双齿甜菜碱衍生物大环及双环镉(Ⅱ)配位化合物的合成与晶体结构
2012-09-15辛清萍李松林刘雪佳
辛清萍李松林*,刘雪佳
(1天津大学理学院化学系,天津 300072)
(2北京航空材料研究院,北京 100095)
双齿甜菜碱衍生物大环及双环镉(Ⅱ)配位化合物的合成与晶体结构
辛清萍1李松林*,1刘雪佳2
(1天津大学理学院化学系,天津 300072)
(2北京航空材料研究院,北京 100095)
利用甜菜碱衍生物1,5-二(4-羧基吡啶基)-N-甲基二乙胺(L)合成了两种镉(Ⅱ)的配位化合物[Cd2Cl4(H2O)2L2]·2H2O和[Cd2(SCN)4(μ-H2O)L2]。用X-射线单晶衍射仪测定了配合物的单晶结构,并对它们进行了元素分析、红外光谱、1H NMR、热重等表征。结构分析表明,前者具有三十六元大环框架,而后者为双环结构。由于缺乏分子之间的强烈相互作用,弱相互作用决定了这两种化合物在晶格中的堆积模式:前者由相邻分子间的π-π和C-H…π相互作用而堆积形成二维砖墙的结构;后者由配位的SCN-阴离子通过S…S弱相互作用联接成一维链状结构。
双齿甜菜碱衍生物;镉(Ⅱ)配合物;π-π堆积;C-H…π作用;S…S作用
0 Introduction
Recently,considerable progress has been made in the design and engineering of supramolecular compounds with regard to their potential applications in the fields of electronic,magnetic,optical,absorbent and catalytic areas[1-5].Much effort has been devoted to the investigation of the supramolecular contacts that areresponsible for the construction of multi-dimensional frameworks.It has been concluded that strong,selective,and directional hydrogen bonding acts as a most powerful organizing force in molecular assemble,and the use of the counter anions to link organic molecules is an alternative method for the design of new inorganic and organometallic supramolecules[6].Meantime,the other non-bonding interactions such as π-π stacking,C-H…π,S…S weak interactions,etc.also have impacted importantinfluences on the topology,functionalities and stabilization.Various structures with interesting compositions and topologies can be produced through a rational choice of ligand and metal precursor geometry.
In the present study a bident betaine derivative 1,5-bis(4-carboxypyridinium)-N-methyl-diethylamine(L)was prepared and adopted as organic building block.The compound has a pair of naked carboxylate groups,and a pair of pyridyl rings.In addition,the carboxylate group and N+atom on the pyridyl ring make the α-H more acidic.
Owing to the varietycoordination modesof carboxylate group toward metal ions,and the ability to form strong hydrogen bonds,carboxylic acid plays a very important role in constructing coordination and hydrogen bonded supramolecules.The new bidentate betaine derivative 1,5-bis(4-carboxypyridinium)-N-methyl-diethylamine (L),which bears a pair of naked carboxylate groups,a pair of pyridyl rings,and a flexible chain,was synthesized and allowed to react with cadmium salts.Two coordination compounds of cadmium(Ⅱ),namely[Cd2Cl4(H2O)2L2]·2H2O(1)and[Cd2(SCN)4(μ-H2O)L2](2),have been prepared and their crystal and molecular structures were determined.The results show that in compound 1 a pair of cadmium(Ⅱ)atoms are bridged by a pair of molecules of L forming a discrete 36-membered Cd2L2ring and in compound 2 those of atoms are bridged by a pair of molecules of L and one ligating water molecule featuring a bicyclo[17.17.1]structure.The anions(Cl-in 1,SCN-in 2)are also involved in coordination to the metal ions.Whereas,in 2 there is only one ligating water molecule which acts in a μ2-mode bridging the two cadmium (Ⅱ)atoms.In addition,the small size of the Cl-anions allows the discrete 36-membered rings to approach close enough enabling π-π stacking and C-H … π interactions between them,thus results a brick wall pattern.However,the weak S… S interactions are responsible for the zigzag chain pattern in 2.
