1,10-邻菲咯啉-5,6-二酮铁(Ⅱ)配合物的合成、结构及与DNA相互作用的研究
2012-09-09赵海燕马晶军杨芙丽
赵海燕马晶军杨芙丽
(1河北科技大学理学院,石家庄 050018)
(2河北农业大学理学院,保定 070001)
(3河北化工医药职业技术学院化学与环境工程系,石家庄 050026)
1,10-邻菲咯啉-5,6-二酮铁(Ⅱ)配合物的合成、结构及与DNA相互作用的研究
赵海燕*,1马晶军2杨芙丽3
(1河北科技大学理学院,石家庄 050018)
(2河北农业大学理学院,保定 070001)
(3河北化工医药职业技术学院化学与环境工程系,石家庄 050026)
在乙腈体系中合成了Fe(Ⅱ)的配合物[Fe(phon)3](ClO3)2·2CH3CN(phon=1,10-邻菲咯啉-5,6-二酮)。X-射线衍射单晶结构表明:配合物[Fe(phon)3](ClO3)2·2CH3CN属于正交晶系,P212121空间群,Fe(Ⅱ)与3个phon配体的6个氮原子配位,形成变形的八面体结构。配合物[Fe(phon)3](ClO3)2·2CH3CN中的氢键和C=O…π相互作用将配合物连接成二维网络结构。用红外光谱、紫外-可见光谱对配合物[Fe(phon)3](ClO3)2·2CH3CN进行了表征,并用紫外光谱法对配合物与ct-DNA的相互作用做了初步研究,结果表明配合物与ct-DNA的结合常数(Kb)为2.2×105L·mol-1。
1,10-邻菲啰啉-5,6-二酮;铁(Ⅱ)配合物;晶体结构;弱相互作用;ct-DNA
As a versatile ligand for the assembly of metal organicmaterials,1,10-phenanthroline-5,6-dione(phon) is linked tometals with an N-N chelate with a free oquinoid group or an O-O chelate with two free diiminic nitrogen atoms and a bridgingmode with two different coordination sites[1-6].This ligand has the ability to form stable complexes with a wide variety of metal inos and carries an o-quinone moiety with pH-dependentelectroactivity[7-8].
Metal complexes of the type[M(LL)3]n+,where LL is either phon or 1,10-phenanthroline ligand,are particularly attractive species for the development of new diagnostic and therapeutic agents that can recognize and cleave DNA.The ligand or themetal in these complexes can be varied in an easily controlled manner to facilitate an individual application,thus providing an easy access to the details involved in DNA-binding and cleavage[9-11].In the present study, the synthesis,characterization,crystal structure,and the interaction with DNA of the complex[Fe(phon)3] (ClO3)2·2CH3CN is reported.
1 Experimental
1.1 Materials and methods
All reagents for syntheses and analyses were of analytical grade and the aqueous solution was prepared using doubly distilled water.Phon was synthesized according to the method described in a previous study[12].Elemental analyses were conducted using a Perkin-Elmer 240C analyzer.FT-IR spectra (KBr pellet)were obtained using a FT-IR 170 SX (Nicolet)spectrometer.The electronic absorption spectra were obtained via UV-Vis spectroscopy using a Jasco V-570 spectrophotometer.
1.2 Synthesis of[Fe(phon)3](ClO3)2·2CH3CN
A solutionofphon(0.0630g,0.3mmol)in ethanol (5 mL)was added with Fe(ClO3)3·9H2O(0.038 5 g,0.1 mmol)dissolved in 5 mL ethanol.The reaction mixture was stirred at room temperature for 30 min to produce a red solution.An aqueous solution of NaN3(0.0070 g,0.2 mmol)was added to the reaction mixture to obtain the Fe-phon-N3mixed-ligand complex.However the product precipitated as a deep red solid was the title complex.The complex was collected via suction filtration,washed with cold water and diethylether,and then air-dried.Crystals for the determination were prepared by recrystallizing the above material from acetonitrile.Yield:42%.Anal.Calcd.For C37H24Cl2FeN8O14(%):C,47.69;H,2.48;N, 11.23.Found(%):C,47.19;H,2.58;N,11.23.FT-IR (KBr,cm-1):3 369w,1 701s,1 569s,1 429s,1 299s, 1 122s,1 089s,941m,813m,720m,626s.
