Temperature-triggered Protein Adsorption and Desorption on Temperature-responsive PNIPAAm-grafted-silica: Molecular Dynamics Simulation and Experimental Validation*
2012-10-31KANGKai康锴LUDiannan卢滇楠andLIUZheng刘铮DepartmentofChemicalEngineeringTsinghuaUniversityBeijing100084China
KANG Kai (康锴), LU Diannan (卢滇楠)** and LIU Zheng (刘铮)**Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
Temperature-triggered Protein Adsorption and Desorption on Temperature-responsive PNIPAAm-grafted-silica: Molecular Dynamics Simulation and Experimental Validation*
KANG Kai (康锴), LU Diannan (卢滇楠)** and LIU Zheng (刘铮)**Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
Poly(N-isopropylacrylamide) (PNIPAAm) grafted onto silica, which may be used for reverse phase chromatography (RPC), was simulated and synthesized for protein separation with temperature-triggered adsorption and desorption. Molecular dynamics simulation at an all-atom level was performed to illustrate the adsorption/desorption behavior of cytochrome c, the model protein, on PNIPAAm-grafted-silica, a temperature responsive adsorbent. At a temperature above the lower critical solution temperature (LCST), the PNIPAAm chains aggregate on the silica surface, forming a hydrophobic surface that is favorable for the hydrophobic adsorption of cytochrome c, which has a high exposure of hydrophobic patches. At temperatures below the LCST, the PNIPAAm chains stretch, forming hydrophilic surface due to hydrogen bonding between PNIPAAm and surrounding water. Desorption of cytochrome c on the PNIPAAm-grafted-silica surface occurs as a result of competition with water, which forms hydrogen bonds with the protein. The conformational transitions of both cytochrome c and PNIPAAm are monitored, providing molecular insight into this temperature-responsive RPC technique. PNIPAAm-grafted-silica beads were synthesized and used for the adsorption and desorption of cytochrome c at approximately 313 K and 290 K, respectively. The experimental results validate the molecular dynamics simulation. In comparison to conventional RPC, using temperature as a driving force for RPC reduces the risk of protein denaturation caused by exposure to chaotropic solvents. Moreover, it simplifies the separation process by avoiding the buffer exchange operations between the steps.
reverse phase chromatography, PNIPAAm-grafted-silica, cytochrome c, molecular dynamics simulation, atom transfer radical polymerization (ATRP)
1 INTRODUCTION
Reverse phase chromatography (RPC) has been extensively used for protein separation at different scales [1]. Selective adsorption and desorption are based on the hydrophobic interaction between hydrophobic patches on the protein and those on the chromatographic adsorbent. Such interaction can be enhanced by using salt or polar solvents that preferentially extract water molecules from the protein and adsorbent surface and thus intensify the adsorption.The replacement of bound protein requires the introduction of an elution buffer that dissociates the hydrophobic pair via preferential hydration. The column is regenerated and subsequently re-equilibrated to prepare for the next run.
The concept of chromatography based on the use of SMART polymers was proposed by Ivanov et al [2].In this technique, adsorption and desorption are conveniently accomplished by changing the surface property of adsorbent that is responsive to environmental stimuli. Poly(N-isopropylacrylamide) (PNIPAAm) is perhaps the most extensively studied temperatureresponsive polymer, which has a lower critical solution temperature (LCST) of approximately 32 °C [3]. Our previous study using PNIPAAm-grafted dextran as an artificial chaperone to assist protein refolding [4] showed that the hydrophobic interaction between polymer and protein could be manipulated by altering the temperature. We extended our studies to examine the possibility of applying such a changeable interaction, which is hydrophobic in nature, to reverse phase chromatography. In comparison to conventional RPC, which is based on the use of different solvents, temperatureresponsive RPC has following major advantages.Firstly, the separation is greatly simplified because buffer exchange between the steps is not necessary, as both adsorption and desorption are only a function of temperature. Secondly, use of organic solvents, particularly chaotropic ones that denature the protein, is avoided. Kanazawa et al. [5] developed an aqueous chromatographic system with a temperature-responsive polymer-modified stationary phase for separation.Maharjan et al. [6] developed a temperature-responsive ion-exchange resin for the fractionation of whey proteins. Akiyama et al. designed novel acryl amide-based thermo-responsive polymer analogues [7], which could be used in a temperature-responsive chromatographic system, and investigated the adsorption and desorption of proteins driven by temperature. In addition, Kidoaki et al. proposed the use of PNIPAAm brushes, in which PNIPAAm was grafted from silica and employed for the fabrication of functional surfaces capable of reversible transition between hydrophilic and hydrophobic forms [8]. Other researchersalso tried to graft PNIPAAm onto different surfaces such as a hydrogen-terminated Si(100) surface [9],anodic aluminum oxide [10], and agarose. Balamurugan et al. grafted PNIPAAm on mixed self-assembled monolayers of 11-mercaptoundecanol and 11-mercaptoundecane on gold and showed the collapse of PNIPAAm brushes as a function of temperature [11].The effects of changes in brush conformation and wettability of the PNIPAAm-grafted cellulose surface[12] as well as the brush height [13] were also examined. Lindqvist et al. monitored the change on the basis of static water contact angle (CA) measurements at temperatures above and below the LCST [14].
With respect to the temperature-responsive mechanism of PNIPAAm, Lin et al. showed that PNIPAAm is hydrophilic below its LCST due to hydrogen bonding with water, while above its LCST water molecules dissociate from polymer due to enhanced movement at higher temperature, leading to significant aggregation of polymer, which increases the entropy of the system and leads to a spontaneous process [15]. Longhi et al. applied a 50-unit oligomer to simulate poly(N-isopropylacrylamide) in a dilute aqueous solution at 300 K and 310K to obtain microscopic details on the conformation change [16].Gangemi et al. investigated the influence of different comonomers on PNIPAAm [17]. Lu et al. performed dynamic Monte Carlo simulations for the interactions between PNIPAAm and protein during protein folding[18]. However, molecular dynamics simulation for the interaction between proteins and PNIPAAm at all-atom level has not been reported.
The objective of this study is to obtain molecular insights into the interaction between PNIPAAm and protein during RPC by changing temperature to trigger the adsorption and desorption of protein. A 50-unit PNIPAAm chain is built and uniformly grafted onto a silica surface to form a temperature-responsive adsorbent for RPC. The PNIPAAm-silica surface is then placed in an aqueous solution, and cytochrome c is used as the model protein. The native conformation of the protein is obtained from the Protein Data Bank.The temperature of the solution is carefully adjusted according to the LCST of PNIPAAm to initiate the alteration in the surface hydrophobicity, conformational transition of PNIPAAm, formation or breakage of hydrogen bonding and change of overall energy of the system, and most importantly, to adsorb and desorb the protein. To validate the molecular dynamics simulation for the RPC process operated with temperature as the driving force, PNIPAAm-silica beads are synthesized via atom transfer radical polymerization (ATRP) method and used for the adsorption and desorption of cytochrome c.