1 Experimental
All chemicals were commercially available and used as received.1H NMR spectra were recorded with a Varian INOVE-500 spectrometer with chemical shifts reported relative to tetramethylsilane.The IR spectra were recorded from KBr pallet in the range of 4 000~400 cm-1with a Bio-Rad Exalibur FTS3000 spectrometer.Elemental analyses were performed on a Perkin-Elmer 2400 CHN element analyzer.TGA and DSC was performed on a Germany Netzsch STA409 PC/PG instruments with a heating rate of 10℃·min-1(N2atmosphere).
1.1 Synthesis
1.1.1 Synthesis of 1,5-bis(4-carboxypyridinium)-N-methyl-diethylamine(L)
N-methyl-N-bis(2-bromoethyl)amine hydrobromide was synthesized by the literature method[7].To the solution ofN-methyl-N-bis (2-bromoethyl)amine hydrobromide(13.03 g,0.04 mol)in ethanol(60 mL),ethyl isonicotinate(13.30 g,0.088 mol,excess 10%)was added.The mixture was refluxed for 36 hours and the resulting precipitate was filtered,rinsed with acetone for several times and air-dried.The product was dissolved in HCl(15%,60 mL)and refluxed for 5 h.After removal of the solvent under reduced pressure,the residue was dissolved in minimum amount of water and treated with 1,2-epoxypropane at c.a.30℃until pH≈7.Remove the solvent under reduced pressure,dissolve the syrupy residual in DMF and vigorously stir the mixture until the precipitate formed.The raw product was recrystallized in H2O/DMF.Yield:68%.1H NMR(400Hz,D2O,δ,ppm)2.39(s,3H,N-CH3),2.99~3.02(t,J=4 Hz,8 Hz,4H,CH2-N-CH2),4.57~4.59(t,J=4 Hz,4H,N-CH2),8.07~8.08(d,J=4Hz,4H,CH=N),8.65~8.65(d,J=4Hz,4H,OOCC=CH).Anal.Calcd.for C17H19N3O4·3H2O(%):C,53.26;H,6.57;N,10.96.Found(%):C,53.10;H,6.52;N,10.86.IR:1640(carboxylate Ⅴasym),1 360(carboxylate Ⅴsym),2 102 cm-1(NC Ⅴsym(-NCS)).
1.1.2 Synthesis of[Cd2Cl4(H2O)2L2]·2H2O(1)and[Cd2(SCN)4(μ-H2O)L2](2)
The preparation procedure of 1 and 2 were similar:a solution of L (192 mg,0.5 mmol)and the corresponding cadmium salts(CdCl2·2.5H2O(228 mg,1 mmol)for 1,CdCl2·2.5H2O (228 mg,1 mmol)and NH4SCN(76 g,1 mmol)for 2)in distilled water(c.a.5 mL)was heated under stirring at c.a.60℃for 15 min and filtered.The crystals of 1 and 2 available for X-ray structure analysis were obtained after standing the filtration at ambient temperature for several days.For 1,colorless prismatic crystals,yield 78%.Anal.Calcd.for C34H46Cd2Cl4N6O12(%):C,37.21;H,4.23;N,7.66.Found(%):C,35.83;H,4.35;N,7.31.IR:1629 cm-1(carboxylate Ⅴasym),1 377 cm-1(carboxylate Ⅴsym);for 2,colorless prismatic crystals,yield 42%.Anal.Calcd.for C38H40Cd2N10O9S4(%):C,40.25;H,3.56;N,12.35.Found(%):C,40.14;H,3.69;N,12.24.IR:1 610(carboxylate Ⅴasym),1391 cm-1(carboxylate Ⅴsym).
1.2 X-ray diffraction data collection,structure determination and refinement
Information concerning X-ray data collection and structure refinement is summarized in Table 1.The intensities for both 1 and 2 were collected at 293(2)K on a Bruker SMART ApexⅡdiffractometer(graphitemonochromated Mo Kα radiation:λ=0.071 073 nm,ωscan).Adsorption correction based on Multi-scan was applied to the intensity data processing.