1.3 Solution preparation of DNA
Calf thymus DNA(ct-DNA,purchased from Siama)was dissolved in Tris-HCl buffer(5 mmol·L-1Tris-HCl,50 mmol·L-1NaCl,pH 7.2,Tris=Tris (hydroxymethyl)aminomethane).ct-DNA was purified following standard procedures.A UV-Vis spectrophotometer was employed to check DNA purity(A260/ A280>1.84)[13]and concentration(ε=6 600 L·mol-1·cm-1at 260 nm)[14].Stock solution was stored at 4℃and used in nomore than 4 d.
1.4 Crystal structure determ ination
Diffraction intensities for complex[Fe(phon)3] (ClO3)2·2CH3CN was collected on a Bruker Smart 1000 CCD area detector using graphite-monochromatized Mo Kαradiation(λ=0.071 073 nm)withω scan mode at 293(2)K.Unit cell dimensions were obtained with least-squares refinements.Semiempirical absorption corrections were applied using SADABS program[15].The structures were solved by direct method using the SHELXS-97[16]and refined by fullmatrix least-squaresmethods on F2by SHELXL-97[17]. Hydrogen atoms were included in calculated positions and refined with fixed thermal parameters riding on their parent atoms.Further crystallographic data and experimental details for structural analyses are summarized in Table 1.The selected bond lengths and bond angles are listed in Table 2.
CCDC:672079.
2 Results and discussion
2.1 Synthesis and characterization
The elementalanalyses of the complex[Fe(phon)3] (ClO3)2·2CH3CN is consistentwith a 1∶2 anion:cation stoichiometry,as confirmed via X-ray crystal structuredetermination,which indicates Fe(Ⅱ)regardless of starting from Fe(ClO3)3·9H2O.

Table 1 Crystal data and structure refinement summary for[Fe(phon)3](ClO3)2·2CH3CN
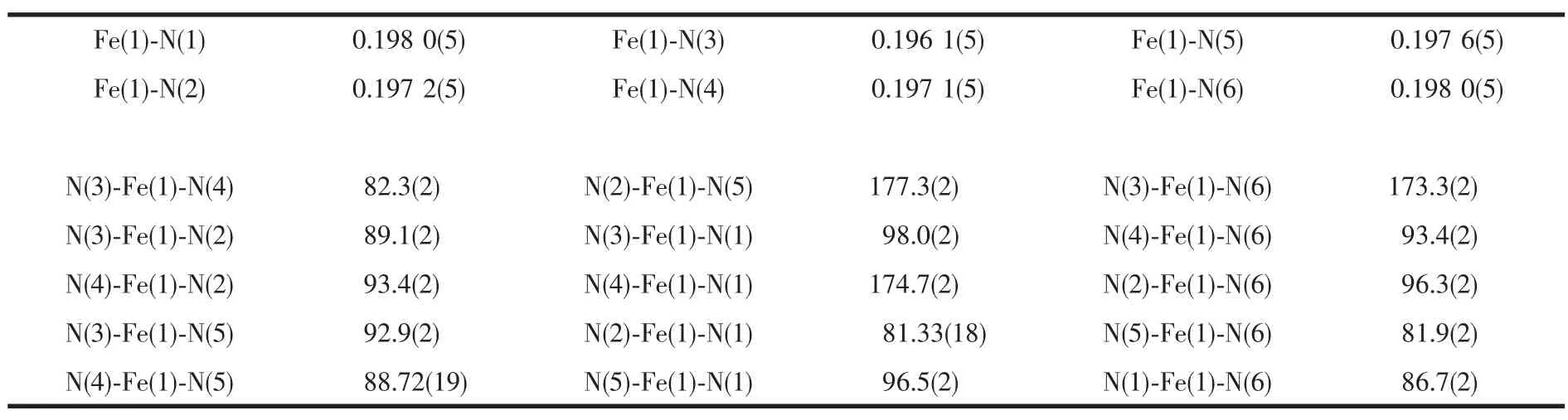
Table 2 Selected bond lengths(nm)and angles(°)for[Fe(phon)3](ClO4)2·2CH3CN
