Adsorptive Thermodynamic Properties and Kinetics of trans-1,2-Cyclohexandiol onto AB-8 Resin
2012-10-31XIEYanxin谢艳新HOULili侯丽丽YANGQian杨倩andJIANGDenggao蒋登高CollegeofChemicalEngineeringandEnergyZhengzhouUniversityZhengzhou45000ChinaDepartmentofChemistryZhengzhouUniversityZhengzhou45000ChinaCommitteeofBaoshanCircularEconomyI
XIE Yanxin (谢艳新)*, HOU Lili (侯丽丽), YANG Qian (杨倩) and JIANG Denggao (蒋登高) College of Chemical Engineering and Energy, Zhengzhou University, Zhengzhou 45000 China Department of Chemistry, Zhengzhou University, Zhengzhou 45000 China Committee of Baoshan Circular Economy Industrial Agglomeration, Hebi 45800, China
Adsorptive Thermodynamic Properties and Kinetics of trans-1,2-Cyclohexandiol onto AB-8 Resin
XIE Yanxin (谢艳新)1,*, HOU Lili (侯丽丽)2, YANG Qian (杨倩)3and JIANG Denggao (蒋登高)11College of Chemical Engineering and Energy, Zhengzhou University, Zhengzhou 450001, China2Department of Chemistry, Zhengzhou University, Zhengzhou 450001, China3Committee of Baoshan Circular Economy Industrial Agglomeration, Hebi 458030, China
AB-8 resin was used as an adsorbent for the removal of trans-1,2-cyclohexandiol (CHD) from aqueous solutions. Batch experiments were carried out to investigate the effect of contact time and temperature on sorption efficiency. The adsorptive thermodynamic properties and kinetics of CHD from water onto AB-8 resin were studied.The Langmuir and Freundlich isotherm models were employed to discuss the adsorption behavior. Thermodynamic parameters such as ∆GӨ, ∆HӨand ∆SӨwere calculated. The results indicate that the equilibrium data are perfectly represented by Langmuir isotherm model. Thermodynamic study reveals that it is an exothermic process in nature and mainly physical adsorption enhanced by chemisorption with a decrease of entropy process. The kinetics of CHD adsorption is well described by the pseudo second-order model. The adsorbed CHD can be eluted from AB-8 resin by 5% ethanol aqueous solution with 100% elution percentage.
AB-8 resin, trans-1,2-cyclohexandiol, adsorption, isotherm, thermodynamics, kinetics
1 INTRODUCTION
Various processes have been employed for the removal of low concentration organic pollutants from aqueous solutions including advanced oxidation, membrane filtration, biological degradation, electrochemical oxidation, photocatalytic degradation, and adsorption [1, 2]. Compared to other methods, adsorption is receiving more attention in environmental treatment applications because it is simple, relatively low-cost and efficient [3, 4]. Active carbons are widely used adsorbents due to their adsorption abilities for many organic pollutants, but its low adsorption selectivity,difficult desorption, short operation life, high initial cost, and the need for a costly regeneration system make it less economically viable as an adsorbent. In recent years, non-ionic macroporous crosslinked polystyrene resins are considered as an alternative to active carbons for the removal of organic pollutants from waste streams [5]. Previous studies indicate that the macroporous resins have excellent adsorption ability for most organics, compared to active carbons. The wide variations in functionality, specific surface area and porosity make resins possible to remove specific organics selectively. Furthermore, the regeneration of resins can be easily accomplished with solvents, while a high temperature and/or steam is needed for regeneration of active carbon.
The adsorption performance of macroporous resins for some adsorbates, including phenols, metal ions,low carbon alcohols and dyes, was reported [6, 7]. We developed a green process to oxidize cyclohexene synthesising epoxycyclohexane with hydrogen peroxide and self-made catalyst [8]. Since the epoxycyclohexane production wastewater contains low concentration trans-1,2-cyclohexandiol (CHD, epoxycyclohexane hydrolysis), it is necessary to find low-cost and effective method to resolve the pollution problem. In this work, the removal of CHD from aqueous solutions with macroporous AB-8 resin is investigated. Experiments for adsorption isotherms and kinetics are carried out. Thermodynamic parameters for adsorption of CHD onto AB-8 resin are calculated. The experimental results may provide a way for the removal and recovery of CHD from aqueous solutions for environmental protection.
