Polymer/Ceramic Composite Membranes and Their Application in Pervaporation Process
2012-08-02LIUGongping刘公平WEIWang卫旺JINWanqin金万勤andXUNanping徐南平
LIU Gongping (刘公平), WEI Wang (卫旺), JIN Wanqin (金万勤)* and XU Nanping (徐南平)
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing University of Technology, Nanjing 210009, China
Polymer/Ceramic Composite Membranes and Their Application in Pervaporation Process
LIU Gongping (刘公平), WEI Wang (卫旺), JIN Wanqin (金万勤)* and XU Nanping (徐南平)
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing University of Technology, Nanjing 210009, China
Pervaporation (PV), as an environmental friendly and energy-saving separation technology, has been
polymer/ceramic composite membrane, pervaporation, bio-fuel recovery, solvent dehydration, PV coupled process
Chinese Journal of Chemical Engineering,20(1) 62—70 (2012)
1 INTRODUCTION
During the past decades, membrane-based technology has been widely used in various applications, such as water treatment, food industry, biochemical engineering and pharmaceuticals, etc. Pervaporation (PV) is a promising membrane-based technique for the separation of liquid mixtures, especially in azeotropic or close-boiling solutions. The advantage of this process lies in the energy-saving, economic and environment friendly, and so on.
Nowadays, the applications of pervaporation separation can be classified into following three major fields: (1) removal of dilute organic compounds from their mixtures (e.g., recovery of bio-fuels from fermentation broth, sulfur removal from gasoline, and removal of volatile organic compounds); (2) dehydration of organic solvents (e.g., alcohols, esters, acids); and (3) organic-organic mixtures separation. The preparation and application of pervaporation membranes have been discussed in several recent reviews [1-4].
A key point of pervaporation process is the pervaporation membrane with high performance (permeate flux, selectivity and stability). For a specific separation system and operating condition, the membrane PV performance is determined by membrane materials and configurations. On the one hand, according to the material’s hydrophobicity, PV membranes can be divided into hydrophobic and hydrophilic membranes. The most representative hydrophobic membranes are polydimethylsiloxane (PDMS) membrane and silicalite-1 membrane, while poly(vinyl alcohol) (PVA) and NaA zeolite membrane are the most commonly used hydrophilic membranes. On the other hand, the membrane configurations of PV membrane include symmetric and composite membrane. The former is usually used in the laboratory research to study the intrinsic properties of the membrane materials, but low flux and mechanical strength of the symmetric membranes limit their industrial application. Nevertheless, a composite membrane generally has a thin dense skin layer on a porous organic or inorganic support, and thus its flux can be increased a lot. Compared with organic support, inorganic support exhibits superior chemical, mechanical and thermal stability, as well as negligible transport resistance. The ceramicsupported composite membranes have attracted tremendous attention for their significant performances in ultrafiltration [5], pervaporation [6-10], gas separation [11], membrane-based reactor [12], membrane chromatography [13], etc.