The IR spectrum of phon clearly exhibits a band at1 675 cm-1thatisascribable toastretching frequency of the C=O bands on the ligand[2,10].This band was observed to be not shifted much in the corresponding complexes,which is reasonable because the C=O moieties are far from the coordination site of the ligand with themetal ion[1,6].The IR spectrum of the complex showed a band approximately 1 701 cm-1, whichwasassigned to theν(C=O)band ofan o-quinoid group of the phon ligand.In the M(O,O′-phon)3(M=V and Ti)and M(O,O′-phon)3(M′Ln)3(M=V,M′Ln=TiCl4, M=Ti,M′Ln=TiCp2 and M=V,M′Ln=TiCp2),phon ligand was coordinated to the metal ion as a phensemiquinonate or phen-diolate via oxygen atoms.In these complexes,the carbon-oxygen stretching vibration of approximately 200 to 300 cm-1shifted to the lower wave number[3].The strong absorption bands at 1 089 and 626 cm-1were assigned to ClO4-and demonstrated the presence of ClO4-as a counter ion.
Theelectronic spectrum of the complex[Fe(phon)3] (ClO3)22CH3CN recorded in dimehtylformamide solution exhibited three absorption bands in the UV region and one band in the visible region.These bands centered at 257,298 and 311 nm and were assigned to ligandcentered(π→π*)transition[18-19].The absorption band centered at 485 nm was assigned to the intense d→π*metal-to-ligand charge transition.This band is similar to that of the corresponding phenanthroline complexes except that there is a blue shift in the maximum wavelength of absorption[20].This effect essentially reflects the enhancedπacidity of the phon ligand relative to thatof phenanthroline[1].
2.2 Crystal structure
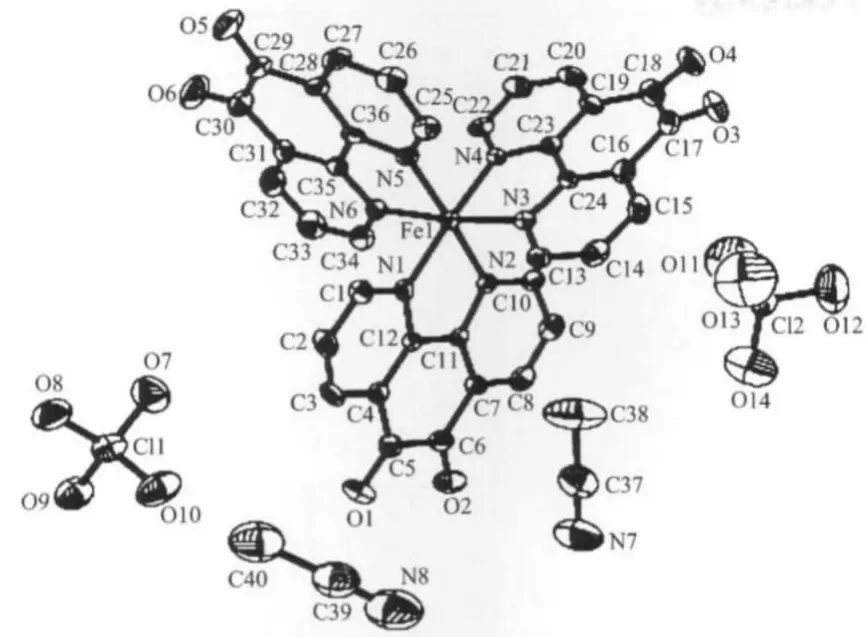
Fig.1 ORTEP view of the complex[Fe(phon)3](ClO3)2·2CH3CN showing the labeling of atomswith thermal ellipsoids at30%probability
The ORTEP structure of[Fe(phon)3](ClO3)2· 2CH3CN with atom labeling is shown in Fig.1.The complex[Fe(phon)3](ClO3)22CH3CN crystallizes in the orthorhombic P212121space group.The analogous complex[Fe(phon)3](ClO3)2·H2O crystallizes in the same space group.However,[Fe(phon)3](ClO3)2·H2O was weekly reflecting[21].The complex[Fe(phon)3] (ClO3)2·2CH3CN consists of a chiral octahedral [Fe(phon)3]2+cation,non-coordinated ClO4-counterious and acetonitrilemolecules.The crystal structure of the complex[Fe(phon)3](ClO3)2·2CH3CN