2 MATERIALS AND METHODS
2.1 Materials and preparation of CHD solutions trans-1,2-Cyclohexanediol (C6H12O2, purity >99%)was purchased from Acros organics, USA. The stock solution of CHD was prepared by dissolving a required amount of CHD in distilled water. The experimental solutions were obtained by diluting stock solution of CHD with distilled water to the desired concentration.All the other reagents such as ethanol, acetone, pyridine used in this work were analytically pure grade.HPD-417, HPD-500, HPD-826, HPD-950 and polyamide resins were supplied by Cangzhou Bon Adsorber Technology Co., Ltd., Hebei Province, China.AB-8, NKA-9, ADS-12 and ADS-17 resins were supplied by Tianjin Nankai Hecheng Technology Co.,Ltd., Tianjin, China. Scanning electron microscope(JEOL JSM-7500F, Japan) was used to examine the resin surface.
2.2 Experimental methods
The adsorption experiments were carried out by
adding 1 g treated resin into each 250 ml stoppered conical flask containing 80 ml CHD solution with certain concentration. The flasks were completely sealed and placed in a thermostat shaker at a pre-set temperature with shaking speed of 140 r·min−1. The adsorption equilibrium experiments were carried out at 293.15, 298.15 and 303.15 K with initial CHD concentration of 3-40 mg·ml−1, separately. The concentrations of CHD in the solutions before and after adsorption were determined by gas chromatography. The adsorption capacity is calculated by following equation
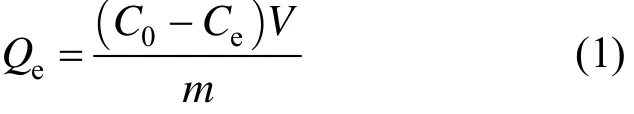
where Qe(mg·g−1) is the adsorption capacity, C0(mg·ml−1) and Ce(mg·ml−1) are the initial and equilibrium CHD concentrations, respectively, V (ml) is the volume of the solution and m (g) is the mass of dry adsorbent.
3 RESULTS AND DISCUSSION
3.1 Selection of resins
Typical properties and adsorption capacity of CHD on different resins are shown in Table 1. It is well known that pore diameter and specific surface area of resin affect its adsorption capacity. As shown in Table 1, AB-8 resin has excellent selective adsorption capacity for CHD, since it has larger specific surface area and smaller average pore size. Thus AB-8 resin was chosen in the following study.
3.2 Characteristics of AB-8 resin
Figures 1 and 2 show the surface morphological characteristic of AB-8 resin before and after the adsorption of CHD using scanning electron microscope(SEM) technology, indicating that the AB-8 resin adsorbs CHD effectively.
3.3 Effect of contact time and temperature
Figure 3 shows the adsorption capacity versus contact time at different temperatures. The adsorption equilibrium is established at 120 min, which was taken as the adsorption equilibrium time for all other experiments. The adsorption process can be divided into three stages. The first stage is the rapid initial adsorption within 40 min. The second stage is a slow adsorption process in 40-100 min, in which the increase of adsorption capacity becomes much slower.After 100 min, the adsorption capacity does not vary significantly, so the adsorption may be in a dynamic equilibrium. The reason is that during the adsorption of CHD, initially the CHD molecules rapidly reach the surface by external diffusion, then they have to diffuse slowly into the porous structure of the adsorbentbecause many of the available external sites are occupied. Fig. 3 also shows that at lower temperature,more CHD is adsorbed onto AB-8 resin, probably because that the bonds between CHD molecules and active sites of adsorbent are strengthened at low temperature. It suggests that the adsorption of CHD is exothermic in nature.