In this review, we summarize our progress on the preparation of polymer composite membranes using macroporous ceramic membrane as the support (membrane morphology and structure are shown in Fig. 1), and their application in pervaporation process. The separation layer includes PDMS [8, 14-17], PVA [9], chitosan (CS) [9] and polyelectrolytes [10, 18]. The applications of these composite membranes include bio-fuel recovery from fermentation broth [19-21], desulfuration of gasoline [22] and dehydration of alcohols and esters [23, 24], and to be coupled with acetone butanol ethanol (ABE) fermentation [25] and ethyl lactate hydrolysis process [26]. Table 1 lists the PV performance of polymer/ceramic composite membranes and some typical PV membranes reported in the literature applied in various applications.
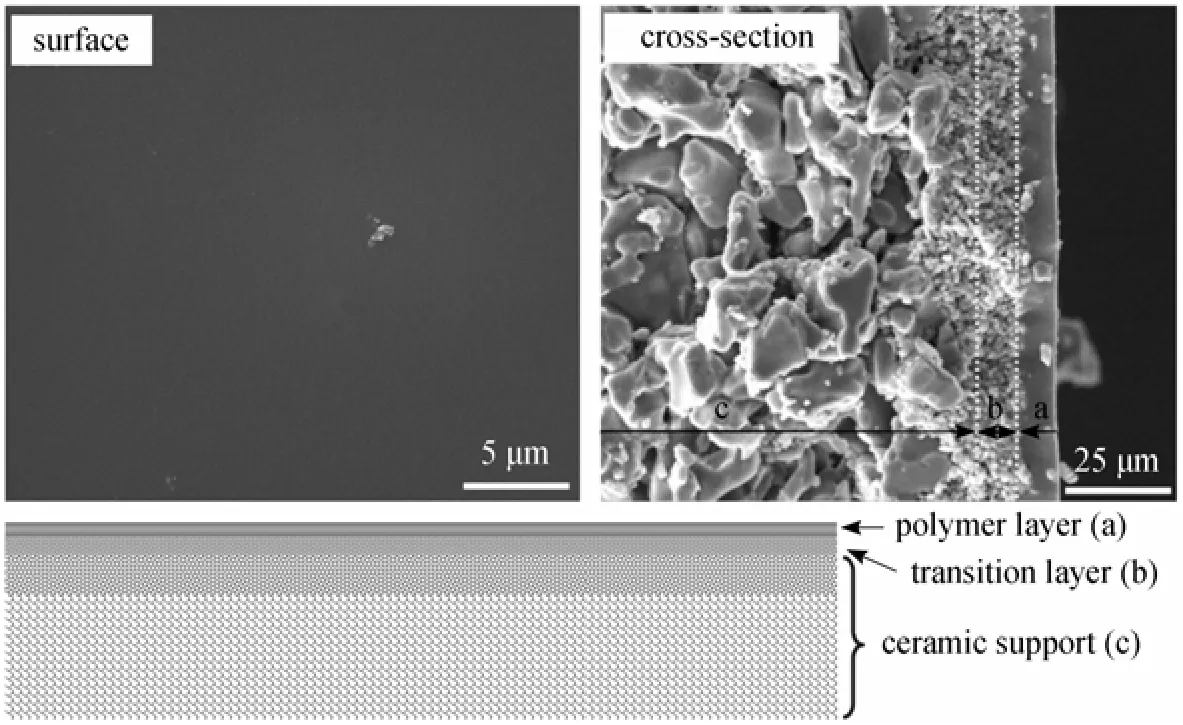
Figure 1 Typical SEM images and schematic structure of the polymer/ceramic composite membrane
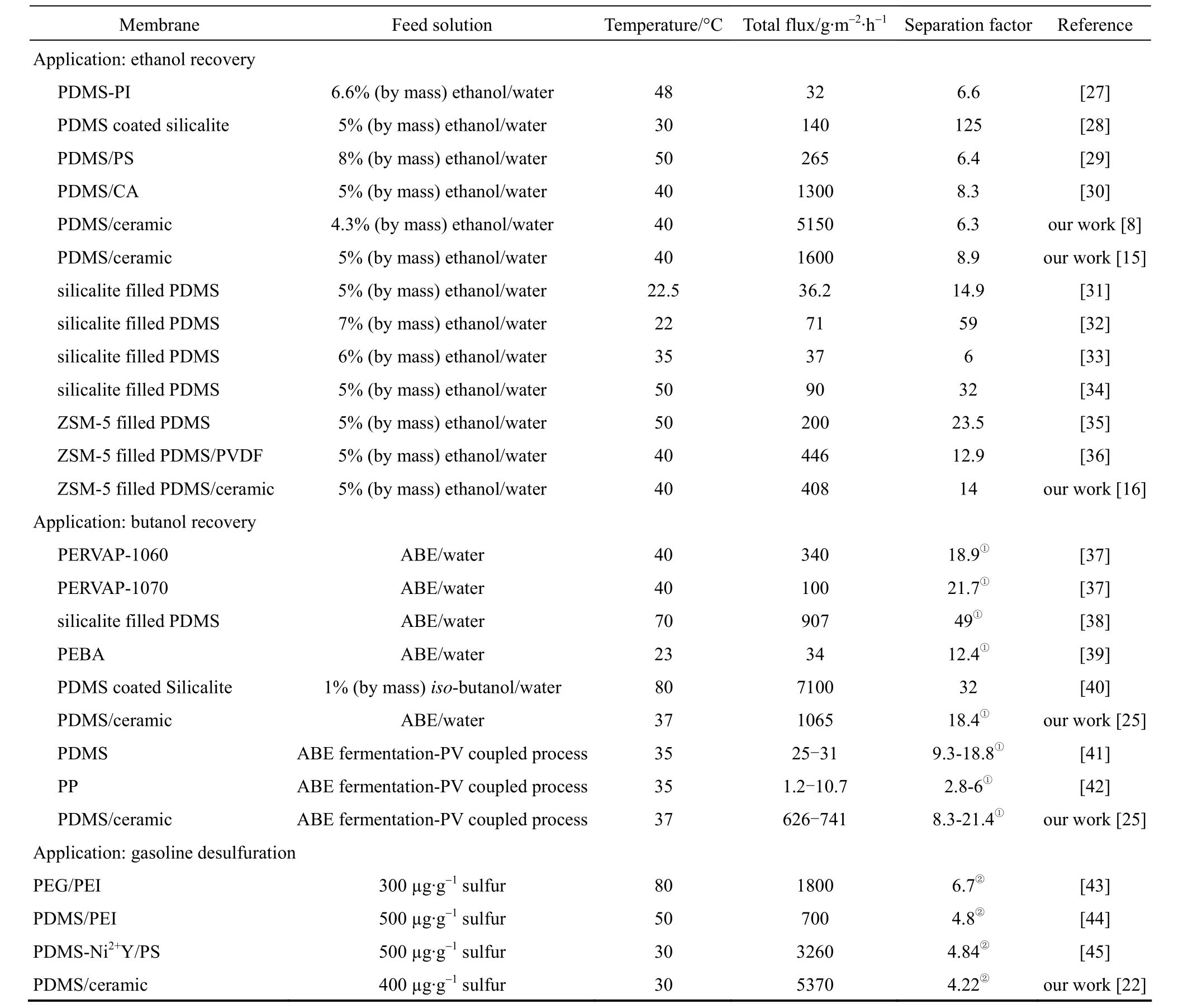
Table 1 PV performance of polymer/ceramic composite membranes and some typical PV membranes reported in the literature applied in various applications
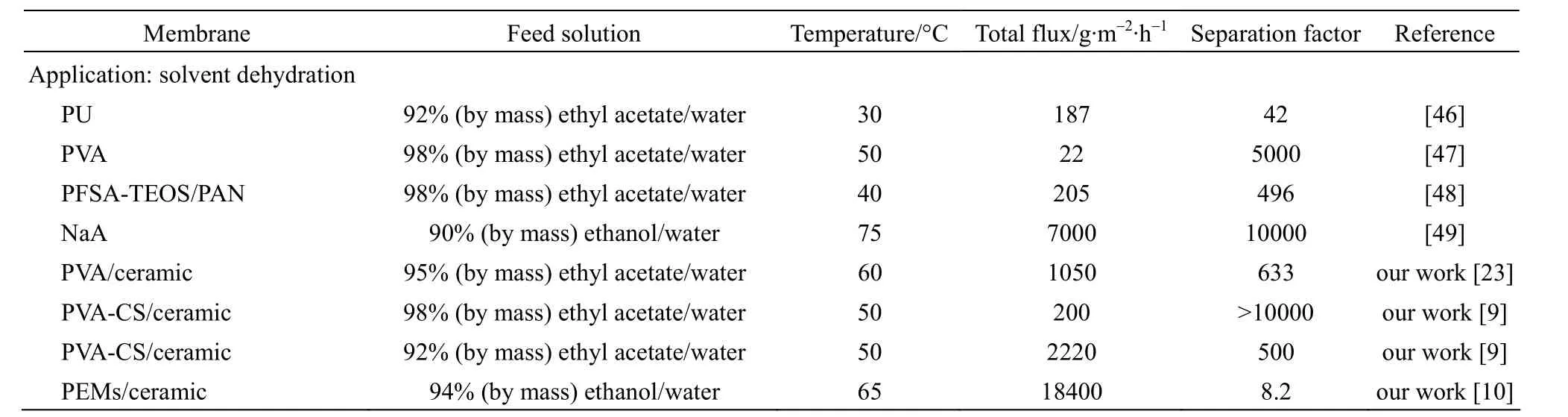
Table 1 (Continued)
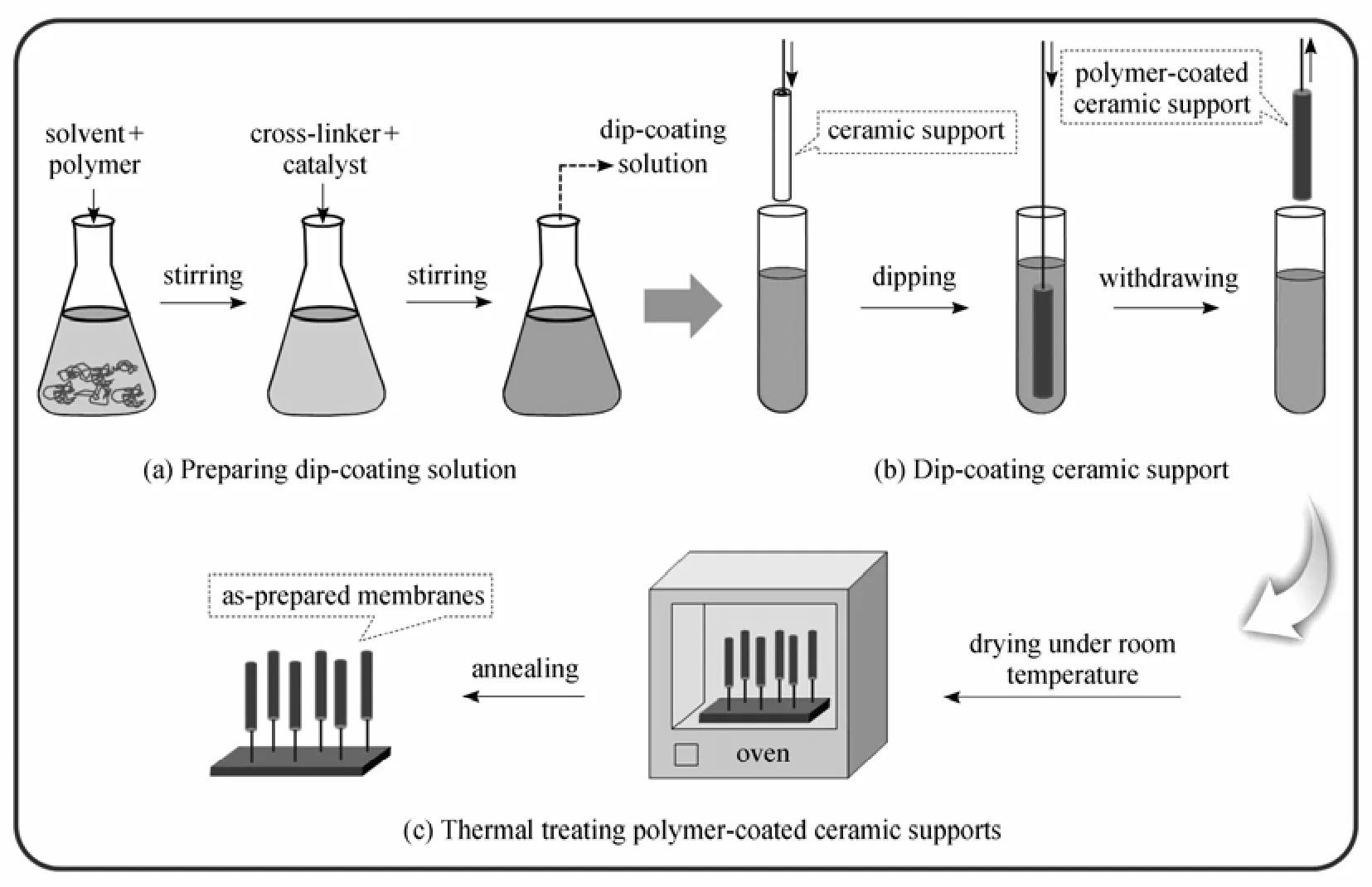
Figure 2 General procedure of preparing polymer/ceramic composite membrane
2 PREPARATION OF POLYMER/CERAMIC PV COMPOSITE MEMBRANES
2.1 PDMS/ceramic composite membranes
As a benchmark hydrophobic membrane, PDMS pervaporation membrane is widely applied in removal of dilute organic compounds from their mixtures and organic-organic mixtures separation. The supports of most PDMS composite membranes in the literature are polymeric supports such as polystyrene (PS) [29], cellulose acetate (CA) [30], polyetherimide (PEI) [42], poly(vinylidene fluoride) [36] and so on. The stability of these polymer-supported composite membranes was still of great concern in organic solvent solutions due to the interfacial shear stress induced by the membrane swelling. Recently, we have developed a type of PDMS/ceramic composite membranes using commercialized macroporous ceramic supports, which make PDMS composite membranes with high mechanical strength, solvent resistant, low transport resistance and being operated at higher temperature [8, 14-17].
The PDMS/ceramic composite membrane was prepared with cross-linked PDMS solution coated on the outer surface of the tubular ceramic support by dip-coating method. The PDMS casting solution was prepared by dissolving PDMS in n-heptane and then adding the cross-linker TEOS (tetraethylorthosilicate) and catalyst dibutyltin dilaurate [8]. The general procedure of preparing polymer/ceramic composite membranes is shown in Fig. 2.