is not resoluted and racemic mixtures,which is confirmed by the Flack parameter(0.44).The Fe(Ⅱ)atom is sixcoordinated with an approximately ideal octahedral geometry,by six nitrogen donors of three ligands.In the complex cation,the Fe(Ⅱ)atom is constrained to lie on the least-squares plane defined by N(1),N(3), N(4)and N(6)set of atoms with the largest deviation being 0.008 40 nm at the N(1)atom,which deviates by only 0.000 86 nm form the mean plane.The sum of the bond angles within the plane is very close to 360°with N(1)-Fe(1)-N((6)87.6(2)°,N(6)-Fe(1)-N(4) 93.4(2)°,N(4)-Fe(1)-N(3)82.2(2)°and N(1)-Fe-N(1) 98.0(2)°,respectively.The N(5)-Fe(1)-N(2)bond angle deviates from linearity(180°)by only 2.7°in this complex,which can be defined as the apex of the octahedron.The bite angles for all three bidentate ligands are in the range of 81.33°to 82.3°.The Fe(Ⅱ)-N bond lengths range from 0.196 1(5)to 0.198 0(5)nm, with an average of 0.197 3 nm.Themean Fe-N distances and the N-Fe-N angles are in the range usually found in other Fe-phenanthroline derivatives[22-23].
The most interesting feature of the crystal structure of the complex is the presence of intermolecular hydrogen bonds and C=O…πinteractions.The CH2group of one ligand and the O atom of the adjacent C=O group form the intermolecular C-H…O hydrogen bond.The C…O and H…O distances are 0.300 5 and 0.235 0 nm,respectively,falling into the normal range of hydrogen bond interactions.The bond angle is 127.2°.The C=O group of one ligand and the adjacent aromatic cycle(the ring,comprising atoms C16-C19,C23 and C24,is designated as ring 1 with centroid Cg)involved in the C=O…πinteractions. The C…centroid and O…centroid distance is 0.423 1 and 0.320 1 nm,respectively,wherein the angle at the O atom is 145.5°,which fall into the normal range of C=O…πinteractions[24].The C-H…O hydrogen bonds are responsible for the formation of infinite chains along the b axis;the C=O…πinteractions join along the c axis,producing 2D layers to the a axis of the crystal(Fig.2).