Table 1 Typical properties and adsorption capacity of resins (CHD concentration 7.823 mg·ml−1, 298.15 K)

Figure 1 SEM image of raw AB-8 resin

Figure 2 SEM image of AB-8 resin after adsorption CHD

Figure 3 Effect of contact time on CHD adsorption onto AB-8 at initial concentration of 25 mg·ml−1 at 293.15,298.15 and 303.15 KT/K: ■ 293.15; ● 298.15; ▲ 303.15
3.4 Adsorption isotherms
For solid-liquid adsorption system, adsorption isotherm is important to describe adsorption behavior.When the adsorption reaches equilibrium state, the adsorption isotherm can be used to indicate the distribution of adsorbate molecules in solid and liquid phases. In this paper, Langmuir and Freundlich plural are employed to investigate the adsorption behavior.
Figure 4 shows the CHD uptake, Qe(mg·g−1), on AB-8 resin versus initial concentration at different temperatures after adsorption for 120 min. The CHD uptake decreases as temperature increases, which confirms the exothermic adsorption process.

Figure 4 Adsorption isotherm of CHD on AB-8 resin T/K: ■ 293.15; ● 298.15; ▲ 303.15
3.4.1 Langmuir isotherm
The Langmuir adsorption isotherm is most widely used for the adsorption of solute from liquid solutions. It plays an important role in determining the maximum capacity of adsorbent. The Langmuir isotherm is based on the assumptions that adsorption takes place at homogeneous sites, the interactions among adsorbate molecules are negligible, and the adsorbent surface is saturated with monolayer. The Langmuir isotherm equation is expressed as [9]

where Qe(mg·g−1) is the equilibrium adsorption capacity per unit mass adsorbent, Ce(mg·ml−1) is the equilibrium concentration of solution, Qm(mg·g−1) is the theoretical maximum adsorption capacity per unit mass adsorbent, and KL(L·g−1) is the Langmuir isotherm constant.
The plot of 1/Qeversus 1/Cefor the adsorption of CHD on AB-8 resin at 293.15, 298.15 and 303.15 K is shown in Fig. 5. The parameters calculated from linear regressive analysis are given in Table 2. The values of R2>0.996 indicate that the Langmuir isotherm model is suitable to describe the adsorption equilibrium of CHD on AB-8 resin in 293.15-303.15 K. Moreover,Qmand KLdecrease as temperature increases. KLrelates to adsorption heat, so higher value of KLindicates a favorable adsorption process. These results further verify that the adsorption of CHD on AB-8 resin is an exothermic process.

Figure 5 Adsorption isotherm fitted with Langmuir model T/K: ■ 293.15; ● 298.15; ▲ 303.15
3.4.2 Freundlich isotherm
The Freundlich isotherm is an empirical equation based on the assumption that the adsorption takes place on heterogeneous surfaces of solids and in multilayer sorption manner. The Freundlich isotherm equation is expressed as [10]

where KF(L·g−1) and 1/n are Freundlich constants.
The plot of lnQeversus lnCeat different temperatures is presented in Fig. 6. The Freundlich parameters and correlation coefficients (R2) evaluated

Table 2 Fitting results of adsorption isotherm equation at different temperatures
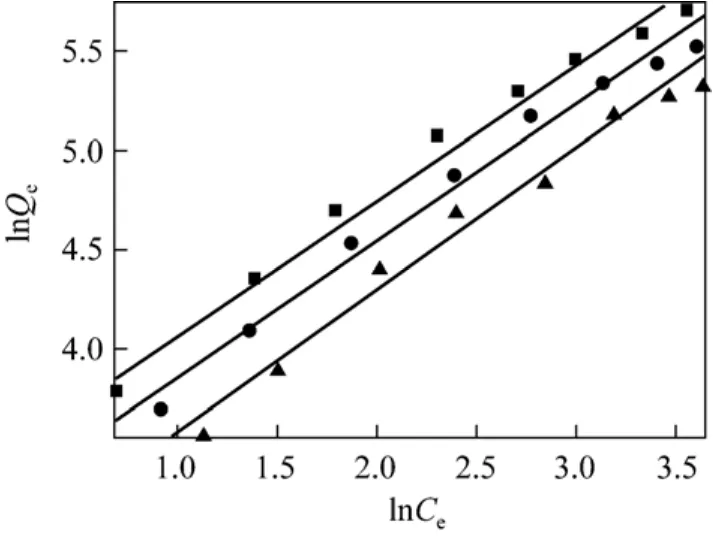
Figure 6 Adsorption isotherm fitted with Freundlich model T/K: ■ 293.15; ● 298.15; ▲ 303.15
from the linear plot are listed in Table 2. The Freundlich parameter 1/n relates to the surface heterogeneity.When 0<1/n<1, the adsorption is favorable; when 1/n=1, the adsorption is homogeneous and there is no interaction among the adsorbed species; when 1/n>1,the adsorption is unfavorable [11]. The value of KFrelates to adsorption capacity, so these values of KFand 1/n indicate that the adsorption occurs easily.Table 2 shows that Langmuir isotherm presents higher regression coefficient R2than Freundlich isotherm, so the surface of AB-8 resin is homogeneous with monolayer adsorption.
3.5 Adsorption thermodynamics
Based on fundamental thermodynamic concept, it is assumed that in an isolated system, energy cannot be gained or lost and the entropy change is the only driving force [12]. In applications, both energy and entropy are considered in order to determine which process will occur spontaneously. The thermodynamic parameters that must be considered in adsorption processes are the changes in standard enthalpy (∆HӨ),standard entropy (∆SӨ), and standard free energy(∆GӨ)due to transfer of unit mole of solute from solution to solid-liquid interface. The thermodynamic parameters∆GӨ, ∆HӨand ∆SӨcan be determined by [13]