2.1.1Ceramic support treatment
One challenge of using macroporous supports to prepare pervaporation composite membrane is to form a dense and defect-free separation layer on it, because the polymer solution is prone to penetrate into pores of the support. Generally, one or more additional transitionlayers on ceramic support are fabricated prior to the preparation of the final separation layer [6]. Here, we took a simple water pre-wetting strategy to treat the ceramic supports. As the pores in the support were still occupied by water, the penetration of coating solutions was significantly reduced. Moreover, the bound water could increase the polarity of the ceramic support and then increased the thermodynamic gradient between the support and PDMS solution, which would increase the leveling ability of PDMS solution, improving the coverage of PDMS solution on the surface of ceramic support [15]. Another factor that influences the formation of a thin and defect-free PDMS separation layer on the ceramic support is the surface roughness of the support. Before dip-coating, the ceramic supports are usually polished to reduce the surface roughness to a suitable level, which is proved to be the prerequisite to obtain high-quality polymer/ceramic composite membranes.
2.1.2Effect of PDMS solution properties on PV performance
As displayed in Fig. 1, for a polymer/ceramic composite membrane, the influence of the properties of polymer casting solution on PV performance should be considered in two aspects: the separation layer itself and the transition layer which is formed at the interface between the active layer and the porous support due to the penetration of casting solution. We have studied the effects of several key parameters that would affect the PDMS solution properties on the PV performance of PDMS/ceramic composite membranes, such as PDMS molecular weight [15], PDMS concentration, cross-linking degree and dip-coating time [14].
Molecular weight has a remarkable influence on the packing of polymer chains which affects the rheology and osmotic pressure of polymer casting solution. The penetration degree of PDMS solution into the porous ceramic support could be controlled by its osmotic pressure. Polymer solution with high PDMS molecular weight has a good leveling property and avoids excessive penetration, resulting in a uniform coverage of PDMS solution on the support surface and getting a defect-free PDMS separation layer. The EDX (energy dispersive X-ray spectroscopy) results also indicated that penetration degree decreased with increasing PDMS molecular weight. PV experimental results showed that the PDMS/ceramic composite membrane prepared from PDMS with higher molecular weight exhibited higher separation factor and lower permeation flux [15].