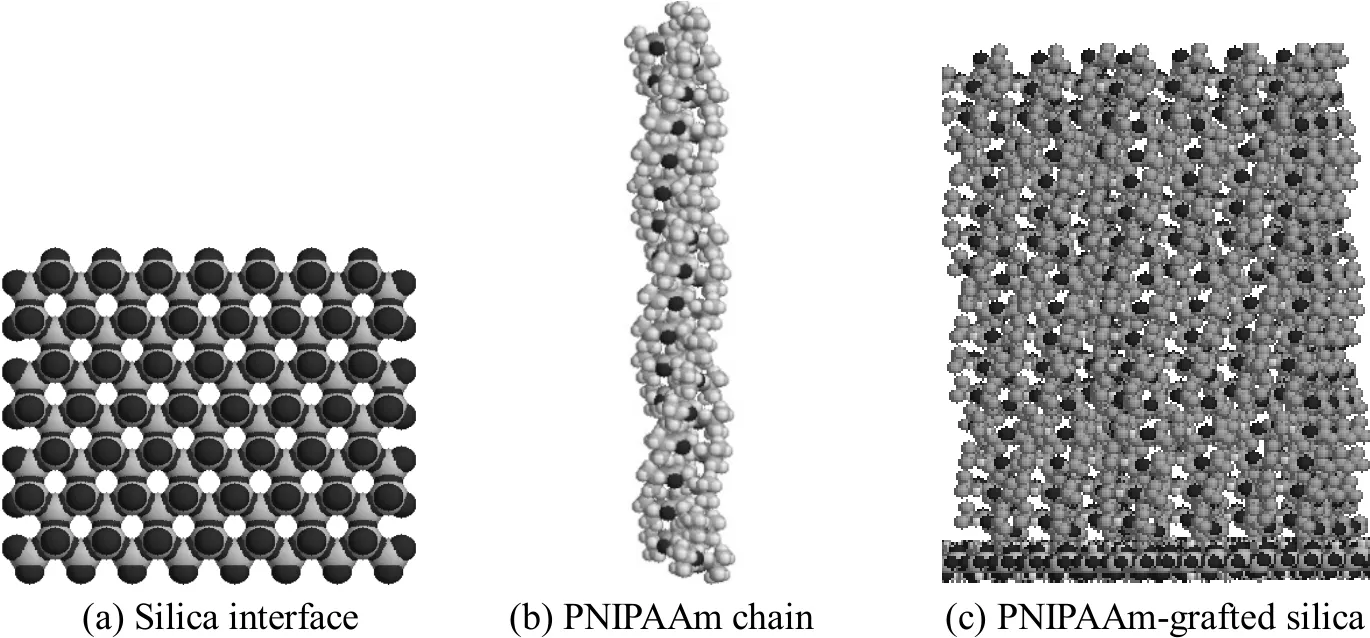
Figure 1 Representation of the 50-unit PNIPAAm-modified interface
2 MODELS AND METHODS
2.1 Modelling of PNIPAAm-grafted-silica
The model of PNIPAAm-grafted-silica is shown in Fig. 1. The silica surface is composed of silicon and oxygen, as described by Giovambattista et al [19]. The geometry and energy of PNIPAAm monomer are calculated at the B3LYP/6-31+G**//B3LYP/3-21G level with the Gaussian 03 software. The oligomer of PNIPAAm is composed of 50 monomers, as shown in Fig. 1 (b). The PNIPAAm chain is then grafted onto the silica surface, as shown in Fig. 1 (c), with final grafting density of 2.24 chains/nm2, which is determined according to the surface area of silica available for exclusively grafting polymer chains. The chromatographic process is simulated by loading the model protein onto the surface of the matrix at a given distance toward the surface of the matrix.
2.2 Modelling of cytochrome c
Cytochrome c is chosen as the model protein,whose native structure is obtained from Protein Data Bank (entry 1AKK) as shown in Fig. 2. Cytochrome c is composed of 104 amino acids with molecular mass of 12344. As shown in Fig. 2 (a), cytochrome c is rich in α-helix, with heme and Fe in active sites. Non-polar residues are widely distributed among the peptide chains,forming hydrophobic patches as shown in Fig. 2 (b).
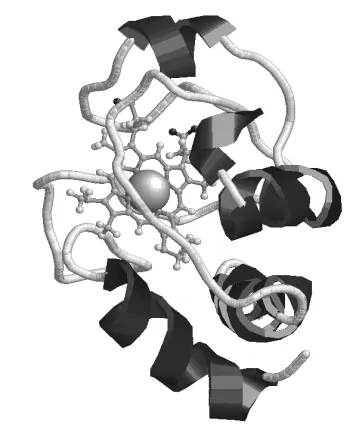
(a) Cartoon structure of cytochrome c black: α-helix; grey:random coil; ball & stick: heme group; ball: Fe

Figure 2 Model of cytochrome c
2.3 Molecular dynamics simulation
Molecular dynamics simulations of PNIPAAmgrafted silica with and without cytochrome c are conducted using Gromacs 4.0 as platform. This package is a collection of programs and libraries for MD simulation and the subsequent analysis of trajectory data.Simulations are performed using general triclinic cell geometry. Pressure and temperature coupling is implemented for all simulation cells. The Berendsen’s weak coupling algorithm scheme is used for both pressure and temperature. The size of the simulation box is 8.83 nm×8.98 nm×20.0 nm. The cutoffs of both neighbor atom list and LJ potential are set as 1.2 nm.Particle-mesh Ewald (PME) method is adopted for the coulomb interaction. The temperature is controlled by the V-rescale method with a time constant of 0.1 ps.And the Gromos96 (ffG43a1) force field is used in the simulation. All snapshots are prepared using the Rasmol program. The temperature-responsive behavior of the 50-unit PNIPAAm is simulated for 10 ns MD at temperatures 290 K and 313 K, which are below and above the LCST of PNIPAAm, respectively. At these temperatures, cytochrome c maintains its stability and activity [20]. The SPC/E water model [21] is chosen to describe the water behavior. The concentration of NaCl is set as 0.15 mol·L−1, which is widely used in the elution buffer during hydrophobic interaction chromatography (HIC). The system containing a PNIPAAm-grafted-silica surface with or without cytochrome c above this surface and a certain number of water molecules are submitted to 500 steps of steepest descent minimization converging to a value of 2000 kJ·mol−1·mol−1. Then a 10 ps position-restrained MD simulation is performed by keeping the protein coordinate fixed and allowing the water molecules to equilibrate themselves. Then a 10 ns MD is performed and a leap-frog algorithm is used for integrating the Newtonian equations of motion for 2×106simulation steps, with a time step of 0.002 ps.
For adsorption of cytochrome c onto the PNIPAAmgrafted-silica surface, the native cytochrome c is placed above the grafted surface with a distance of 0.5 nm. The distance and orientation are important parameters determining the protein adsorption. The configuration and orientation of protein are altered when it accesses the adsorbent to achieve a reduced free energy while increasing the entropy of the system. For the present study, the initial distance is chosen according to the computational trial, which allows an adequate parallel sampling that establishes a molecular insight into the adsorption. The MD simulation is performed for 10 ns at 313 K, which is above LCST.Once the distance between cytochrome c and silica surface is lower than that between PNIPAAm and silica surface, the protein is adsorbed on the PNIPAAmgrafted-silica surface. In the desorption of cytochrome c from the PNIPAAm-grafted-silica surface, once the distance between cytochrome c and silica surface is larger than 0.5 nm, the protein is totally desorbed from the surface.