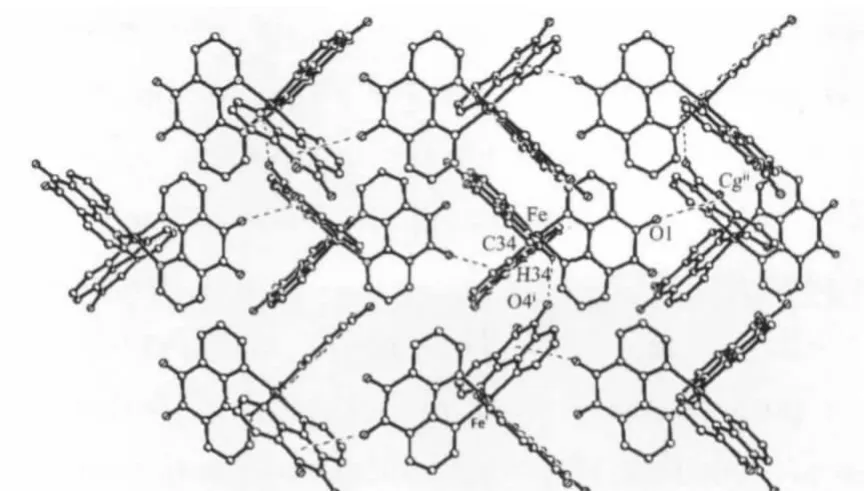
Fig.2 2D structure linked by C-H…O and C=O…π interactions in[Fe(phon)3](ClO3)2·2CH3CN
2.3 Interaction with ct-DNA
DNA binding is a critical step in DNA cleavage. Therefore,the potential DNA binding caability of the complex[Fe(phon)3](ClO3)2·2CH3CN was studied via UV spectroscopy following the intensity changes of the π-π*transition band at 225 nm.The electronic spectra of the complex[Fe(phon)3](ClO3)2·2CH3CN in the absence and the presence of ct-DNA are illustrated in Fig.3.Upon the addition of an increasing amount of DNA(from 0 to 6.95×10-6mol·L-1)to the complex(1.00×10-5mol·L-1),a 74%hypochromism and a red shift(of 5 nm)were observed.These spectral changes are consistent with the intercalation of the Fe(Ⅱ)complex into the DNA base stack[25].
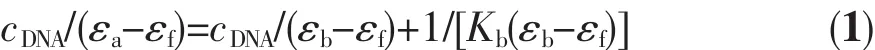
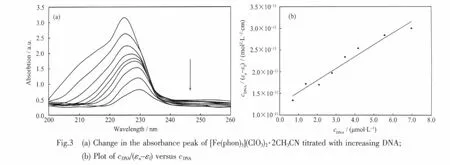
where cDNAis the total concentration of base pairs,and εa,εfandεbcorrespond to the extinction coeffi-cients for the absolutely bound Fe(Ⅱ)complex,free Fe(Ⅱ)complex,and the actually bound Fe(Ⅱ)complex, respectively.In the plots of cDNA/(εa-εf)versus cDNA, Kbis given by the ratio of the slope to the intercept. The Kbvalue obtained for the complex is 2.2×105L· mol-1,which is higher than those observed for [Fe(phen)]32+(4.68×103L·mol-1)[27]and[Ru(phen)]32+(5.5×103L·mol-1)[28].This result is expected,since phon possesses a greater planar area and extendedπ system than that of phen,leading to phon penetrating more deeply into andmakes stackingmore strongly.
[1]Hadadzadeh H,Olmsted M M,Rezvani A R,et al.Inorg. Chim.Acta,2006,359:2154-2158
[2]Goss C A,Abruna H D.Inorg.Chem.,1985,24:4263-4267
[3]Calderazzo F,Pampaloni G,Passarelli V.Inorg.Chim.Acta, 2002,330:136-142
[4]Nargiotta N,VertolasiV,Capitelli F,etal.Inorg.Chim.Acta, 2004,357:149-158
[5]Fujihara T,Okamuka R,Wada T,etal.J.Chem.Soc.,Dalton Trans.,2003:3221-3226
[6]Saravani H,Rezvani A R,Mansouri G,et al.Inorg.Chim. Acta,2007,3609:2829-2834
[7]Yamada Y,SakuraiH,Miyashita Y,etal.Inorg.Chim.Acta, 2006,359:807-814
[8]Fox G A,Bhattacharya S,Pierpont C G.Inorg.Chem.,1991, 30:2895-2899
[9]Bhattacharya P K,Lawson H J,Barton J K.Inorg.Chem., 2003,42:8811-8817
[10]Boghaei D M,Behzadian A F.J.Coord.Chem.,2007,60: 1629-1635
[11]Pascaly M,Yoo J,Barton JK.J.Am.Chem.Soc.,2002,124: 9083-9092
[12]Hiort C,Lincoln P,Norden B.J.Am.Chem.Soc.,1993, 115:3448-3454
[13]Marmur J.J.Mol.Biol.,1961,3:208-211
[14]Reichmann M E,Rice SA,Thomas CA,etal.J.Am.Chem. Soc.,1954,76:3047-3053
[15]Sheldrick G M.SADABS:Program for Expirical Absorption Correction of Area Detector Data,University of Göttingen, Germany,1996.