The ∆HӨand ∆SӨvalues are calculated from the slope and intercept from the plot of lnKLversus 1/T by linear regression analysis. The thermodynamic parameters are listed in Table 3. The ∆GӨvalues are negative,revealing the spontaneous nature of adsorption. The negative value of ∆HӨindicates the exothermic nature of adsorption, in agreement with the experimental observation. Generally, the magnitude of ∆HӨvalue is in the range of 2.1-20.9 and 80-200 kJ·mol−1for physical and chemical adsorptions, respectively [14]. In this work, the absolute value of ∆HӨis 38.371 kJ·mol−1,indicating that the adsorption is mainly physical in nature enhanced by chemisorption. Negative ∆SӨvalue suggests that the adsorbate molecules at the solid/solution interface are less random. It is also supposed [15] that the change of ∆SӨvalue is related to the displacement of the adsorbed water molecules by the adsorbate. In this study, the negative ∆SӨvalue may reveal that the AB-8 resin surface does not prefer CHD molecules over adsorbed water molecules. Thus,the adsorption of CHD on AB-8 resin under the condition is considered as an enthalpy driven process.

Table 3 Thermodynamic parameters for the adsorption of CHD on AB-8
3.6 Adsorption kinetics
The kinetics describes the adsorption rate of adsorbate on adsorbent and controls the equilibrium time[16], which gives important information for designing and modeling the processes. In this study, the adsorption data is analyzed using two kinetic models: the pseudo first-order and pseudo second-order model,which are extensively used in kinetic study. The intraparticle diffusion model is also used to determinethe diffusion mechanism of the adsorption system.

Table 4 Fitting results of adsorption kinetic equation
3.6.1 Pseudo first-order model
The pseudo first-order kinetic model is based on the assumption that the rate of change of adsorbed solute with time is proportional to the difference in equilibrium adsorption capacity and the adsorbed amount, which can be expressed as [17]
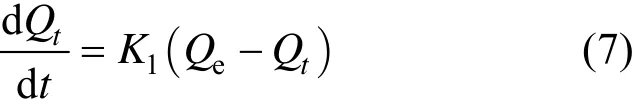
With the boundary condition Qt=0 at t=0, the integration of Eq. (7) is [18]

where Qt(mg·g−1) is the adsorption capacity per unit mass of adsorbent at time t (min) and K1(min−1) is the pseudo first-order rate constant.
The plot of ln(1−Qt/Qe) against t is shown in Fig. 7.The kinetic parameters in the pseudo first-order model(Eq. 8) are tabulated in Table 4. Low R2values and notable variances between the experimental and theoretical uptakes indicate the poor fitting of the model.

Figure 7 Adsorption kinetics fitted by pseudo first-order modelT/K: ■ 293.15; ● 298.15; ▲ 303.15
3.6.2 Pseudo second-order model
The pseudo second-order model is based on the assumption that the rate-limiting step involves chemisorption. The equation can be expressed as [19]

With the initial condition Qt=0 at t=0, the integration can be rewritten as [20]

where K2(g·mg−1·min−1) is the pseudo second-order rate constant.
The linear plot of t/Qtversus t (Fig. 8) is analyzed by linear regression to obtain parameters Qeand K2. The results are given in Table 4. The calculated Qevalues are close to the experimental values, so the pseudo second-order model fits the experimental data quite well. The correlation coefficients (R2) are higher than 0.997, indicating that the adsorption of CHD on AB-8 follows the pseudo second-order model. According to this model, boundary layer resistance is not the rate-limiting step, the external resistance model cannot adequately describe the adsorption, and the rate-controlling step is chemical adsorption involving valency forces through exchange or sharing of electrons between CHD molecules and adsorbent.3.6.3 Intraparticle diffusion model