In order to optimize the membrane preparation conditions, a response surface methodology (RSM) was employed to establish the regression equation between the preparation parameters and the membrane performance [14]. For fabricating PDMS/ceramic composite membranes, polymer concentration, cross-linking degree and dip-coating time were considered as dominant preparation parameters in controlling PV performance. We investigated the main effects, quadratic effects and interactions of the three variables on the flux and separation factor of PDMS/ceramic composite membranes. It was proved that polymer concentration was the most significant variable that influenced the PV performance among three variables. Meanwhile, the experimental results were in good agreement with those predicted by the obtained regression models, indicating that the regression models can be expected to have application in the preparation of PDMS/ceramic membranes and reasonably predict and optimize the membrane performance.
2.1.3Interfacial adhesion between PDMS layer and ceramic support
The interfacial adhesion between separation layer and support plays an important role in the structural stability of composite membrane. We proposed an in-situ method to characterize the mechanical and adhesive properties of tubular PDMS/ceramic composite membranes by the nanoindentation and nanoscratch tests [17]. It was found that the interfacial adhesion between PDMS layer and ceramic support could be controlled by the viscosity of casting solution and the support roughness. Generally, lower viscosity of PDMS solution and higher roughness of ceramic support are propitious to increase the interfacial adhesion strength.
The adhesion of PDMS separation layer on ceramic support relies on three mechanisms: adsorption, chemical bonding and mechanical interlocking. First, the ceramic support has higher surface energy than the PDMS solution, thus the coated PDMS layer could adhere to the surface of ceramic support. Second, the hydrogen bond occurs between OH group in the ceramic support and oxygen atom of PDMS. Third, the ceramic pores form the enchased structure between the ceramic support and PDMS layer. The in-situ characterization of interfacial adhesion suggests that the mechanical interlocking dominates the adhesion between the PDMS separation layer and the porous ceramic support [17].
For a polymer/ceramic composite membrane, polymer in thin film which adheres on a substrate may have different physical properties from those in the bulk. As presented in Fig. 3, based on the PV experimental results and FESEM (field emission scanning electron microscopy) images, we proposed a confinement effect (also called constrained swelling effect) of PDMS layer in the PDMS/ceramic composite membranes [15]. Compared with the conventional polymeric support, the ceramic support exhibits a unique advantage in constraining the swelling of the PDMS separation layer, which results in higher separation factor at high operating temperature, as well as significantly improves the PDMS membrane stability.
2.1.4Zeolite filled PDMS/ceramic composite membranes
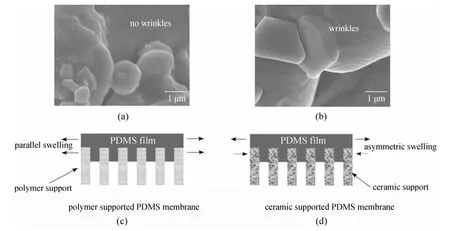
Figure 3 FESEM images of the penetrated PDMS in the pore of two supports: (a) BCA (blend cellulose acetate) support and (b) ceramic support. Schematic drawings of the parallel swelling and asymmetric swelling for (c) polymer-supported PDMS membrane and (d) ceramic-supported PDMS membrane. Reproduced from Ref. [15]
Although the PDMS/ceramic composite membrane has a high permeate flux, its selectivity is still insufficient for practical applications in bio-ethanol production, according to the economic analysis reported by O’Brien et al [50]. By utilizing the high selectivity of MFI zeolite [28, 40], we have incorporated hydrophobic zeolite particles into PDMS membrane to prepare zeolite filled PDMS/ceramic composite membranes via a facile and cost-effective way to improve the membrane selectivity. ZSM-5 particles were homogeneously dispersed in PDMS matrix by a surface graft/ coating approach: ZSM-5 surface was first grafted with n-octyl chains using n-octyltriethoxysilane and then coated with a thin PDMS polymer layer [16]. The molecular dynamics simulation results suggested that the interaction between ZSM-5 particles and PDMS matrix was highly improved by the surface modification of zeolite. The PV experiment in ethanol-water solution indicated that the uniform-dispersed ZSM-5 particles greatly improved the selectivity of PDMS/ceramic composite membrane, and the separation factor of surface-modified ZSM-5 filled PDMS membrane increased gradually with zeolite loading.
2.2 Ceramic-supported PVA, CS and PVA-CS composite membranes
Hydrophilic polymeric materials have been widely investigated for pervaporation dehydration, among which poly(vinyl alcohol) (PVA) and chitosan (CS) are the most commonly studied. To date, porous polymeric membranes are commonly used as the substrates for PVA or CS composite membranes. However, they often show a lack of chemical, mechanical and thermal stability. Conversely, ceramic membranes have unique stable structure, and thereby they can withstand various kinds of organic solvents and higher temperature. Thus, we have fabricated ceramic-supported PVA or CS composite membrane by dip-coated cross-linked PVA or CS [7% (by mass) PVA or 2% (by mass) CS solution with cross-linker maleic anhydride and catalyst sulfuric acid] separation layer on the surface of tubular porous ceramic membrane [9]. The ceramicsupported PVA or CS composite membranes show high separation factors in the dehydration of t-butanol/ water mixture, but their permeate fluxes are too low.
Since PVA has semicrystalline structure while CS has less compact structure and relatively larger free volume, blending of PVA with CS can reduce the crystallinity, and as a result, flux can be improved. Thus, a ceramic-supported PVA-CS blending composite membrane has been developed [9]. We found that chitosan played an important role in increasing the membrane permeation flux. With blending of CS into PVA, intramolecular hydrogen bonds are substituted by intermolecular hydrogen bonds between CS and PVA, so the membrane structure becomes less compact and the free volume in membrane increases, thus the permeation flux increases with CS content. Meanwhile, as the hydrophilic groups in PVA and CS form intermolecular hydrogen bonds, hydrophilicity of the membranes decreases, thus decreases the separation factor slightly.
2.3 PEMs/ceramic composite membranes
The layer-by-layer (LBL) alternative deposition of polyanion-polycation on porous supports to form polyelectrolyte multilayers (PEMs) has attracted much attention due to their extremely low thickness, high homogeneity and nice tunability on molecular dimensions. One of the applications of PEMs is for separation of nanoscale species, for example, pervaporation of organic-water mixtures.