Both structures of 1 and 2 were solved by the direct method,and the non-hydrogen atoms were refined anisotropically by full-matrix least squaresmethod based on all the diffraction data using the SHELXTL program package[8].All hydrogen atoms were placed at their calculated positions,allowed to ride on their respective parent atoms and included in the structure factor calculations.Final atomic coordinates and equivalent isotropic thermal parameters along with their estimated standard deviation,a listing of observed and calculated structure factors,anisotropic thermal parameters and H-atom coordinates as supplementary material are available on request.
CCDC:860582,1;860583,2.
2 Results and discussion
NH4SCN was added to the aqueous solution of L and CdCl2·2.5H2O (nSCN-∶nCl-=1∶2)intending to bridge different cadmium(Ⅱ)carboxylate moieties using SCN-,however,only SCN-is involved in the ligation and compound 2 is obtained.
The IR spectra of L,1 and 2 were measured,and the differences of asymmetric and symmetric vibration wave numbers of carboxylate group ΔⅤ=Ⅴasym-Ⅴsymare 280 cm-1,252 cm-1and 219 cm-1,respectively,which indicates that the coordination modes of carboxylate groups in 1 and 2 are different:in 1 the mode is probably unidentate and in 2 it may be chelate one[9].
2.1 Crystal structure of compound 1
Compound 1 crystallizes in space group P1(No.2),and consists of discrete[Cd2Cl4(H2O)2L2]·2H2O molecules and solvent water molecules packed together in the crystal lattice.The selected bond lengths and angles are listed in Table 2 and 3.
As depicted in Fig.1,the discrete 36-membered ring molecule composes a pair of Cd(Ⅱ) atoms(Cd…Cd:0.5482(2)nm)bridged by a pair of L molecules in a trans fashion with each carboxylate groups of L acting in a unidentate coordination mode (Cd-O:0.224 9(3),0.222 8(3)nm;O-Cd-O:157.1(1)°). The distorted trigonal bipyramidal coordination geometry of Cd(Ⅱ)atom (bond angles:77.6°~157.1°)is fulfilled by the ligation of a pair of Cl-anions and an aqua ligand.Aninteresting feature of 1 is that,both aqua ligands are positioned in the ring,and each forms strong hydrogen bonds with the pendent oxygen atoms of carboxylate groups ligating to the other Cd(Ⅱ)atom (O1W…O2A:0.286 3(4),O1W…O3 0.277 5(4)nm;O3…O1W…O2A:108.2(1)°).These strong hydrogen bonds play an important role in stabilizing the ring structure[10],and are responsible for the large deviation of O1-Cd1-O4A angle(157.1(1)°)from 180°.

Table 2 Selected bond lengths(nm)and angles(°)for 1 and 2

Table 3 Bong length and bond angle of hydrogen bond in complex 1 and 2
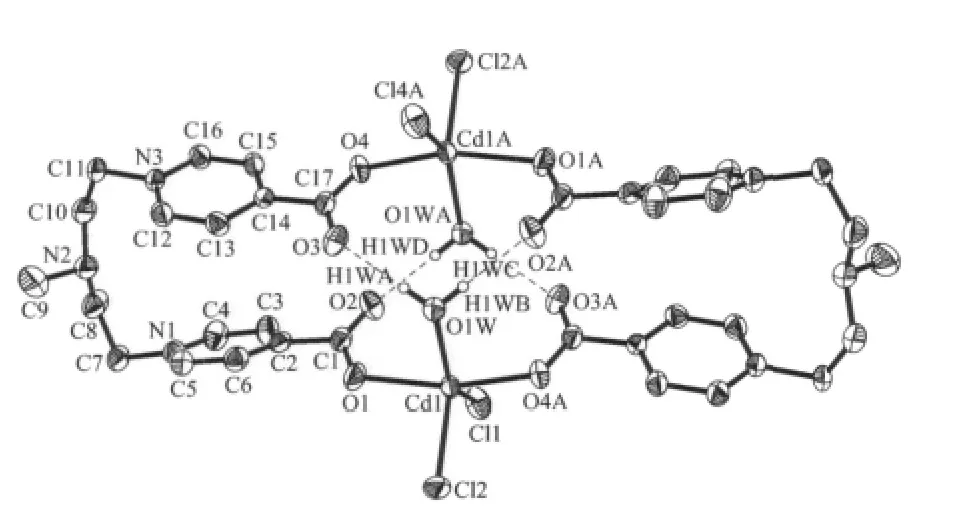
Fig.1 Structure and atom numbering scheme of compound 1
In addition,the strong hydrogen bonds also exert a great effect on the configuration of the L:they cause O1C1C2C6 torsion angle to be as large as 32.4(5)°,which significantly weakens the conjugation between the carboxylate group and the pyridyl ring,and forces the pair of pyridyl rings of L approaching each other close enough to allow a π-π interaction between them(centroid-centroid distance(Cg2…Cg3):0.399 0 nm;centroid-plane distance:0.370 7 nm;dihedron angle:27.64(1)°).
