The Research Progress of CO2Capture with Ionic Liquids*
2012-08-02ZHAOZhijun赵志军DONGHaifeng董海峰andZHANGXiangping张香平
ZHAO Zhijun (赵志军), DONG Haifeng (董海峰)and ZHANG Xiangping (张香平),**
1Beijing Key Laboratory of Ionic Liquids Clean Process, State Key Laboratory of Multiphase Complex System, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
2College of Chemistry and Chemical Engineering, Graduate University of Chinese Academy of Sciences, Beijing 100049, China
The Research Progress of CO2Capture with Ionic Liquids*
ZHAO Zhijun (赵志军)1,2, DONG Haifeng (董海峰)1and ZHANG Xiangping (张香平)1,**
1Beijing Key Laboratory of Ionic Liquids Clean Process, State Key Laboratory of Multiphase Complex System, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
2College of Chemistry and Chemical Engineering, Graduate University of Chinese Academy of Sciences, Beijing 100049, China
Due to their negligible volatility, reasonable thermal stability, strong dissolubility, wide liquid range and tunability of structure and property, ionic liquids have been regarded as emerging candidate reagents for CO2capture from industries gases. In this review, the research progresses in CO2capture using conventional ionic liquids, functionalized ionic liquids, supported ionic-liquids membranes, polymerized ionic liquids and mixtures of ionic liquids with some molecular solvents were investigated and reviewed. Discussion of relevant research fields was presented and the future developments were suggested.
CO2, capture, absorption, separation, ionic liquids, desorption, solubility, selectivity
Chinese Journal of Chemical Engineering,20(1) 120—129 (2012)
1 INTRODUCTION
The emission of carbon dioxide (CO2) from utilization of fossil fuels has received worldwide attention due largely to the rapid growth in worldwide CO2emissions predicted to 40.2 Gt by 2030 [1]. Therefore, there is a growing interest in developing technologies for efficient capture and sequestration of large quantities of CO2. By far, a number of CO2capture technologies which have already being practiced on laboratory scale or industrially are processes based on physisorption/chemisorption, membrane separation or molecular sieves, carbamation, amine physical absorption, amine dry scrubbing, mineral carbonation [2]. The traditional technology for CO2capture in industry is chemical adsorption by an aqueous solution of amine, which has some advantages such as its maturity, stable operation, good reactivity, and high capacity [3]. However, using aqueous amines like monoethanolamine (MEA) CO2capture consumes almost 30% of the energy that is needed to run a power plant [4], in addition to other drawbacks like insufficient CO2capture capacity, high solvent losses caused by evaporation, degradation and poor thermal stability, as well as the equipment corrosion [5, 6]. Therefore, developing the economical and energy efficient CO2capture technologies is urgently needed.
Ionic liquids (IL) with a low melting point (<373.15 K) have been emerging as nonvolatile and reversible absorbents for CO2capture. Compared to traditional organic solvents, the non-volatile ILs are more environmentally friendly [7, 8]. This paper reviews the latest research progress of CO2capture with ILs, and also proposes some research prospects.
2 RESEARCH PROGRESS OF CO2CAPTURE WITH IONIC LIQUIDS
2.1 CO2capture with conventional ionic liquids (CIL)
Blanchard et al. [9] reported firstly that CO2is highly soluble in ionic liquid of 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]), reaching a mole fraction of 0.6 at 8 MPa (Fig. 1), while the solubility of [C4mim][PF6] in CO2is less than 10−5mole fraction at 13.8 MPa and 40 °C. In six ILs such as 1-n-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]), 1-n-octyl-3-methylimidazoliumhexafluorophosphate ([C8mim][PF6]), 1-n-octyl-3-methylimidazoliumtetrafluoroborate ([C8mim][BF4]), 1-n-butyl-3-methylimidazoliumnitrate ([C4mim][NO3]), 1-ethyl-3-methylimidazolium ethyl sulfate ([C2mim][EtSO4]), and N-butylpyridiniumtetrafluoroborate ([N-bupy][BF4]) at high-pressure, a large quantities of CO2was found to dissolve in the ionic liquid phase, while no appreciable amount of ionic liquid solubilized in the CO2phase [10]. At present, the CO2capture by ILs received much attention.