We have prepared a type of PEMs/ceramic composite membrane by means of electrostatic self-assembly of polyelectrolytes on silica sol-gel modified macroporous ceramic supports [10]. In order to study thegrowth of polyelectrolytes on ceramic supports, an in-situ characterization method has been conducted by applying transmembrane streaming potential measurement via surface charge monitoring [18]. The top-assembled materials and numbers of deposited cycles could influence the surface charge of PEMs/ ceramic composite membranes.
The separation performance of PEMs/ceramic composite membrane was optimized by coordinating the support modification cycle, polyelectrolyte molecule structure, self-assemble conditions and thermal treatment conditions [10]. It was found that the polyelectrolyte molecular structure was the most important factor to control the membrane performance. The PEMs/ceramic composite membrane deposited by 60 pairs of polyethylenimine/poly(vinylsulfate) (PEI/PVS) layer shows a high flux of 18.4 kg·m−2·h−1in ethanol/ water mixtures with an enhancement of water concentration from 6.2% (by mass) in the feed to 35.3% (by mass) in the permeate at 65 °C.
3 APPLICATION OF POLYMER/CERAMIC PV COMPOSITE MEMBRANES
3.1 Recovery bio-fuels
Owing to the shortage of crude oil and the environmental requirement, the utilization of renewable resources for energy production, such as using biomass fermentation processes to produce bio-fuels, has been received increasing attention in recent years. As an environment friendly and energy-saving separation technology, pervaporation is a potential process for recovering alcohol from biomass fermentation broth [51]. The research progresses of pervaporation applied in bio-ethanol and bio-butanol production have been reviewed [52, 53]. As listed in Table 1, several hydrophobic membranes such as PDMS with or without zeolite fillers [27, 29-38], poly(ether block amide) (PEBA) [39], polypropylene (PP) [42] and silicalite-1 [28, 40] membranes, were employed to recover ethanol or butanol from model solution or fermentation broth. We have investigated extensively the performance of PDMS/ceramic composite membranes for alcohol recovery in various feed systems, e.g., ethanol aqueous solution [8, 14, 15], n-butanol aqueous solution [19], ABE (acetone butanol ethanol) aqueous solution, model fermentation broth and real fermentation broth [25].
As for ethanol or n-butanol aqueous solution, with increasing the operating temperature, the PDMS/ceramic composite membrane shows an increased total flux, while a nearly constant separation factor, which does not follow the trend of the polymeric-supported PDMS membranes [15, 19]. This phenomenon should be attributed to the confinement effect existed in the polymer/ceramic composite membranes. As temperature increased, the swelling of PDMS polymer that penetrated into the porous ceramic support was constrained by the rigid ceramic support. Additionally, the continuous PV experiments [see Fig. 4 (a)] confirmed that this membrane shows good long-term stability [14]. Compared with other PDMS membranes in the literature, the PDMS/ceramic composite membrane shows a high flux and good separation factor: 1.6-5.15 kg·m−2·h−1and 6.3-8.9 with 5% (by mass) ethanol in feed at 40 °C, respectively; 0.46 kg·m−2·h−1and 26 with 1% (by mass) n-butanol in feed at 40 °C, respectively. This high performance is owing to the thin and defect-free PDMS separation layer (ca. 5 μm) and high porosity ceramic support with low transport resistance.
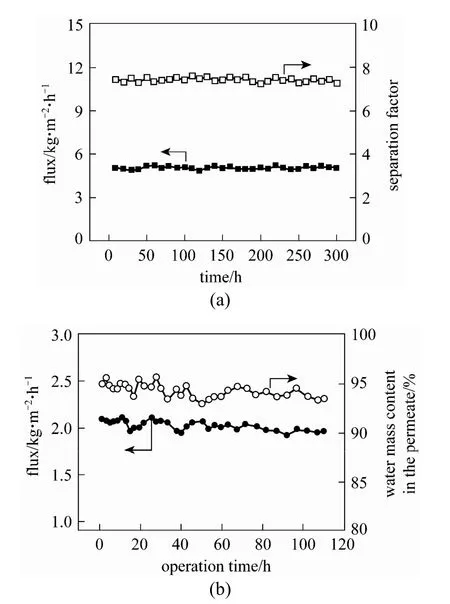
Figure 4 Long-term stability of polymer/ceramic composite membranes (a) PDMS composite membrane in 5% (by mass) ethanol/water solution at 60 °C; (b) PVA composite membrane in 9.18% (by mass) water, 4.47% (by mass) ethanol and 86.35% (by mass) ethyl acetate mixtures at 60 °C. Reproduced from Refs. [14] and [24]
In the ethanol fermentation broth, it was found that the other components such as glucose, glycerol and succinic acid have little effects on the PV performance of PDMS/ceramic composite membrane [20]. Nevertheless, the inorganic salts in the broth could improve the permeate flux and separation factor, resulted from the increase of ethanol activity, which could be calculated by Setschenow equation [21]. In the ABE fermentation broth, we also observed that the inorganic salts favored the membrane selectivity whereas the microbial cells resulted in reduced performance [25].
年需要红土镍矿50万t(干基),其主要成分见表1。红土镍矿含自由水33%,结晶水10%,矿石粒度≤100 mm。
3.2 Desulfuration of gasoline
PDMS/ceramic composite membranes were employed for removal of sulfur impurities out of model gasoline (n-octane/thiophene mixtures) by pervaporation process [22]. The PV performances of the membranes under various crosslinking agent amounts, feed sulfur content, feed temperature, permeate pressure and feed flow rate were investigated systematically. Compared with the performance of those composite membranes with polymeric supports reported in the literature, the ceramic-supported PDMS membrane exhibits a higher permeation flux of 5.37 kg·m−2·h−1and an acceptable sulfur enrichment factor of 4.22 for 400 μg·g−1sulfur in feed at 30 °C. The main reason for this high flux is that the porous ceramic support possesses a low mass transfer resistance in the PV process.
3.3 Dehydration of alcohols and esters
The widest application of pervaporation is the dehydration of organic mixtures, especially for azeotropes or the close-boiling mixtures, for the reason of important commercial benefits. For example, PV could be applied to replace the azeotropic distillation or extractive distillation in the further purification of ethanol or ethyl acetate. The most commonly used and also industrialized hydrophilic membranes for solvent dehydration are polymeric PVA membrane [47-49] and inorganic NaA zeolite membrane [49], as shown in Table 1.