All simulations are performed at least three times for average.
2.4 Analysis methods
Two order parameters, i.e., radius of gyration (Rg)and the number of hydrogen bonds (H-bond), are used to evaluate the simulations. Rgreflects the size of the specific conformation cytochrome c, which can be calculated by Eq. (1):

where miis the mass of atom i in cytochrome c and riis the internal coordinate of atom i of conformation.The Rgvalue of native cytochrome c is 1.38 nm. The increase of Rgindicates the denaturation of cytochrome c.
Hydrogen bonds are determined based on cutoffs for the angle (30°) Acceptor-Donor-Hydrogen and the distance Hydrogen-Accepter (0.35 nm).
The interaction energies of PNIPAAm and water,PNIPAAm and silica surface, and the protein and PNIPAAm are calculated, which is helpful in elucidating the molecular fundamentals underlying the conformational transitions of PNIPAAm and cytochrome c, hydrophobic interactions, and subsequent adsorption or desorption behavior. The interaction energy is determined using the energy program of Gromacs 4.0, as detailed in Ref. [22].
3 EXPERIMENTAL
3.1 Materials
The chromatography silica gel was purchased from Meigao Group Co., Ltd. (China). 3-Glycidoxypropyltrime-thoxysilane, 4-dimethylamino-pyridine,1,4-dioxane, tetrahydrofuran, 2-bromoisobutyryl bromide, N,N-dimethyl formamide, and 1,1,4,7,7-pentamethyldiethyl-enetriamine were obtained from Sigma.Cytochrome c was also from Sigma. Vitamin C was obtained from Biodee. PNIPAAm was obtained from Aldrich and recrystallized prior to use. CHF3O3S, toluene, acetone, ethanol, diethanolamine, triethylamine,and CuBr were of analytical grade and purchased from standard suppliers.
3.2 Synthesis of PNIPAAm-grafted silica
PNIPAAm-grafted-silica was synthesized according to following procedure. 5 g of silica gel was suspended in 50 ml of 5% CHF3O3S solution to generate reactive hydroxyl groups. 5 g of the activated silica gel was suspended in 50 ml toluene with 5 ml 3-glycidoxypropyltrime-thoxysilane and a spot of triethylamine at 85 °C for 24 h. The product, named“coupling silica”, was repeatedly washed with toluene and acetone. Coupling silica (5 g) was suspended in 50 ml 1,4-dioxane containing 5 ml diethanolamine at 60 °C for 6 h. The product named “hydroxyl silica”was repeatedly washed with ethanol and acetone. Hydroxyl silica (5 g) was suspended in 50 ml tetrahydrofuran (THF) with 1.5 ml triethylamine and 40 mg 4-dimethylamino-pyridine. After dropwise addition of 1 ml 2-bromoisobutyryl bromide, the reaction was continued for 24 h at room temperature. The product,named “initiator silica”, was repeatedly washed with THF, water and acetone. Initiator silica (5 g) was suspended in 50 ml of a solvent composed of N,N-dimethyl formamide and water (volume ratio of 50︰50),and this was followed by the addition of 700 μl of the catalyst (composed of 0.136 mol·L−1CuBr and 0.136 mol·L−11,1,4,7,7-pentamethyldiethylenetriamine), 23.6 g NIPAAm, and 10 ml vitamin C solution (200 mg·ml−1).The reaction was carried out at room temperature for 8 h. The final product, PNIPAAM-grafted- silica, was repeatedly washed with water, methanol and acetone at least for three times.
3.3 Adsorption of cytochrome c at 290 K and 313 K
Cytochromec was chosen as the model protein to illustrate the use of tunable hydrophobic interactions between the protein, PNIPAAm, and water in RPC.The experiment was designed to reproduce the simulation results. In the experiment, 10 ml aqueous cytochrome c solution (0.2 mg·ml−1) was added to a 50-ml flask, and this was followed by the addition of 1.0 g PNIPAAm-grafted silica. The flask was incubated at 290 K or 313 K at a rotation speed of 100 r·min−1for 10 min before examining the cytochrome c concentration in the supernatant. The concentration could be used to calculate the cytochrome c adsorption capacity of PNIPAAm-grafted-silica at different temperatures.Then the flask incubated at 313 K was cooled down to 290 K with and without rotation.
3.4 Assays
3.4.1 Elemental analysis
The elemental analysis was carried out using a Vario EL-III elemental analyzer (Elementar Co., Germany). The change in the content of different elements at different steps is indicative for the success of the reaction.
3.4.2 Differential scanning calorimetry (DSC)
The temperature-responsive character of PNIPAAmgrafted-silica was analyzed with a Shimadzu DSC-60 Differential Scanning Calorimeter (Shimadzu Co.,Japan).
3.4.3 Thermogravimetric analyzer (TGA)
A Shimadzu DSC-60 (Shimadzu Co., Japan)thermogravimetric analyzer (TGA) was used to determine the thermogravimetry of silica after each modification step. The grafting rate (GR) is interpreted from the gravimetry loss in the temperature range of 200-900 °C, using the following equation:

where W1and W2represent the mass loss compared to initial mass in successive steps, and Wgis the mass of final residual mass.
3.4.4 Protein concentration
The concentration of cytochrome c was determined by the bicinchoninic acid colorimetric assay(BCA) using bovine serum albumin (BSA) as the standard protein.
4 RESULTS AND DISCUSSION
4.1 Temperature-responsive behavior of the PNIPAAm grafted on silica surface
The simulated morphology of PNIPAAm chains on the surface of silica at different temperature is shown in Fig. 3. The distance between the mass center of PNIPAAm layer and silica surface at 290K is larger than that at 313 K, indicating that PNIPAAm chains collapse on the silica surface at higher temperature.This may be attributed to the water-swelling effects[23]. Moreover, this simulation reproduces the experimental results of Wang et al. [24] and offers molecular insight into the temperature-responsive behavior of PNIPAAm.
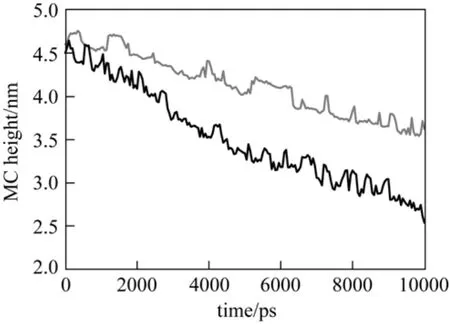
Figure 3 The distance between the mass center of PNIPAAm chains and silica surface at different temperatures T/K: 290; 313
The simulated surface morphological change of the PNIPAAm-grafted silica surface at 290 K and 313 K is shown in Fig. 4. At 313 K, above the LCST of PNIPAAm (around 305 K), the PNIPAAm chains are twisted due to increased hydrophobic interactions among the polymer chains and between the polymer chain and silica surface. Consequently, the final maximum height of the PNIPAAm layer is 6.382 nm at 313 K,which is lower than that at 290 K (8.231 nm).