On the other hand,the existence of pyridyl rings in the discrete molecule determines the packing pattern of the compound in the crystalline state:when packing in the lattice all the pyridyl rings of adjacent molecules are mutually parallel to each other with the centroidcentroid distances being 0.381 4 (Cg1i…Cg2),0.427 6(Cg3…Cg4ii)nm and centroid-plane distances 0.372 9,0.338 1 nm,respectively.The former represents a typical π-π interaction,but the latter is best classified as a C-H…π interaction[11-12].With these non-covalent interactions,the molecules pack in a two-dimensional bridge wall pattern as depicted in Fig.2.
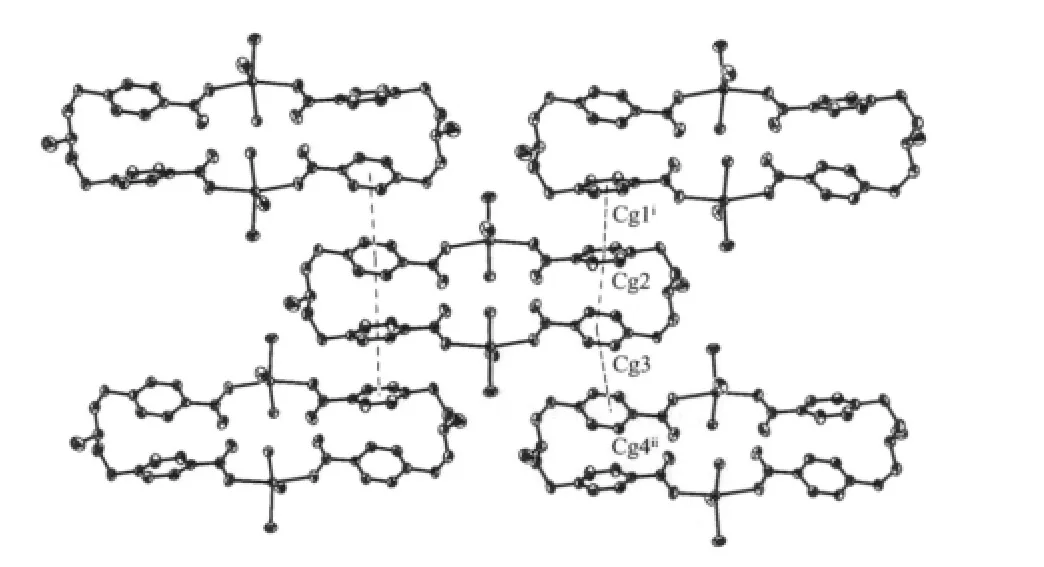
Fig.2 Two-dimensional brick-wall packing pattern in 1 showing π-π interactions between pyridyl rings of adjacent molecules
Furthermore, the three-dimensional crystal structure of the compound is furnished by the weak CH…Cl hydrogen bonds between the α-hydrogen atoms of the pyridyl rings and the coordinated Cl-anions(C…Cl distance 0.348 nm,C-H…Cl angle 118.0°][13].