Pervaporation dehydration of ethyl acetate-water mixtures was conducted using above-mentioned PVA/ ceramic composite membrane [23]. Swelling experiments were performed to evaluate the membrane sorption characteristic, and then Flory-Huggins and NRTL theories were applied to analyze the interactions of the membrane and penetrants and the mutual interaction between penetrants. This membrane shows higher PV performance than the reported tartaric acid cross-linked PVA, PFSA (perfluorosulfonic acid) and PU (polyurethane) membranes, with total flux of 1.05 kg·m−2·h−1and separation factor of 633, with 5.1% (by mass) water in the feed at 60 °C. Furthermore, the PVA/ceramic composite membrane shows good stability during the pervaporation of the product from the reactive distillation of ethyl acetate, as shown in Fig. 4 (b) [24].
PVA-CS/ceramic composite membranes were also used for the dehydration of organic/water mixtures [9]. In the alcohol/water mixtures, with increasing the operating temperature or feed water concentration, the permeation flux increased without sacrifice in separation factor. This abnormal trend between flux and separation factor suggested that the non-deformable ceramic support had a constrained swelling effect on the PVA-CS separation layer, which also existed in the PDMS/ceramic composite membranes. Furthermore, in the ester/water mixtures with water mass content less than 5%, this ceramic-supported PVA-CS composite membrane shows superior performance with the separation factor larger than 10000.
3.4 Pervaporation coupled process
In the production of bio-fuel, several solvent recovery technologies have been coupled with biomass fermentation process to reduce the effect of product inhibition, in order to improve the solvent productivity and realize continuous fermentation. Pervaporation is considered to be one of the most potential technologies owing to its energy-saving and efficiency, as well as no harmful effects on the microorganisms.
In addition, pervaporation or vapor permeation (VP) also can be used to overcome the reversible chemical equilibrium limitation by removal of the products as soon as it is produced. For instance, we coupled PDMS/ceramic composite membrane with the hydrolysis of ethyl lactate (a reversible reaction) to in-situ remove the ethanol product via VP, which has significantly enhanced the conversion [26]. The final conversion of the ethyl lactate increased from 77.1% to 98.2% with the initial water/ethyl lactate molar ratio of 10∶1 at 85 °C. Moreover, larger initial water/ethyl lactate molar ratio performed more effectively in the ethyl lactate hydrolysis-VP coupled process.
4 CONCLUSIONS AND PROSPECTS
By matching the properties of the ceramic support and polymer separation layer, the polymer casting solution could be deposited on the surface of macroporous ceramic support via dip-coating or electrostatic self-assembly method to fabricate polymer/ceramic composite membranes. These composite pervaporation membranes have been widely used in alcohol recovery, desulfuration, solvent dehydration and coupled processes, in which the composite membranes exhibithigh pervaporation performance, especially high permeate flux. This is attributed to the thin polymer separation layer and ceramic support with low transport resistance. Meanwhile, the confinement effect in the ceramic-supported composite membranes is in favor of keeping the membrane with high selectivity at higher operating temperature and long-term stability.
The future of polymer/ceramic pervaporation composite membranes lies in development of new membrane materials and fabrication techniques, as well as broadening their feasible applications. For the PDMS/ ceramic composite membrane, efforts should be devoted continuously to improve the alcohol-selectivity without sacrificing the flux. Until now, an effective way is preparing nano-sized zeolite filled PDMS mixed matrix membranes with thin active layer. As to the ceramic-supported PVA-CS composite membranes, their permeate flux is still not attractive for practical application, whose further enhancement could be anticipated. Further research is also required in optimization of the polymer-ceramic interface behaviors, in order to improve the PV performance and stability.
REFERENCES
1 Lipnizki, F., Hausmanns, S., Ten, P.K., Field, R.W., Laufenberg, G.,“Organophilic pervaporation: Prospects and performance”, Chem. Eng. J.,73, 113-129 (1999).
2 Shao, P., Huang, R.Y.M., “Polymeric membrane pervaporation”, J. Membr. Sci.,287, 162-179 (2007).
3 Chapman, P.D., Oliveira, T., Livingston, A.G., Li, K., “Membranes for the dehydration of solvents by pervaporation”, J. Membr. Sci.,318, 5-37 (2008).
4 Smitha, B., Suhanya, D., Sridhar, S., Ramakrishna, M., “Separation of organic-organic mixtures by pervaporation—A review”, J. Membr. Sci.,241, 1-21 (2004).
5 Matsumoto, Y., Sudoh, M., Suzuki, Y., “Preparation of composite UF membranes of sulfonated polysulfone coated on ceramics”, J. Membr. Sci.,158, 55-62 (1999).
6 Peters, T.A., Poeth, C.H.S., Benes, N.E., Buijs, H.C.W.M., Vercauteren, F.F., Keurentjes, J.T.F., “Ceramic-supported thin PVA pervaporation membranes combining high flux and high selectivity; contradicting the flux-selectivity paradigm”, J. Membr. Sci.,276, 42-50 (2006).
7 Yoshida, W., Cohen, Y., “Removal of methyl tert-butyl ether from water by pervaporation using ceramic-supported polymer membranes”, J. Membr. Sci.,229, 27-32 (2004).
8 Xiangli, F.J., Chen, Y.W., Jin, W.Q., Xu, N.P., “Polydimethylsiloxane (PDMS)/ceramic composite membrane with high flux for pervaporation of ethanol-water mixtures”, Ind. Eng. Chem. Res.,46, 2224-2230 (2007).
9 Zhu, Y.X., Xia, S.S., Liu, G.P., Jin, W.Q., “Preparation of ceramic-supported poly(vinyl alcohol)-chitosan composite membranes and their applications in pervaporation dehydration of organic/water mixtures”, J. Membr. Sci.,349, 341-348 (2010).
10 Chen, Y.W., Xiangli, F.J., Jin, W.Q., Xu, N.P., “Organic-inorganic composite pervaporation membranes prepared by self-assembly of polyelectrolyte multilayers on macroporous ceramic supports”, J. Membr. Sci.,302, 78-86 (2007).
11 Randon, J., Paterson, R., “Preliminary studies on the potential for gas separation by mesoporous ceramic oxide membranes surface modified by alkyl phosphonic acids”, J. Membr. Sci.,134, 219-223 (1997).
12 Peters, T.A., Benes, N.E., Keurentjes, J.T.F., “Preparation of aberlystcoated pervaporation membranes and their application in the esterification of acetic acid and butanol”, Appl. Catal. A,317, 113-119 (2007).
13 Nova, C.J.M., Paolucci-Jeanjean, D., Belleville, M.P., Barboiu, M., Rivallin, M., Rios, G., “Elaboration, characterization and study of a new hybrid chitosan/ceramic membrane for affinity membrane chromatography”, J. Membr. Sci.,321, 81-89 (2008).