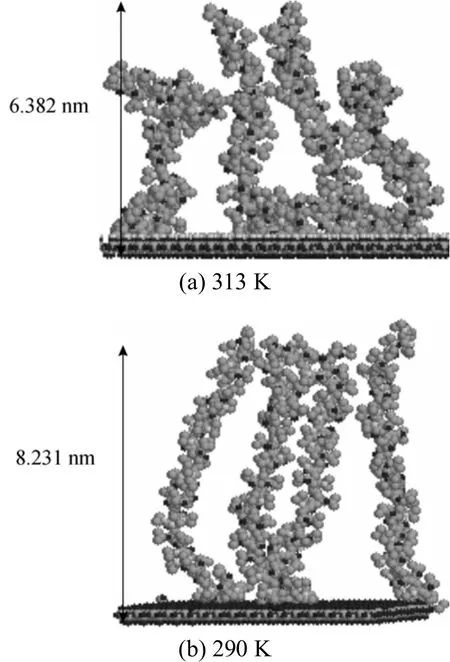
Figure 4 Snapshots of the PNIPAAm-grafted-surface at different temperatures
To probe the molecular mechanism underlying the topological change of PNIPAAm chain, the hydrogen bond number is calculated, as shown in Fig. 5. Above the LCST, i.e., at 313 K, the total number of hydrogen bonds (HB) between PNIPAAm and water decreases,while that of intramolecular hydrogen bonds of PNIPAAm increases. This leads to the aggregation of PNIPAAm and simultaneous release of water molecules from the polymer surface, as reported by Lindqvist et al [14].
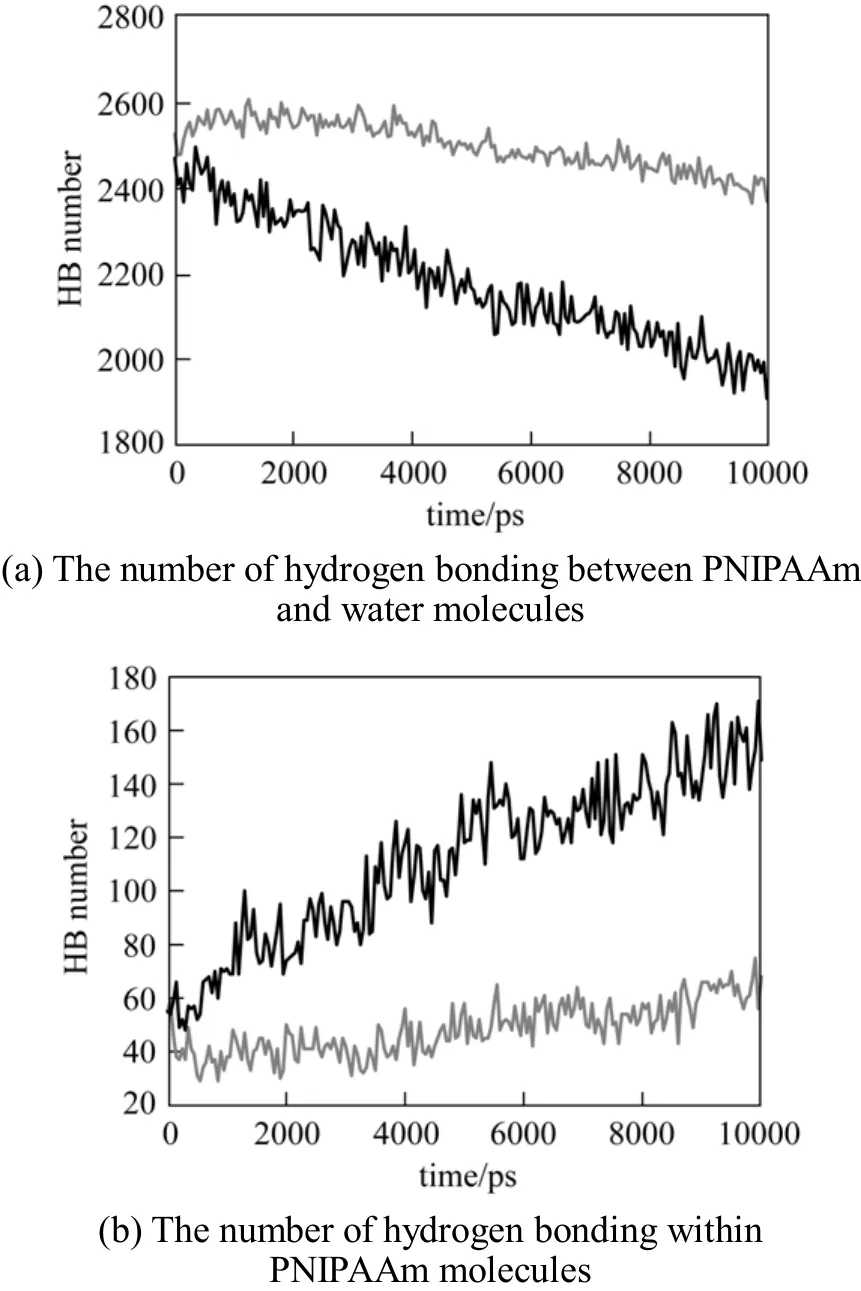
Figure 5 The formation of hydrogen bonding at different temperaturesT/K: 290; 313
The molecular interaction energy between PNIPAAm and the silica surface as well as that between PNIPAAm and water is calculated as a function of temperature, which is shown in Fig. 6. When the temperature increases from 290 K (below the LCST of PNIPAAm) to 313 K (above the LCST of PNIPAAm),the interactions between PNIPAAm and silica, PNIPAAm and PNIPAAm are strengthened and the interaction between PNIPAAm and water is weakened.From the energy of these interactions, we can also see that the interaction between PNIPAAm and water plays the major role in the main interaction. Combined with the change of hydrogen bond number in Fig. 5,we can conclude that the stability of hydrogen bond between PNIPAAm and water decreases when the temperature increase makes the interaction between them weakened. Thus PNIPAAm chain twists and intramolecular hydrogen bonds of PNIPAAM form,which further strengthens the twist. Moreover, the interaction between PNIPAAm chain and the surface as a function of temperature accounts for the reduction in the distance from the mass center of the polymer to the silica surface. Although an extension of the computational time favors the equilibrium acquisition of parameters for the adsorption, the present simulation is performed for 10 ns to display the adsorption/desorption behavior as a function of temperature.