2.2 Crystal structure of compound 2
Compound 2 crystallizes in space group C2/c(No.15)with discrete[Cd2(SCN)4(μ-H2O)L2] moleculespacking in the lattice.The ORTEP drawing and atom numbering scheme are shown in Fig.3,and the selected bond lengths and angles are listed in Table 2 and 3.

Fig.3 Structure and atom numbering scheme of compound 2
Similar to compound 1,in this compound two Cd(Ⅱ)atoms are also bridged by a pair of L ligands leading to a 36-membered ring.However,in 2 one of the carboxylate groups of L acting in a monodentate coordination mode and the other in an asymmetric chelate coordination mode (Cd-O distances:0.2337(2)and 0.249 1(2)nm).The aqua ligand locates at the inverse center and acts in a μ2-mode bridging the pair of Cd(Ⅱ)atoms thus forming a bicyclo[17.17.1]structure with a Cd…Cd separation of 0.401 8(1)nm,which is much shorterthan thatin 1(0.548 2(2)nm).The distorted octahedral coordination sphere of the Cd(Ⅱ)atom is completed by the ligation of a pair of SCN-anions to Cd(Ⅱ)atom through their nitrogen atoms,which point in opposite directions with an angle of 125.0°between them.
The μ2-aqua ligand is linked with free oxgen atoms from monodenate carboxylate groups by hydrogen bonds(O1W…O2:0.264 3(2)nm;O2…O1W…O2A:121.1(1)°),which are somewhat shorter than that in 1.The plausible reason is that,ligating to two Cd(Ⅱ)atoms enhances the acidity of the hydrogen atoms of the aqua ligand,hence allows stronger hydrogen bond formation.On the other hand,compared to 1,the stronger hydrogen bond in 2 results in a larger twist of the carboxylate group with respect to the pyridyl ring(O1C1C2C6 torsion angle:47.7(3)°),which further reducestheconjugation between them.However,although it is expected that the destruction of the conjugation between the carboxylate group and the pyridyl ring would shorten the length of the py-C(O2)bond,the results show that the non-coplanarity of these two moieties has no significant effect on the bond length.
The substitution of Cl-anions in 1 with SCN-anions in 2 not only drastically affects the structure of the discrete molecule:it repels one of the aqua ligands from the coordination sphere and enables the formation of aqua-bridge between two Cd(Ⅱ)atoms,but also is responsible for the packing pattern of 2.In compound 2 the SCN-anions point out of the bicyclo[17.17.1]structure,and their larger volume (compared with Cl-1anion)prevents the discrete molecules approaching each other close enough to enable π-π or C-H … π interactions between them.However,they approach to each other in such a fashion that the S…S distances between adjacent discrete molecules are 0.3572(2)nm as shown in Fig.4,which is shorter than the sum of the radius of two sulfur atoms(0.368 nm).
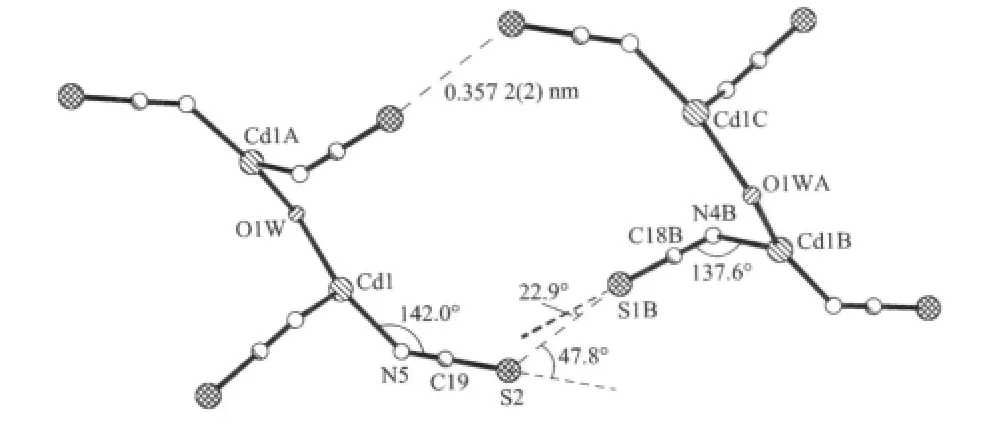
Fig.4 Drawing showing S…S interactions in 2
The S…S weak interaction has well been established for divalent sulfur(X-S-Y)by examining the published crystal structures[14].The results indicate that for the interacted pair of sulfur atoms one of which acts as an electrophile and the other as a nucleophile,and the directional preferences of nonbonded atomic S…S contacts are consistent with their frontier orbital orientations.In compound 2,the Cd-N-C angles for the two SCN-anions are significantly different(142.03(2)°and 137.6(2)°,respectively),and which indeed affectsthe Cd-N,N-C and C-S bond lengths despite the effects are very small.Obviously,the non-equivalence of these two SCN-anions determines their roles in the S…S contactpair:thesulfuratom S1B may be the electrophile and S2 the nucleophile.With these weak S…S interactions compound 2 packs in a linear fashion in the crystal as shown in Fig.5.

