[6]GAO Feng(高峰),ZHENG Mian-Ping(郑绵平),NIE Zhen(乜贞),et al.Acta Geoscientica Sinica(Diqiu Xuebao),2011,32(4):483-492
[7]SUN Shu-Ying(孙淑英),ZHANG Qin-Hui(张钦辉),YU Jian-Guo(于建国).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(4):567-572
[8]Wang L,Ma W,Liu R,et al.Solid State Ionics,2006,177(17/18):1421-1428
[9]JiZY,YuanJS,XieYH.Adv.Mater.Res.,2010,96,233-236
[10]SUN Shu-Ying(孙淑英),YE Fan(叶帆),SONG Xing-Fu(宋兴福),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(3):439-444
[11]JIA Xu-Hong(贾旭宏),LI Li-Juan(李丽娟),ZENG Zhong-Min(曾忠民),et al.Inorg.Chem.Ind.(Wujiyan Gongye),2011,43(8):29-32
[12]Gabra G G,Torma A E.Hydrometallurgy,1978,3(1):23-33
[13]Bukowaky H,Uhlemann E,Gloe K,et al.Hydrometallurgy,1992,28(3):323-329
[14]Zhang Q H,Li S P,Sun S Y,et al.Adv.Powder Technol.,2009,20(5):432-437
[15]Zhang Q H,Sun S Y,Li S P,et al.Chem.Eng.Sci.,2007,62(18/20):4869-4874
[16]Zhang Q H,Li S P,Sun S Y,et al.Ann.N.Y.Acad.Sci.,2009,1161(1):500-507
[17]Zhang Q H,Li S P,Sun S Y,et al.Chem.Eng.Sci.,2010,65(1):169-173
[18]Paulsen J M,Dahn J R.Chem.Mater.,1999,11(11):3065-3079
[19]Feng Q,Miyai Y,Kanoh H,et al.Langmuir,1992,8(7):1861-1867
[20]OoiK,MiyaiY,SakakiharaJ.Langmuir,1991,7(6):1167-1171
[21]SHI Xi-Chang(石西昌),YU Liang-Liang(余亮良),CHEN Chen-Zhen(陈白珍),et al.J.Central South Univ.:Sci.Technol.(Zhongnan Daxue Xuebao:Ziran Kexue Ban),2011,42(8):2198-2203
[22]Özgür C.Solid State Ionics,2010,181(31/32):1425-1428
[23]Yang X J,Kanoh H,Tang W P,et al.J.Mater.Chem.,2000,10(8):1903-1909
[24]LU Hong-Yan(陆红岩),YANG Li-Xin(杨立新),WU Sai-Xiang(邬赛祥),et al.Chem.J.Chinese Universities(Gaodeng Xuexiao Huaxue Xuebao),2011,32(10):2268-2273
[25]WANG Lu(王 禄),MA Wei(马 伟),HAN Mei(韩梅),et al.Acta Chim.Sin.(Huaxue Xuebao),2007,65(12):1135-1139
[26]Chitrakar R,Kanoh H,Miyai Y,et al.Chem.Mater.,2000,12(10):3151-3157
[27]Chitrakar R,Kanoh H,Miyai Y,et al.Ind.Eng.Chem.Res.,2001,40(9):2054-2058
[28]MA Li-Wen(马立文),Chen Bai-Zhen(陈白珍),SHI Xi-Chang(石西昌),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(4):697-703
[29]Dziembaj R.Solid State Ionics,2003,157(1/2/3/4):81-87
[30]Dziembaj R,Molenda M.J.Power Sources,2003,119-121:121-124
[31]Molenda M,Dziembaj R,Majda D,et al.Solid State Ionics,2005,176(19/20/21/22):1705-1709
[32]Park Y,Kim J,Kim M,et al.Solid State Ionics,2000,130(3/4):203-214
[33]Yang X J,Tang W P,Liu Z H,et al.J.Mater.Chem.,2002,12(3):489-495
[34]Sun S Y,Song X F,Zhang Q H,et al.Adsorption,2011(17):881-887
[35]Onodera Y,Iwasaki T,Hayashi H,et al.Chem.Lett.,1990,1801-1804
[36]Sagara F,Ning W,Yoshida I,et al.Sep.Sci.Technol.,1989,24(14):1227-1243
[37]Ooi K,Miyai Y,Katoh S.Sep.Sci.Technol.,1986,21(8):755-766
[38]Chung K,Lee J,Kim W,et al.J.Membr.Sci.,2008,325(2):503-508