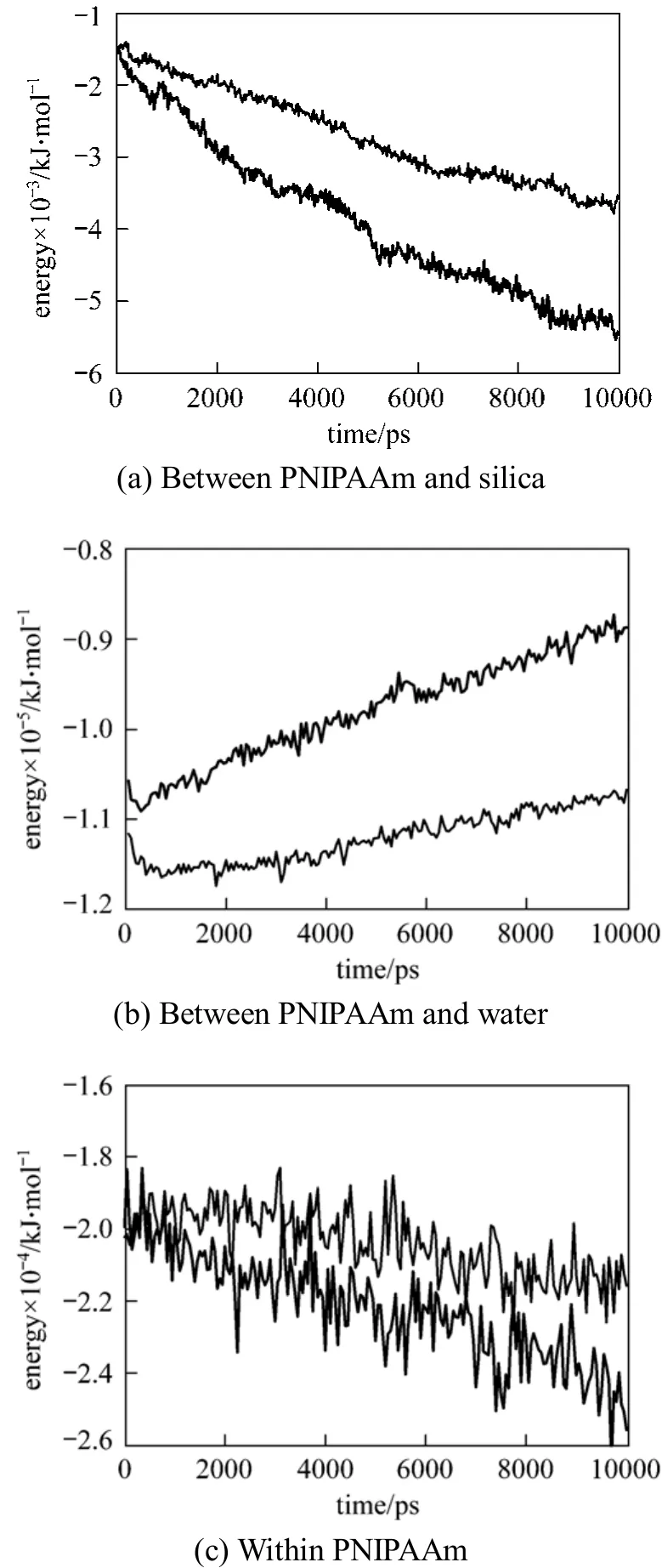
Figure 6 Interaction energy T/K: 290; 313
4.2 Adsorption behavior of cytochrome c onto PNIPAAm-grafted silica
The temperature-triggered adsorption of cytochrome c onto PNIPAAm-grafted silica is simulated using the above-mentioned procedure, and the MD simulation is performed for 10 ns at each temperature.We concern the adsorption ability of PNIPAAmgrafted-silica at different temperatures. The adsorption process is characterized by the distance between cytochrome c and silica.
In Fig. 7, the distance between cytochrome c and the silica surface decreases at 313 K, while it remains unchanged at 290 K. At 313 K, the distance between cytochrome c and silica surface is about 6.0 nm,which is lower than 6.38 nm as shown in Fig. 3 (a),indicating that cytochrome c is entangled by PNIPAAm chains, which is the driving force for adsorption. At 290 K, however, the distance between cytochrome c and silica surface is about 10.0 nm, which is larger than 8.23 nm as shown in Fig. 3 (b), indicating that cytochrome c cannot be adsorbed due to hydrophilic repulse.
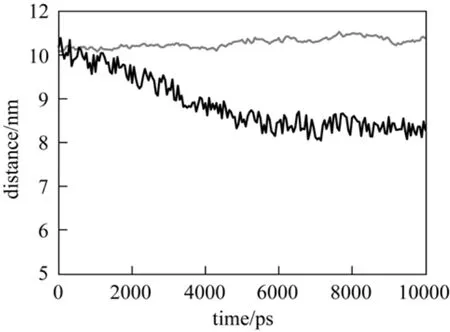
Figure 7 Distance between cytochrome c and silica at different temperaturesT/K: 290; 313
The conformational variations of PNIPAAm and cytochrome c as a function of temperature are recorded and shown in Fig. 8. At 313 K, cytochrome c is stretched with an enlarged hydrophobic patch exposed to the polymer chains [Fig. 8 (a)]. This favors the hydrophobic interactions for adsorption. In contrast, at 290 K, cytochrome c maintains its globular configuration with less exposure of its hydrophobic patches when the polymer extends outwards [Fig. 8(b)]. This hinders the adsorption of cytochrome c, resulting in unchanged distances from the protein to the silica surface, as shown in Fig. 7.
The gyration radius of protein, Rg, is a parameter related to protein conformational transition. As shown in Fig. 9, Rgof cytochrome c fluctuates more at 313 K than at 290 K, which may be attributed to the adsorption of protein. Such a fluctuation can be altered by adjusting temperature to prevent irreversible conformation changes in the protein when using chaotropic solvents,as shown in a previous simulation study [25]. This is an essential advantage of temperature-triggered RPC.
Figure 10 shows the interaction energy between cytochrome c and the PNIPAAm-grafted surface. At 313 K, the interaction energy substantially increases in the first 5 ns, indicating that protein adsorption occurs on the surface. However, such a significant change is not observed at 290 K, so that the adsorption does not occur. In other words, the protein is in the free form at 290 K. This allows adsorption at 313 K and desorption at 290 K.