[16]Sheldrick G M.SHELXL-97,Program for X-ray Crystal Structure Solution,Gottingen University,Germany,1997.
[17]Sheldrick G M.SHELXL-97,Program for X-ray Crystal Structure Refinement,Gottingen University,Germany,1997.
[18]Stephenson M D,Prior T J.Hardie M.J.Cryst.Growth. Des.,2008,8:643-653
[19]Larsson K,Ohrstrom L.Inorg.Chim.Acta,2004,357:657-664
[20]Mudasir,Wijaya K,Yoshioka N,et al.J.Inorg.Biochem.,
2003,94:263-271
[21]Gaspar A B,Muoz M,Real J A.C.R.Acad.Sci.Paris, Chimie/Chemistry,2001,4:193-196
[22]Kulkarni P,Padhye S,Sinn E.Polyhedron,1998,17:2623-2626
[23]Filippova IG,Simonov Y A,Gdanets M,et al.Journal of Structure Chemistry,2005,46:1095-1098
[24]Ma Q,Zhu M L,Yuan C X.Cryst.Growth.Des.,2010,10: 1706-1714
[25]Tysoe SA,Morgan R J,Baker A D,et al.J.Phys.Chem., 1993,97:1707-1711
[26]Wolf A,Shimer Jr G H,Meehan T.Biochemistry,1987,26: 6392-6396
[27]Mudasir,Yoshioka N,Inoue H.J.Inorg.Biochem.,1999,77: 239-247
[28]Pyle A M,Rehmann JP,Meshoyrer R,et al.J.Am.Chem. Soc.,1989,111:3051-3058
Synthesis,Crystal Structure and the Interaction w ith DNA of the Fe(Ⅱ)Comp lex w ith Tris-(1,10-phenanthroline-5,6-dione)
ZHAO Hai-Yan*,1 MA Jing-Jun2 YANG F
u-Li3 (1College of Scie
nce, Hebei University of Science and Technology, Shijiazhuang 050018, China) (2College of Science,
Hebei University of Agriculture, Baoding, Hebei 070001, China)
(3Department of Chemical and Environmental Engineering, Hebei Chemical & Pharmaceutical College, Shijiazhuang 050026, China)
The crystal structure of the complex[Fe(phon)3](ClO3)22CH3CN(phon=1,10-phenanthroline-5,6-dione) has been determined via the X-ray diffractionmethod and refined via the full-matrix least-squaresmethods to R= 0.069 1 and wR2=0.143 7 using4 966 reflectionswith I>2σ(I).This complex crystallizes in theorthorhombic system, space group P212121with a=1.381 8(4),b=1.416 7(4),c=2.053 3(6)nm and Z=4.The X-ray analysis revealed that the Fe(Ⅱ)ion is coordinated by the six nitrogen atoms from three ligands in a slightly distorted octahedral arrangement.The C-H…O and C=O…πinteractions extend the complex into a quasi 2D networks,and such weak interactions further stabilized the complex.The complex was characterized via FT-IR and UV-Vis.The interaction between the complex and calf thymus DNA was investigated using UV spectra.The intrinsic binding constant(Kb)for the complex to DNA was calculated to be 2.2×105L·mol-1.CCDC:672079.
1,10-phenanthroline-5,6-dione;Fe(Ⅱ)complex;crystal structure;weak interactions;calf thymus DNA
book=0,ebook=60
O614.81+1
A
100-4861(2012)11-2468-05
2011-11-15。收修改稿日期:2012-06-09。
河北省教育厅青年基金(No.2011129)、河北科技大学校立科研基金(No.XL201044)资助项目。
*通讯联系人。E-mail:hbhaiyanzh@163.com;会员登记号:S06N6511M1009。