Fig.5 S…S linked chain of compound 2 in crystalline state
2.3 Thermal property
The thermal behavior of compounds was examined by TG-DSC.The ligand(L)losses 13.21%of its weight at in the first step(calcd.13.47%),about 73.64%of its weigth at in the second step.The conrresponding DSC curve shows intense exothermic peaks recorded at 118℃ (melting and decomposing)and 243℃(decomposing)respectively.The TG curve of sample 1 also shows two weigth losses,the first one,6.02%of its weight,is attributed to the removal of both the coordinated water molecules and free water molecules (calcd.6.56%),another weight loss of due to the decomposing of sample 1 at 285℃,The conrresponding DSC curve shows intense exothermic peaks recorded at 123℃and at 292℃for sample 1,at 218℃and at 276℃for sample 2.and they are attributed to the melting and composing.Comparing the TG curves of ligand and sample 1,sample 2,one can see a significant change on the thermal behavior of the materials.The thermal stability of sample 2 is slightly increased when it may contains water-bridge withoutfree water molecules.The decomposing temperature of sample 2 is much higher than free ligand(L)and sample 1.
3 Conclusions
A new bidentate betaine derivative 1,5-bis(4-carboxypyridinium)-N-methyldiethyl-amine was prepared and allowed to react with cadmium(Ⅱ)slates.By analyzing the crystalstructuresofthe resulting coordination compounds it has been found that the flexible chain of the compound permits its molecule adopt a clamp configuration which is advantageous for it to form large ring coordination compounds by bridging metal ions.In addition,both the positively charged quaternary nitrogen atom and the electron withdrawing carboxylate group drastically reduce the πelectron density of the pyridyl rings which enhances their ability to form π-π and C-H … π interactions.These characters make the compound a suitable candidate study the weak interactions between different moieties and their role in determining the packing fashion of the compound in crystalline state.
[1](a)Janiak C.Dalton Trans.,2003:2781-2804(b)Zhang Q C,Bu X H,Zhang J,et al.J.Am.Chem.Soc.,2007,129:8412-8413
[2](a)Yang P P,Song X Y,Liu R N,et al.Dalton Trans.,2010,39:6285-6294(b)He L,Hu Y X,Kim H,et al.Nano Lett.,2010,10:4708-4714(c)Takamizawa S,Nakata E,Akatsuka T.Angew.Chem.Int.Ed.,2006,45:2216-2221
[3](a)Wang M S,Guo G C,Zou W Q,et al.Angew.Chem.Int.Ed.,2008,47:3565-3567(b)LI Hui(李辉),ZHU Ya-Lin(朱亚林),ZHENG Lei(郑磊),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(12):2453-2458(c)TAO Wu(陶 武),LIU Jie-Min(刘 杰 民),ZHENG Yan-Jun(郑延军),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(12):2419-2424
[4](a)Pan L,Olson D H,Ciemnolonski L R,et al.Angew.Chem.Int.Ed.,2006,118:632-635(b)Wong-Foy A G,Matzger A J,Yaghi O M.J.Am.Chem.Soc.,2006,128:3494-3495
[5](a)Coates G W,Moore D R.Angew.Chem.Int.Ed.,2004,43:6618-6639(b)Lee J Y,Farha O K,Roberts J.et al.Chem.Soc.Rev.,2009,38:1450-1459
[6](a)Diaz P,Benet-Buchholz J,Vilar R,et al.Inorg.Chem.,2006,45:1617-1626(b)Kumar D K,Das A,Dastidar P.CrystEn Comm,2006,8:805-814(c)Zou R Q,Li J R,Xie Y B,et al.Cryst.Growth Des.,2004,4:79-84(d)Gale P A.Coord.Chem.Rev.,2003,240:191-221(e)Noro S,Kitaura R,Kondo M,et al.J.Am.Chem.Soc.,2002,124(11):2568-2583
[7]Pettit G R,Chamberland M R,Blonda D S,et al.Can.J.Chem.,1964,42:1699-1706
[8]Sheldrick G M.SHELXTL Version 5,Siemens Industrial,Automation Inc.,Madison,WI,1995.