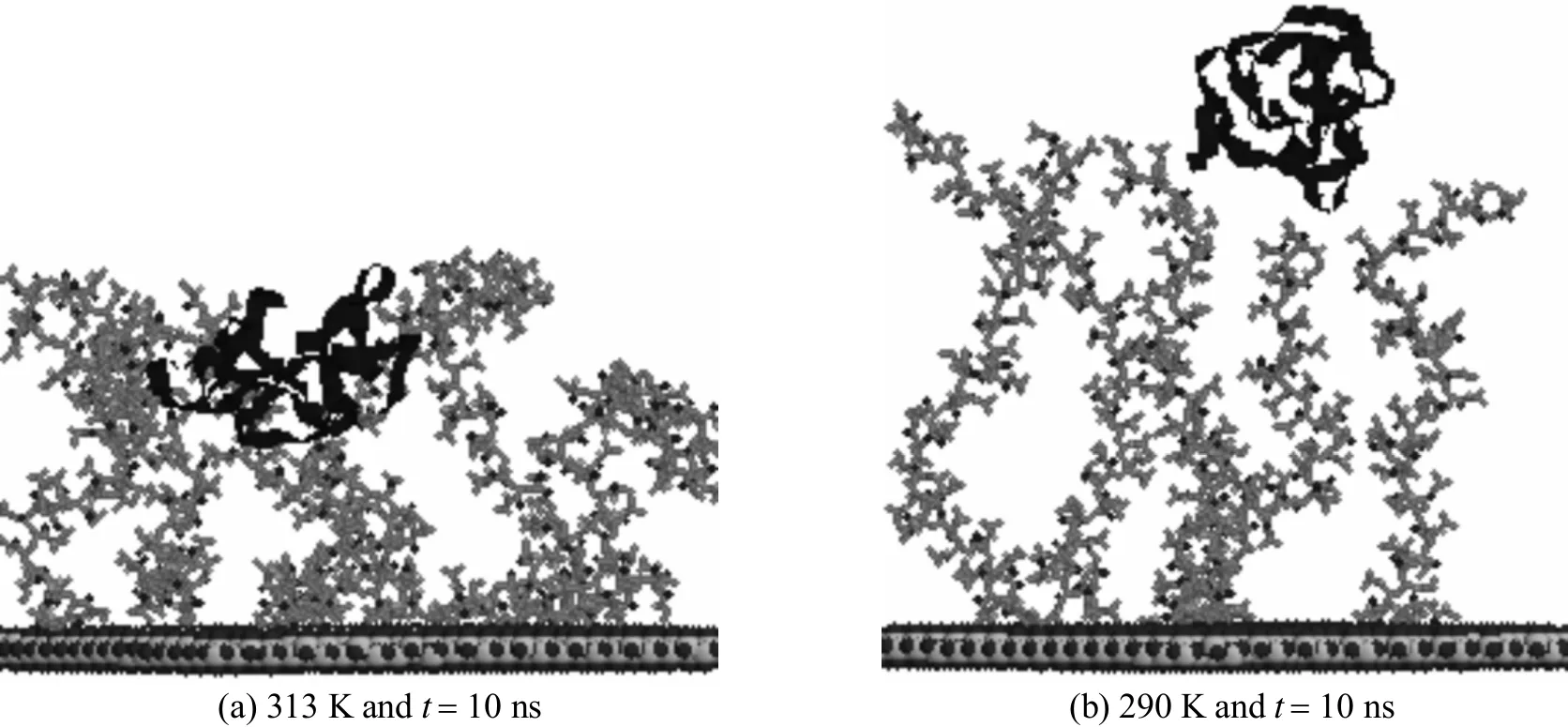
Figure 8 Snapshots of protein on the PNIPAAm-grafted surface at different temperatures
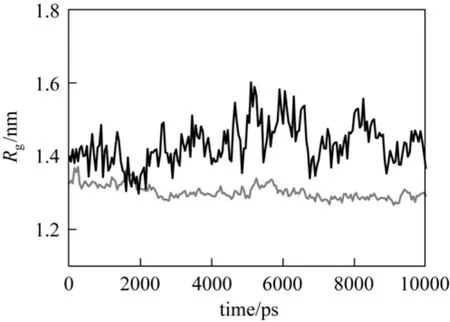
Figure 9 Gyration radius of cytochrome c T/K: 290; 313
4.3 Adsorption and desorption behavior of cytochrome c onto PNIPAAm-grafted-silica
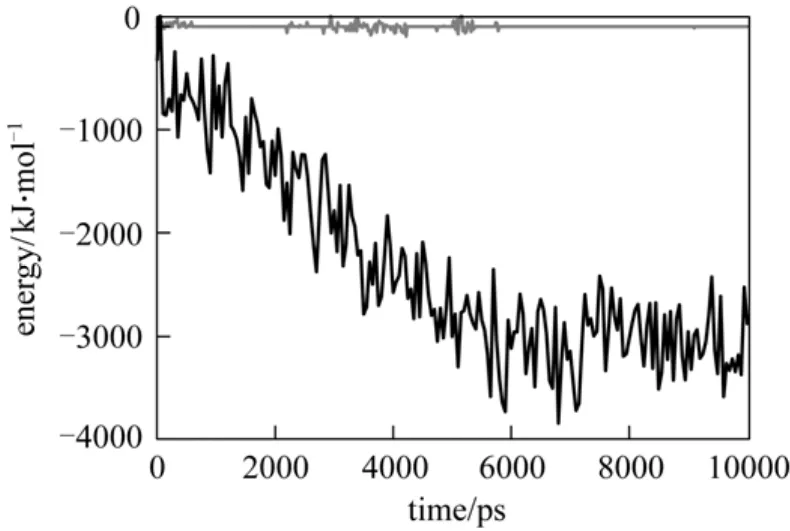
Figure 10 Interaction energy between cytochrome c and PNIPAAm-grafted surface T/K: 290; 313
In order to further prove that the interaction of cytochrome c and PNIPAAm-grafted surface depends on temperature, after the cytochrome c adsorbs on the PNIPAAm-grafted surface at 313 K, we change the simulation temperature to 290 K. Then we examine the distance and interaction energy between cytochrome c and PNIPAAm-grafted surface. We only consider the hydrogen bond number between PNIPAAm and water, because we have demonstrated that the interaction between water and PNIPAAm is the main interaction in this process. The results are shown in Fig. 11. At 313 K, the distance between cytochrome c and silica surface decreases from 10.0 nm to 6.2 nm,which favors the adsorption. When the temperature decreases from 313 K to 290 K, which makes PNIPAAm from hydrophobic to hydrophilic, the distance between cytochrome c and silica surface increases from 6.2 nm to 7.7 nm. This indicates that cytochrome c is excluded from PNIPAAm-grafted-silica surface,which favors the desorption. Fig. 11 (b) gives the interaction energy between cytochrome c and PNIPAAmgrafted-surface. In adsorption process at 313 K, the interaction energy between cytochrome c and PNIPAAmgrafted-surface increases and reaches about 3500 kJ·mol−1. In desorption process at 290 K, however, the interaction energy decreases, indicating a weak interaction between cytochrome c and the surface at lower temperature. Fig. 11 (c) shows that the hydrogen bond number between PNIPAAm and water decreases in adsorption but increases in desorption, indicating a stronger interaction between PNIPAAm and water,which is the driving force for this temperature-triggering chromatography. Above results have demonstrated that at the temperature below LCST, water competitively forms hydrogen bond with PNIPAAm, decreasing the interaction between cytochrome c and the surface. For conventional reverse phase chromatography(RPC), a stronger dissection driving force is obtained by adding eluent that competitively interacts with adsorbent. The results in Fig. 7 show the possibility of using temperature to adjust the interaction between cytochrome c, surface and water for adsorption and desorption.
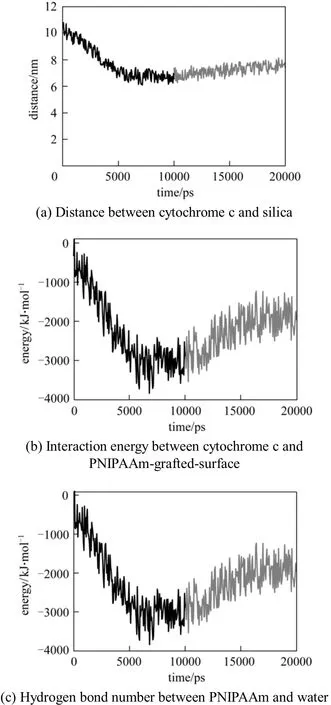
Figure 11 Adsorption and desorption of cytochrome c from the surface of PNIPAAm-grafted-silicaT/K: 313; 290
4.4 Experimental validation of adsorption and desorption behavior of cytochrome c on PNIPAAmgrafted-silica
Using the above-mentioned synthetic procedures,we obtain a high-density PNIPAAm-grafted silica surface. The elemental analysis is shown in Table 1,and the substantial increase in the N and C contents confirms the presence of PNIPAAm on the silica surface.