14 Xiangli, F.J., Wei, W., Chen, Y.W., Jin, W.Q., Xu, N.P., “Optimization of preparation conditions for polydimethylsiloxane (PDMS)/ ceramic composite pervaporation membranes using response surface methodology”, J. Membr. Sci.,311, 23-33 (2008).
15 Wei, W., Xia, S.S., Liu, G.P., Dong, X.L., Jin, W.Q., Xu, N.P., “Effects of polydimethylsiloxane (PDMS) molecular weight on performance of PDMS/ceramic composite membranes”, J. Membr. Sci.,375, 334-344 (2011).
16 Liu, G.P., Xiangli, F.J., Wei, W., Liu, S.N., Jin, W.Q., “Improved performance of PDMS/ceramic composite pervaporation membranes by ZSM-5 homogeneously dispersed in PDMS via a surface graft/coating approach”, Chem. Eng. J.,174, 495-503 (2011).
17 Wei, W., Xia, S.S., Liu, G.P., Gu, X.H., Jin, W.Q., Xu, N.P., “Interfacial adhesion between polymer separation layer and ceramic support for composite membrane”, AIChE J.,56, 1584-1592 (2010).
18 Chen, Y.W., Xiangli, F.J., Jin, W.Q., Xu, N.P., “Streaming potential characterization of LBL membranes on porous ceramic supports”, AIChE J.,53, 969-977 (2007).
19 Liu, G.P., Hou, D., Wei, W., Xiangli, F.J., Jin, W.Q., “Pervaporation separation of butanol-water mixtures using polydimethylsiloxane/ceramic composite membrane”, Chin. J. Chem. Eng.,19, 40-44 (2011).
20 Xu, L.F., Xiangli, F.J., Chen, Y.W., Jin, W.Q., Xu, N.P., “Influence of simulated ethanol fermentation components on pervaporation performance of PDMS/ceramic composite membranes”, J. Chem. Ind. Eng. (China),58, 1466-1472 (2007). (in Chinese)
21 Hou, D., Wei, W., Xia, S.S., Xiangli, F.J., Chen, Y.W., Jin, W.Q.,“Influence of inorganic salts on pervaporation performance of PDMS/ceramic composite membranes”, J. Chem. Ind. Eng. (China),60, 389-393 (2009). (in Chinese)
22 Xu, R., Liu, G.P., Dong, X.L., Jin, W.Q., “Pervaporation separation of n-octane/thiophene mixtures using polydimethylsiloxane/ceramic composite membranes”, Desalination,258, 106-111 (2010).
23 Xia, S.S., Dong, X.L., Zhu, Y.X., Wei, W., Xiangli, F.J., Jin, W.Q.,“Dehydration of ethyl acetate-water mixtures using PVA/ceramic composite pervaporation membrane”, Sep. Purif. Technol.,77, 53-59 (2011).
24 Xia, S.S., Wei, W., Liu, G.P., Dong, X.L., Jin, W.Q., “Pervaporation properties of PVA/ceramic composite membrane for separation of ethyl acetate/ethanol/water ternary mixtures”, Korean J. Chem. Eng., DOI: 10.1007/S11814-011-0154-X (2011).
25 Liu, G.P., Wei, W., Wu, H., Dong, X.L., Jiang, M., Jin, W.Q., “Pervaporation performance of PDMS/ceramic composite membrane in acetone butanol ethanol (ABE) fermentation-PV coupled process”, J. Membr. Sci.,373, 121-129 (2011).
26 Li, W.X., Zhang, X.J., Xing, W.H., Jin, W.Q., Xu, N.P., “Hydrolysis of ethyl lactate coupled by vapor permeation using polydimethylsiloxane/ceramic composite membrane”, Ind. Eng. Chem. Res.,49, 11244-11249 (2010).
27 Kashiwagi, T., Okabe, K., Okita, K., “Separation of ethanol from ethanol/water mixtures by plasma-polymerized membranes from silicone compounds”, J. Membr. Sci.,36, 353-362 (1988).
28 Matsuda, H., Yanagishita, H., Negishi, H., Kitamoto, D., Ikegami, T., Haraya, K., Nakane, T., Idemoto, Y., Koura, N., Sano, T., “Improvement of ethanol selectivity of silicalite membrane in pervaporation by silicone rubber coating”, J. Membr. Sci.,210, 433-437 (2002).
29 Guo, J., Zhang, G., Wu, W., Ji, S., Qin, Z., Liu, Z., “Dynamically formed inner skin hollow fiber polydimethylsiloxane/polysulfone composite membrane for alcohol permselective pervaporation”, Chem. Eng. J.,158, 558-565 (2010).
30 Li, L., Xiao, Z., Tan, S., Pu, L., Zhang, Z., “Composite PDMS membrane with high flux for the separation of organics from water by pervaporation”, J. Membr. Sci.,243, 177-187 (2004).
31 Hennepe, H.J.C., Bargeman, D., Mulder, M.H.V., Smolders, C.A.,“Zeolite-filled silicone rubber membranes. Part 1. Membrane preparation and pervaporation results”, J. Membr. Sci.,35, 39-55 (1987).
32 Jia, M., Pleinemann, K., Behling, R., “Preparation and characterization of thin-film zeolite-PDMS composite membranes”, J. Membr. Sci.,73, 119-128 (1992).
33 Vankelecom, I.F.J., Depre, D., Beukelaer, S. De, Uytterhoeven, J.B.,“Influence of zeolites in PDMS membranes. Pervaporation of water/alcohol mixtures”, J. Phys. Chem.,99, 13193-13197 (1995).
34 Yi, S.L., Su, Y., Wan, Y.H., “Preparation and characterization of vinyltriethoxysilane (VTES) modified silicalite-1/PDMS hybrid pervaporation membrane and its application in ethanol separation from dilute aqueous solution”, J. Membr. Sci.,360, 341-351 (2010).
35 Vane, L.M., Namboodiri, V.V., Bowen, T.C., “Hydrophobic zeolite-silicone rubber mixed matrix membranes for ethanol-water separation: effect of zeolite and silicone component selection on pervaporation performance”, J. Membr. Sci.,308, 230-241 (2008).
36 Zhan, X., Li, J.D., Chen, J., Huang, J.Q., “Pervaporation of ethanol/water mixtures with high flux by zeolite-filled PDMS/PVDF composite membranes”, Chin. J. Polym. Sci.,27, 771-780 (2009).