Figure 8 Adsorption kinetics fitted by pseudo second-order modelT/K: ■ 293.15; ● 298.15; ▲ 303.15
Determination of rate-limiting step is important for an adsorption process. The adsorption of adsorbate molecules from the bulk liquid phase onto adsorbent surface is presumed to involve three stages: (1) mass transfer of adsorbate molecules across the external boundary layer; (2) intraparticle diffusion within the pores of adsorbent; (3) adsorption at a site on the surface. The intraparticle diffusion model can be described as [21]

where Kt(g·mg−1·min−0.5) is the intraparticle diffusion rate constant and C is associated to the boundary layer thickness.
If the adsorption follows the intraparticle diffusion model, the plot of Qtagainst t0.5should show linear relationship and the line passes through the origin,then the rate limiting step is due to the internal diffusion only. Otherwise, some other effects are also involved. Fig. 9 indicates that the intraparticle diffusion model is not appropriate, since the lines of Qtversus t0.5do not pass through the origin and coefficients R2are poor (Table 4). Thus the internal diffusion is not the only rate-controlling step, and other effects such as boundary layer may control the adsorption process to some extent, indicating that CHD molecules adsorb on the sites in a thin region adjacent to the external surface of AB-8 resin rather than diffuse deeply into the pores.

Figure 9 Adsorption kinetics fitted by intraparticle diffusion modelT/K: ■ 293.15; ● 298.15; ▲ 303.15
3.7 Desorption