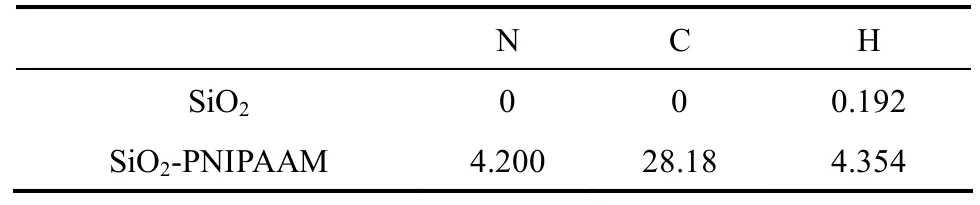
Table 1 Elemental Analysis (%)
The DSC profile of PNIPAAm-grafted-silica is shown in Fig. 12 (a), which confirms that this material exhibits excellent response to temperature, with the LCST value of PNIPAAm-grafted-silica at 307 K. Fig.12 (b) shows the thermogravimetric analysis results.Molecular mass reduction appears at each step, indicating that the reaction proceeded as expected. According to Eq. (2), the grafting ration of PNIPAAmgrafted-silica is calculated as approximately 48%.
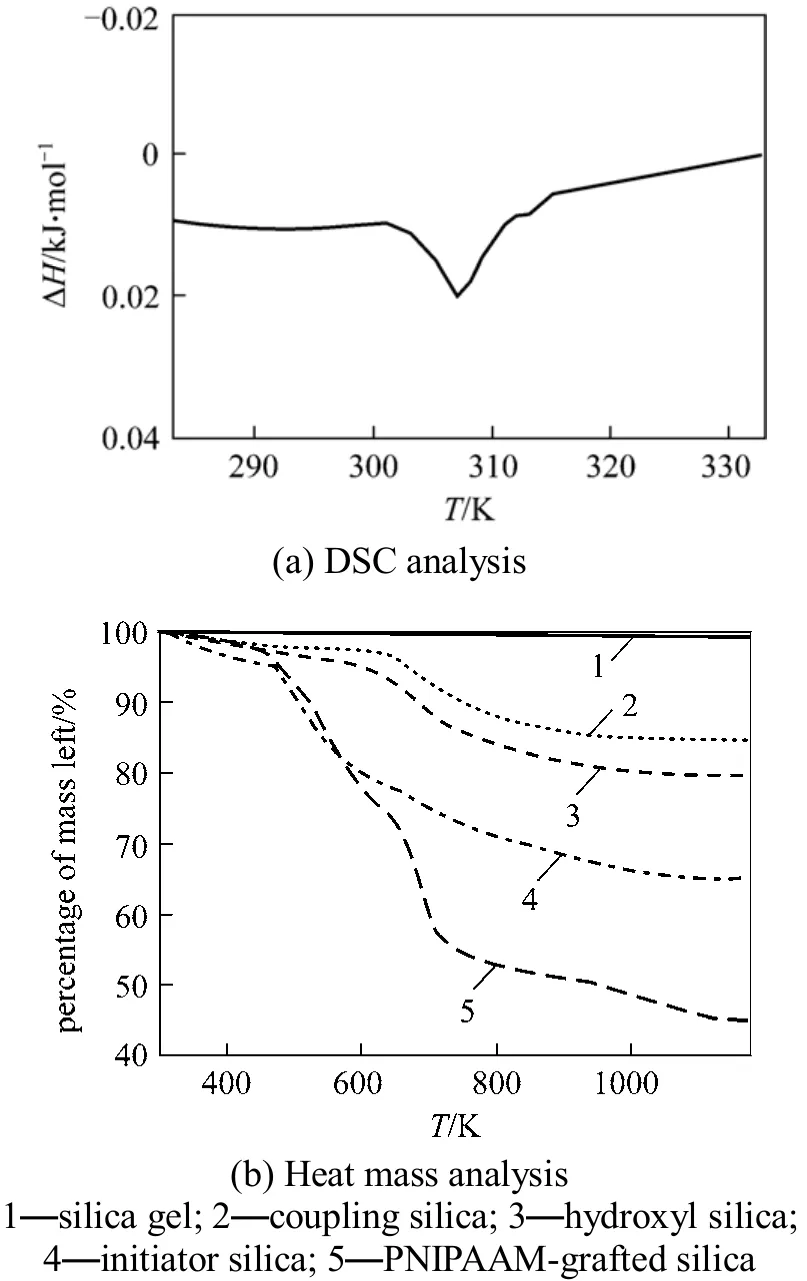
Figure 12 Characterization of PNIPAAm-grafted-silica
Adsorption of cytochrome c onto PNIPAAmgrafted silica was carried out at 290 K and 313 K using the procedures described above. The results given in Table 2 suggest that the adsorption capacity at 313 K is significantly higher than that at 290 K. After cytochrome c adsorbed onto the PNIPAAm-grafted silica,the flask incubated at 313 K was cooled down to 290 K,at which the adsorption capacity of PNIPAAm-grafted silica was decreased. The protein recovery of cytochrome c is 29.2%, so it is possible to recover protein by adjusting the temperature. The experimental results show that the desorption process triggered by temperature is more moderate compared with RPC andare identical with the simulation results.

Table 2 Adsorption capacity of PNIPAAm-grafted silica
This behavior may form the basis of a novel RPC process, in which adsorption and desorption are accomplished by changing the temperature. Moreover,this process differs from conventional RPC, in which it is necessary to apply an elution buffer containing chemicals that preferentially dissolve the adsorbed protein. Another notable advantage of this temperaturebased RPC technique over the conventional one is that it avoids the buffer exchange steps and thus greatly simplifies the operation. This is particularly advantageous for large-scale separation processes.
The adsorption capacity of cytochrome c on PNIPAAm-grafted silica is lower than that on conventional alkyl-based adsorbents such as C8, as shown in Table 2. This can be improved by using a porous matrix as the support or by increasing the grafting density.
5 CONCLUSIONS
PNIPAAm-grafted silica was synthesized for temperature-triggered RPC of proteins. The conformational transitions of PNIPAAm and the protein as a function of temperature, arising from the intra- and inter-molecular interactions between proteins, polymers, and silica surface, were analyzed by molecular dynamics simulation. The hydrogen bonds formed between the polymer and water were also examined as a function of temperature and were found to be responsible for the displacement of the protein. The simulation indicated the possibility of using this type of chromatography for protein separation with temperature as the sole operation parameter for adsorption and desorption. The adsorption of cytochrome c on the synthetic PNIPAAm-grafted silica at temperature above and below the LCST of PNIPAAm confirmed the potential of this method. The temperature-triggered chromatography process greatly simplifies the operation, particularly in comparison with conventional chromatography based on the change of solutions, since it is easier to adjust the temperature. Development of new media with enhanced temperature-responsive behavior and design of a novel column suitable for manipulating temperature are included into the upcoming efforts for development of this novel chromatographic technique.
1 Janson, J.C., “The development of gel media and columns for large-scale chromatography of proteins, a historical review”, Chin. J.Chem. Eng., 10 (6), 690-695 (2002).
2 Ivanov, A.E., Galaev, I.Y., Kazakov, S.V., Mattiasson, B., “Thermosensitive copolymers of N-vinylimidazole as displacers of proteins in immobilized metal affinity chromatography”, J. Chromatogr. A, 907(1/2), 115-130 (2001).