37 Jonquieres, A., Fane, A., “Filled and unfilled composite GFT PDMS membranes for the recovery of butanols from dilute aqueous solutions: influence of alcohol polarity”, J. Membr. Sci.,125, 245-255 (1997).
38 Huang, J.C., Meagher, M.M., “Pervaporative recovery of n-butanol from aqueous solutions and ABE fermentation broth using thin-film silicalite-filled silicone composite membranes”, J. Membr. Sci.,192, 231-242 (2001).
39 Liu, F.F., Liu, L., Feng, X.S., “Separation of acetone-butanol-ethanol (ABE) from dilute aqueous solutions by pervaporation”, Sep. Purif. Technol.,42, 273-282 (2005).
40 Liu, X.L., Li, Y.S., Liu, Y., Zhu, G.Q., Liu, J., Yang, W.S., “Capillary supported ultrathin homogeneous silicalite-poly(dimethylsiloxane) nanocomposite membrane for bio-butanol recovery”, J. Membr. Sci.,369, 228-232 (2011).
41 Qureshi, N., Blaschek, H.P., “Production of acetone butanol ethanol (ABE) by a hyper-producing mutant strain of Clostridium beijerinckii BA101 and recovery by pervaporation”, Biotechnol. Progr.,15, 594-602 (1999).
42 Qureshi, N., Maddox, I.S., Friedl, A., “Application of continuous substrate feeding to the ABE fermentation: relief of product inhibition using extraction, perstraction, stripping, and pervaporation”, Biotechnol. Progr.,8, 382-390 (1992).
43 Chen, J., Li, J.D., Chen, J.X., Lin, Y.Z., Wang, X.G., “Pervaporation separation of ethyl thioether/heptane mixtures by polyethylene glycol membranes”, Sep. Purif. Technol.,66, 606-612 (2009).
44 Li, B., Xu, D., Jiang, Z.Y., Zhang, X.F., Liu, W.P., Dong, X., “Pervaporation performance of PDMS-Ni2+Y zeolite hybridmembranes in the desulfurization of gasoline”, J. Membr. Sci.,322, 293-301 (2008).
45 Zhao, C.W., Li, J.D., Qi, R.B., Chen, J., Luan, Z.K., “Pervaporation separation of n-heptane/sulfur species mixtures with polydimethylsiloxane membranes”, Sep. Purif. Technol.,63, 220-225 (2008).
46 Devi, D.A., Raju, K.V.S.N., Aminabhavi, T.M., “Synthesis and characterization of moisture-cured polyurethane membranes and their applications in pervaporation separation of ethyl acetate/water azeotrope at 30 °C”, J. Appl. Polym. Sci.,103, 3405-3414 (2007).
47 Salt, Y., Hasanoglu, A., Salt, I., Keleser, S., Dincer, S.O.S., “Pervaporation separation of ethylacetate-water mixtures through a crosslinked poly(vinylalcohol) membrane”, Vacuum,79, 215-220 (2005).
48 Yuan, H.K., Xu, Z.L., Shi, J.H., Ma, X.H., “Perfluorosulfonic acid-tetraethoxysilane/polyacrylonitrile (PFSA-TEOS/PAN) hollow fiber composite membranes prepared for pervaporation dehydration of ethyl acetate-water solutions”, J. Appl. Polym. Sci.,109, 4025-4035 (2008).
49 Morigami, Y., Kondo, M., Abe, J., Kita, H., Okamoto, K., “The first large-scale pervaporation plant using tubular-type module with zeolite NaA membrane”, Sep. Purif. Technol.,25, 251-260 (2001).
50 O’Brien, D.J., Roth, L.H., McAloon, A.J., “Ethanol production by continuous fermentation-pervaporation: A preliminary economic analysis”, J. Membr. Sci.,166, 105-111 (2000).
51 Vane, L.M., “A review of pervaporation for product recovery from biomass fermentation processes”, J. Chem. Technol. Biotechnol.,80, 603-629 (2005).
52 Xu, L.F., Xiangli, F.J., Chen, Y.W., Jin, W.Q., Xu, N.P., “Progress in pervaporation for biofuel ethanol”, Chem. Ind. Eng. Prog. (China),26, 788-796 (2007). (in Chinese)
53 Jin, W.Q., Liu, G.P., Xu, N.P., “Progress of pervaporation in bio-butanol production from ABE fermentation”, Membr. Sci. Technol. (China),31, 25-31 (2011). (in Chinese)
54 White, L.S., Wormsbecher, R.F., Lesemann, M., “Membrane separation for sulfur reduction”, U.S. Pat., 6896796 (2005).
of sulfur from gasoline has receivedmore attention with growing environmental awareness. Since Grace Davison Company offered the PV membrane-based S-Brane process in 2002 [54], pervaporation is considered as a promising and feasible technology for desulfurization of gasoline. PV desulfurization demonstrates distinct advantages over conventional technologies such as low energy consumption, simple operation, and little reduction of octane number.
Received 2011-08-03, accepted 2011-11-10.
* To whom correspondence should be addressed. E-mail: wqjin@njut.edu.cn
increasing attention in recent years. This article reviews the preparation and application of macroporous ceramic-supported polymer composite pervaporation membranes. The separation materials of polymer/ceramic composite membranes presented here include hydrophobic polydimethylsiloxane (PDMS) and hydrophilic poly(vinyl alcohol) (PVA), chitosan (CS) and polyelectrolytes. The effects of ceramic support treatment, polymer solution properties, interfacial adhesion and incorporating or blending modification on the membrane structure and PV performance are discussed. Two in-situ characterization methods developed for polymer/ceramic composite membranes are also covered in the discussion. The applications of these composite membranes in pervaporation process are summarized as well, which contain the bio-fuels recovery, gasoline desulfuration and PV coupled process using PDMS/ceramic composite membrane, and dehydration of alcohols and esters using ceramic-supported PVA or PVA-CS composite membrane. Finally, a brief conclusion remark on polymer/ceramic composite membranes is given and possible future research is outlined.
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Festschrift in Honor of the 90thBirthday of Prof. Chen Jiayong
- Ternary System of Fe-based Ionic Liquid, Ethanol and Water for Wet Flue Gas Desulfurization*
- The Research Progress of CO2Capture with Ionic Liquids*
- Synthesis of PGMA Microspheres with Amino Groups for High-capacity Adsorption of Cr(VI) by Cerium Initiated Graft Polymerization*
- Solvothermal Synthesis and Optical Performance of One-dimensional Strontium Hydroxyapatite Nanorod*
- Effects of Additives and Coagulant Temperature on Fabrication of High Performance PVDF/Pluronic F127 Blend Hollow Fiber Membranes via Nonsolvent Induced Phase Separation