Figure 10 The effect of desorption
In this work, desorption of CHD was carried out using ethanol aqueous eluent solutions with various concentrations, and the result is shown in Fig. 10. The elution ratio was different at various eluent concentrations, CHD desorption ratio was 100.0%, 98.9%,91.6% and 88.9% with 5%, 10%, 20% and 30% ethanol aqueous solutions, respectively. Therefore, 5%ethanol aqueous solution is more effective for the desorption of CHD from AB-8 resin. The CHD adsorbed on the resin are desorbed easily, so the resin can be used repeatedly in CHD adsorption.
4 CONCLUSIONS
The present work showed that AB-8 resin can be used as a low cost, efficient adsorbent for the removal of CHD from its aqueous solutions. The equilibrium data were better described by Langnuir isotherm model at 293.15, 298.15 and 303.15 K. Based on the thermodynamic parameters, the adsorption of CHD on AB-8 resin is an exothermic and primarily physical process in nature enhanced by chemisorption with a decrease of entropy. The adsorption kinetics follows the pseudo second-order model and the intraparticle diffusion is not the only rate-controlling step in the CHD adsorption . The CHD adsorption capacity decreases as temperature increases in the range of 293.15-303.15 K. 5% ethanol aqueous solution provides effective desorption of CHD from AB-8 resin.
NOMENCLATURE
Ceequilibrium concentration of CHD, mg·ml−1
C0initial concentration of CHD, mg·ml−1
∆GӨstandard free energy, kJ·mol−1
∆HӨstandard enthalpy, kJ·mol−1
KFFreundlich isotherm constant, L·g−1
KLLangmuir isotherm constant, L·g−1
Ktintraparticle diffusion rate constant, g·mg−1·min−0.5
K1pseudo first-order adsorption rate constant, mg·g−1·min−1
K2pseudo second-order adsorption rate constant, g·mg−1·min−1
m amount of adsorbent, g
n degree of nonlinearity between solution concentration and adsorption
Qeadsorption capacity at equilibrium, mg·g−1
Qmmaximum adsorption capacity of adsorbent, mg·g−1
Qtadsorption capacity at time t, mg·g−1
R universal gas constant, J·mol−1·K−1
∆SӨstandard entropy, J·mol−1·K−1
T absolute temperature, K
V volume of solution, ml
1 Van Hulle, S.W. H., Audenaert, W., Decostere, B., Hogie, J., Dejans,P., “Sustainable wastewater treatment of temporary events: the Dranouter Music Festival case study”, Water Sci. Technol., 8 (8),1653-1657 (2008).
2 Wongsarivej, P., Tongprem, P., Swasdisevi, T., Charinpanitkul, T.,“Adsorption and ozonation kinetic model for phenolic wastewater treatment ”, Chin. J. Chem. Eng., 19 (1), 76-82 (2011).
3 Polaert, I., Wilhelm, A.M., Delmas, H., “Phenol wastewater treatment by a two-step adsorption-oxidation process on activated carbon”, Chem. Eng. Sci., 57, 1585-1590 (2002).
4 Yousef, R., EI-Eswed, B., AI-Muhtaseb, A., “Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: kinetics, mechanism and thermodynamics studies”, Chem. Eng. J., 171, 1143-1149 (2011).
5 Zhang, Q.X., Chen, J.L., Xu, Z.Y., “Application of polymeric resin adsorbent in organic chemical wastewater treatment and resources reuse”, Polymer Bulletin, (4), 116-121 (2005).
6 Yu, Y., Zhuang, Y.Y., Wang, Z.H., Qiu, M.Q., “Adsorption of water-soluble dyes onto modified resin”, Chemosphere., 54 (3),425-430 (2004).
7 Li, A.M., Zhang, Q.X., Wu, H.S., Zhai, Z.C., “A new amine-modified hyper-cross-linked polymeric adsorbent for removing phenolic compounds from aqueous solutions”, Adsorpt. Sci. Technol., 22 (10),807-819 (2004).
8 Fu, L.L., Jiang, D.G., Xie, Y.X., “Synthesis of immobilized quaternary ammonium heteropolyphosphatotungstate catalysts and their activity in the epoxidation of cyclohexene”, Sci. T. J., (4), 52-55(2010). (in Chinese)
9 Langmuir, I., “The adsorption of gases on plane surfaces of glass,mica and platinum”, J. Am. Chem. Soc., 40, 1361-1403 (1918).
10 Freundlich, H.M.F., “Over the adsorption in solution”, J. Phys.Chem., 57, 385-470 (1906).
11 Han, X.L., Wang, W., Ma, X.J., “Adsorption characteristics of methylene blue onto low cost biomass material lotus leaf”, Chem. Eng.J., 171, 1-8 (2011).
12 Kumar, K.V., Kumaran, A., “Removal of methylene blue by mango seed kernel powder”, Biochem. Eng. J., 27, 83-93 (2005).
13 Liu, Q.S., Zheng, T., Wang, P., Jiang, J.P., “Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated fibers”, Chem. Eng. J., 157, 348-356 (2010).
14 Liu, Y., “Is the free energy change of adsorption correctly calculated?” J. Chem. Eng. Data, 54, 1981-1985 (2009).
15 Li, Y.H., Di, Z., Ding, J., Wu, D., Luan, Z., Zhu, Y., “Adsorption thermodynamic, kinetic and desorption studies of Pb2+on carbon nanotubes”, Water Res., 39, 605-609 (2005).
16 Tan, I.A.W., Ahmad, A.L., Hameed, B.H., “Biosorption isotherms,kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon”, J. Hazard. Mater., 164, 473-482 (2009).
17 Ho, Y.S., McKay, G., “Sorption of dye from aqueous solution by peat”, Chem. Eng. J., 70, 115-124 (1998).
18 Tutem, E., Apak, R., Unal, C.F., “Adsorption removal of chlorophenols from water by bituminous shale”, Water. Res., 32, 2315-2324(1998).
19 McKay, G., Ho, Y.S., “Pseudo second-order model for sorption processes”, Process. Biochem., 34, 451-465 (1999).
20 Yang, X., Al-Duri, B., “Kinetic modeling of liquid-phase adsorption of reactive dyes on activated carbon”, J. Colloid Interface Sci., 287,25-34 (2005).
21 Weber, W.J., Morris, J.C., “Kinetics of adsorption on carbon from solution”, J. Sanit. Eng. Div. Am. Soc. Civ. Eng., 89, 31-59 (1963).
2011-11-03, accepted 2012-03-15.
* To whom correspondence should be addressed. E-mail: xyx19830110@yeah.net
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Optimization for Production of Intracellular Polysaccharide from Cordyceps ophioglossoides L2 in Submerged Culture and Its Antioxidant Activities in vitro*
- A Pilot-scale Demonstration of Reverse Osmosis Unit for Treatment of Coal-bed Methane Co-produced Water and Its Modeling*
- ECT Image Analysis Methods for Shear Zone Measurements during Silo Discharging Process*
- Temperature-triggered Protein Adsorption and Desorption on Temperature-responsive PNIPAAm-grafted-silica: Molecular Dynamics Simulation and Experimental Validation*
- Tracking Submicron Particles in Microchannel Flow by Microscopic Holography*
- Oxidation of p/o-Cresols to p/o-Hydroxybenzaldehydes Catalyzed by Metalloporphyrins with Molecular Oxygen*