[9]Nakamoto K.Infrared and Raman Spectra of Inorganic and Coordination Compounds.New Jersey:John Wiley&Son,Inc.,2009:74
[10](a)Calhorda M J.Chem.Commun.,2000:801-809(b)Lu L,Wang J,Bai J W,et al.Cryst.Res.Technol.,2008,43(12):1327-1330
[11]Janiak C.Dalton Trans.,2000:3885-3896
[12](a)He Y H,Feng Y L,Lan Y Z,et al.Cryst.Growth Des.,2008,8(10):3586-3594(b)Janiak C,Temizdemir S,Dechert S,et al.Inorg.Chem.Commum.,2000,3:271-275(c)Hannon M J,Painting C L,Alcock N W.Chem.Commum.,1999:2023-2024
[13](a)Sarma J A R P,Desiraj G R.Acc.Chem.Res.,1986,19:222-228(b)Losier P,Zaworotko M J.Angew.Chem.Int.Ed.Eng.,1996,35(23/24):2779-2782
[14](a)Jr Rosenfield R E,Parthasarathy R,Dunitz J D.J.Am.Chem.Soc.,1978,99:4860-4862(b)Row T N G,Parthasarathy R.J.Am.Chem.Soc.,1981,103:477-479
Synthesis and Crystal Structures of Cadmium(Ⅱ)Coordination Compounds of Large Ring and Bicyclo Framework with a Bidentate Betaine Derivative
XIN Qing-Ping1LI Song-Lin*,1LIU Xue-Jia2
(1Department of Chemistry,Tianjin University,Tianjin 300072,China)
(2Beijing Institute of Aeronautical Materials,Beijing 100095,China)
A new bidentate betaine derivative 1,5-bis(4-carboxypyridinium)-N-methyl-diethylamine(L)was prepared and allowed to react with cadmium salts leading to the formation of two new cadmium coordination compounds[Cd2Cl4(H2O)2L2]·2H2O and[Cd2(SCN)4(μ-H2O)L2],which were structurally characterized by IR spectoroscopy,singlecrystal X-ray diffraction,elemental analysis,thermal analysis,etc.The single crystal X-ray diffraction structure analysis of these compounds shows the molecule of former consists of discrete 36-membered ring and that of latter bicyclo[17.17.1]structure.Owing to lacking strong intermolecular interactions in these compounds,weak interactions determine their packing pattern in crystalline state:the former forms 2D brick-wall network by the ππ stacking and C-H…π interactions between pyridyl rings of adjacent molecules,and the latter zig-zag chains through S…S weak interactions between ligating SCN-anions.CCDC:860582,1;860583,2.
bidentate betaine derivative;cadmium(Ⅱ) coordination compounds;π-π stacking;C-H…π interaction;S…S weak interaction
O614.24+2
A
1001-4861(2012)04-0815-08
2011-05-21。收修改稿日期:2011-12-17。
*通讯联系人。E-mail:sl.li@live.cn