[39]Ma L W,Chen B Z,Chen Y.Microporous Mesoporous Mater.,2011,142(1):147-153
[40]Umeno A,Miyai Y,Takagi N,et al.Ind.Eng.Chem.Res.,2002,41(17):4281-4287
[41]XIAO Guo-Ping(肖国萍),PENG Jie(彭洁),ZHANG Qin-Hui(张钦辉),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(3):435-439
[42]Topp N E,Pepper K W.J.Chem.Soc.,1949(5):3299-3303
[43]DONG Dian-Quan(董殿权),ZHANG Feng-Bao(张凤宝),ZHANG Guo-Liang(张国亮),et al.Acta Phys.-Chim.Sin.(Wuli Huaxue Xuebao),2007,23(6):950-954
[44]Wang L,Meng C G,Ma W.Colloids Surf.A:,2009,334(1/2/3):34-39
[45]Rosseinsky D R.Chem.Rev.,1965,65(4):467-490
Preparation of Spherical PVC-MnO2Ion-Sieve and Its Lithium Adsorption Property
XIAO Guo-Ping TONG Ke-Feng SUN Shu-Ying YU Jian-Guo*
(National Engineering Research Center for Integrated Utilization of Salt Lake Resources,East China University of Science and Technology,Shanghai 200237,China)
Ultrafine powder Li4Mn5O12prepared by our lab has a high adsorption capacity and selectivity for Li+in brine (containing Li+,Na+,K+and Mg2+).However,in the practical application,the ultrafine powder should be formed into the granular adsorbent materials in order to be packed in column with a lower pressure drop of fluid flowing.In this paper,the spherical PVC-Li4Mn5O12ion-sieve with particle diameter 2.0~3.5 mm was prepared,where using Li4Mn5O12ultrafine powder as ion-sieve precursor,poly vinyl chloride (PVC)as binder.And it was treated with HCl solution to obtain spherical PVC-MnO2ion-sieve.The prepared spherical ion-sieve was characterized by Scanning Electron Microscopy (SEM)and X ray Diffraction (XRD).The adsorption/desorption behavior for lithium ion was evaluated by the experimental measurements in the batch and fixed bed adsorbers packed with the spherical PVC-MnO2ion-sieve.It was found that the prepared spherical ion-sieve well maintained the excellent adsorption behavior of the ultrafine powder with a high Li+adsorption capacity of 5.28 mmol·g-1,and also it has a high Li+selectivity when recovering Li+from the solution containing Li+,Na+,K+,Mg2+.With the developed PVC granulation process,the prepared spherical PVC-MnO2ion-sieve (H type of PVCLi4Mn5O12)makes the ion-sieve adsorption potential application in industry for lithium recovery from brine or seawater.
PVC-MnO2;ion-sieve;lithium;adsorption;granulation
The research was supported by NSFC(20906022),National 863 Program (2012AA061601)and the Fundamental Research Funds for the Central Universities.
O614.111;O614.71+1;TQ028.15
A
100-4861(2012)11-2385-10
2012-03-26。收修改稿日期:2012-04-19。
国家自然科学基金(No.20906022);国家863计划(No.2012AA061601);中央高校基本科研业务费专项资金资助项目。
*通讯联系人。 E-mail:jgyu@ecust.edu.cn