3 Schild, H.G., “Poly(N-isopropylacrylamide): experiment, theory and application”, Progress in Polymer Science, 17 (2), 163-249 (1992).
4 Lu, D.N., Liu, Z.X., Zhang, M.L., Wang, X.G., Liu, Z., “Dextrangrafted-PNIPAAm as an artificial chaperone for protein refolding”,Biochem. Eng. J., 27, 336-343 (2006).
5 Kanazawa, H., Nishikawa, M., Mizutani, A., Sakamoto, C., Morita-Murase, Y., Nagata, Y., Kikuchi, A., Okano, T., “Aqueous chromatographic system for separation of biomolecules using thermoresponsive polymer modified stationary phase”, J. Chromatogr. A,1191 (2), 157-161 (2008).
6 Maharjan, P., Hearn, M.T.W., Jackson, W.R., “Development of a temperature-responsive agarose-based ion-exchange chromatographic resin”, J. Chromatogr. A, 1216 (50), 8722-8729 (2009).
7 Akiyama, Y., Shinohara, Y., Hasegawa, Y., Kikuchi, A., Okano, T.,“Preparation of novel acrylamide-based thermoresponsive polymer analogues and their application as thermoresponsive chromatographic matrices”, J. Polym. Sci. A—Polym. Chem., 46 (16),5471-5482 (2008).
8 Kidoaki, S., Ohya, S., Nakayama, Y., Matsuda, T., “Thermoresponsive structural change of a poly(N-isopropylacrylamide) graft layer measured with an atomic force microscope”, Langmuir, 17 (8),2402-2407 (2001).
9 Xu, F.J., Zhong, S.P., Yung, L.Y.L., Kang, E.T., Neoh, K.G., “Surface-active and stimuli-responsive polymer−Si(100) hybrids from surface-initiated atom transfer radical polymerization for control of cell adhesion”, Biomacromolecules, 5 (6), 2392-2403(2004).
10 Fu, Q., Rao, G.V.R., Basame, S.B., Keller, D.J., Artyushkova, K.,Fulghum, J.E., Lopez, G.P.J., “Reversible control of free energy and topography of nanostructured surfaces”, Journal of the American Chemical Society, 126 (29), 8904-8905 (2004).
11 Balamurugan, S., Mendez, S., Balamurugan, S.S., O’Brien, M.J.,Lopez, G.P., “Thermal response of poly(N-isopropylacrylamide)brushes probed by surface plasmon resonance”, Langmuir, 19 (7),2545-2549 (2003).
12 Takei, Y.G., Aoki, T., Sanui, K., Ogata, N., Sakurai, Y., Okano, T.,“Dynamic contact-angle measurement of temperature-responsive surface properties for poly(N-isopropylacrylamide) grafted surfaces”,Macromolecules, 27 (21), 6163-6166 (1994).
13 Jones, D.M., Smith, J.R., Huck, W.T.S., Alexander, C., “Variable adhesion of micropatterned thermoresponsive polymer brushes:AFM investigations of poly (N-isopropylacrylamide) brushes prepared by surface-initiated polymerizations”, Advanced Materials, 14(16), 1130-1134 (2002).
14 Lindqvist, J., Nystrom,D., Ostmark, E., “Intelligent dual-responsive cellulose surfaces via surface-initiated ATRP”, Biomacromolecules,9, 2139-2145 (2008).
15 Lin, S.Y., Chen, K.S., Run-Chu, L., “Thermal micro ATR/FT-IR spectroscopic system for quantitative study of the molecular structure of poly(N-isopropylacrylamide) in water”, Polymer, 40 (10),2619-2624 (1999).
16 Longhi, G., Lebon, F., Abbate, S., “Molecular dynamics simulation of a model oligomer for poly(N-isopropylamide) in water”, Chemical Physics Letters, 386, 123-127 (2004).
17 Gangemi, F., Longhi, G., Abbate, S., Lebon, F., Cordone, R.,Ghilardi, G.P., Fornili, S.L., “Molecular dynamics simulation of aqueous solutions of 26-unit segments of p(NIPAAm) and of p(NIPAAm) ‘Doped’ with amino acid based comonomers”, J. Phys.Chem. B, 112 (38), 11896-11906 (2008).
18 Lu, D.N., Liu, Z., “Molecular simulation of polymer assisted protein refolding”, J. Chem. Phy., 123 (13), 134903 (2005).
19 Giovambattista, N., Rossky, P.J., Debenedetti, P.G., “Effect of pressure on the phase behavior and structure of water confined between nanoscale hydrophobic and hydrophilic plates”, Physical Review E,73 (4), 041604 (2006).
20 Garcia-Arellano, H., Valderrama, B., Saab-Rincon, G., Vazquez-Duhalt, R., “High temperature biocatalysis by chemically modified cytochrome c”, Bioconjugate Chemistry, 13 (6), 1336-1344 (2002).
21 Berendsen, H.J.C., Grigera, J.R., Stroatsma, T.P., “The missing term in effective pair potentials”, J. Phys. Chem., 91, 6269-6271 (1987).
22 Spoel, D., Lindahl, E., Hess, B., Buuren, A.R., Apol, E., Meulenhoff,P.J., Tieleman, D.P., Sijbers, A.L.T.M., Feenstra, K.A., Drunen, R.,Berendsen, H.J.C., Gromacs User Manual version 4.5, www.gromacs.org(2010).
23 Galaev, I.Y., Mattiasson, B., “Smart polymers and what they could do in biotechnology and medicine”, Trends in Biotechnology, 17 (8),335-340 (1999).
24 Wang, X., Xiao, X., Wang, X., Zhou, J., Li, L., Xu, J., Guo, B.,“Reversibly switchable double-responsive block copolymer brushes”,Macromolecular Rapid Communications, 28 (7), 828-833 (2007).
25 Kang, K., Lu, D.N., Zhang, M.L., Liu, Z., “All-atom molecular dynamics simulation of protein separation process by reverse phase liquid chromatography”, CISEC J., 3, 660-667 (2010). (in Chinese)
2011-05-23, accepted 2011-07-07.
* Supported by State Key Laboratory of Chemical Engineering (SKL-ChE-09A05) and the National Excellent Doctoral Dissertation Special Fund (200956).
** To whom correspondence should be addressed. E-mail: ludiannan@tsinghua.edu.cn, liuzheng@mail.tsinghua.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Optimization for Production of Intracellular Polysaccharide from Cordyceps ophioglossoides L2 in Submerged Culture and Its Antioxidant Activities in vitro*
- A Pilot-scale Demonstration of Reverse Osmosis Unit for Treatment of Coal-bed Methane Co-produced Water and Its Modeling*
- ECT Image Analysis Methods for Shear Zone Measurements during Silo Discharging Process*
- Adsorptive Thermodynamic Properties and Kinetics of trans-1,2-Cyclohexandiol onto AB-8 Resin
- Tracking Submicron Particles in Microchannel Flow by Microscopic Holography*
- Oxidation of p/o-Cresols to p/o-Hydroxybenzaldehydes Catalyzed by Metalloporphyrins with Molecular Oxygen*