Figure 1 CO2solubility in [C4mim][PF6] at 25 °C [9]
The solubility of CO2in a series of imidazoliumbased IL at low pressure has been determined by Baltus et al [11]. It was found to increase with the length of the alkyl side chain on the imidazolium ring but the solubility in IL with phenyl groups was lower when compared to that with alkyl groups. The CO2solubility is greater in ionic liquids with Tf2N−anions than that in ILs with6PF−anions. And the imidazolium-based ionic liquids with a fluorine-substituted C8side chain are higher than the corresponding ILs having a non-fluorinated C8side chain. Chen et al. [12] studied the CO2solubility in 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]) and 1,1,3,3-tetramethylguanidium lactate (TMGL) at 297 K to 328 K under 0 to 11 MPa. The experimental results showed that the solubilities of CO2in TMGL are slightly higher than those in [C4mim][PF6]. The solubility of CO2in TMGL is 2.77 mol·kg−1and in [C4mim][PF6] is 2.65 mol·kg−1at 319 K and 5.73 MPa and the selectivity of TMGL toward CO2is much more favorable than other gases such as N2, O2, CH4and H2[13]. Anderson et al. [14, 15] studied the impact of fluorination of the cation on CO2solubility with the conclusion the CO2solubility was higher in [C8H4F13mim][Tf2N] than [C6H4F9mim][Tf2N], and lowest in [C6mim][Tf2N]. In all, the CO2solubility increases as the numbers of fluorine in the alkyl side chain but this tendency is not very visible.
Experimental and the molecular dynamics simulation results from Cadena et al. [16] indicated that the nature of anion has the greatest influence on the solubility of CO2and the bis(trifluoromethylsulfonyl)-imide anion ([Tf2N]−) has the greatest affinity for CO2. Aki et al. [17] studied the CO2solubility in ILs with cation [C4mim]+but different anions, and found CO2solubility increases in the order of [NO3]−<[DCA]−<[BF4]−<[PF6]−<[CF3SO3]−<[Tf2N]−<[methide]−. Therefore, the more fluorine atoms in the anions, the higher CO2solubility is, due to the strong coulombic interactions responsible for the organization of the liquid, which also proved by other groups [18-20].
In order to study the selectivity of separating CO2from mixture gases, Anthony et al. [21] measured the solubilities of CO2, C2H4, C2H6, CH4, CO, O2, H2and N2in [C4mim][PF6], and found that CO2is the most soluble among these gases, and [C4mim][PF6] can absorb CO2selectively (Fig. 2). Regarding the purification of natural gas, certain hygroscopic imidazolium-based ionic liquids like [C4mim][PF6], [C8mim][BF4] and [C8mim][PF6] have ability of dehydration as well [22, 23]. Shiflett et al. [24] developed a ternary equation of state (EOS) model for the N2O/CO2/[C4mim][BF4] system. With this EOS model, for both large and small N2O/CO2feed ratios, the N2O/CO2gas selectivity a is predicted to be 1.4-1.5, compared with (a=0.96-0.98) in the absence of ionic liquid. Anderson et al. [14] measured the solubility of CO2, C2H4, C2H6, CH4, O2and N2in [C6mpy][Tf2N], they also found this IL is a potential CO2absorbent with high selectivity.

Figure 2 CO2, C2H4, C2H6, CH4, Ar and O2solubilityin [C4mim][PF6] at 25 °C [21]● CO2; ▲ C2H4; ■ C2H6; ▼ CH4; ○ Ar; + O2
Thus far, some CIL (especially imidazoliumbased) have diminished corrosion of the equipment, and the heat capacity of CIL is almost one-third of that of aqueous systems, which may have profound effect in reducing the high investment and operation cost [25-28]. As for the regeneration, CIL based materials can be easily recycled either by pressure sweep process coupled with vacuum treatment, or by applying heat or by bubbling nitrogen through the absorbent [29, 30].
In short, fluorination of the cation and anion is an effective way to improve CO2solubility in conventional IL, but the anion got more attention. Though the conventional IL can absorb and separate CO2effectively to a certain extent, it is just by mechanism of physical adsorption. The drawback is that the CO2absorption capacity of these ILs, even in the fluorinated ILs, is far below that of the traditional alkanolamine solutions.
2.2 CO2capture with functionalized ionic liquids (FIL)
As discussed above, the solubility of CO2in the CIL is not satisfying, and the CO2absorption capacity can be further improved by functionalization of IL with a suitable moiety (like amine) [31, 32]. Bates et al. [33] firstly synthesized [NH2p-bim][BF4] consisting of an imidazolium ion to which a primary amine moiety is covalently bonded, and gave the proposed reaction mechanism between FIL and CO2, similar to the reaction between traditional organic amine and CO2(Scheme 1). In 3 h, the CO2capacity in [NH2p-bim][BF4] approached the theoretical maximum (0.5 mol CO2per mol IL), similar to the CO2capacity of alkanolamine (Fig. 3). The process of CO2uptake is reversible, and CO2being depleted from IL upon heating (80-100 °C) in several hours under vacuum. The recovered IL can be recycled for CO2uptake (five cycles) with no detectable loss of efficiency.

Figure 3 CO2/ FIL molar ratio as a function of time [33]
More and more FILs appeared by utilizing two techniques. The first one is to functionalize the anion with alkaline group-NH2such as [NH2p-bim][BF4], and the other is to attach the functional group to the anion.
2.2.1Functionalize the anion of IL with alkaline group-NH2
Zhang et al. [34] functionalized the anion with alkaline group-NH2, and tetrabutylphosphonium amino acid ([P(C4)4][AA]) was synthesized by reaction of tetrabutylphosphonium hydroxide [P(C4)4][OH] with amino acids, including glycine, l-alanine, l-b-alanine, l-serine, and l-lysine. The CO2absorption capacity of this IL is similar with that of the [NH2p-bim][BF4] (0.5 mol CO2per mol IL).
Meindersma et al. [35] studied the kinetics of CO2absorption in amino-functionalized IL such as 1-(3-aminopropyl)-3-methylimidazolium tetrafluoroborate ([APmim][BF4]) at concentrations between 45 and 253 mol·m−3at 303 K and 333 K. The experimental enhancement factors based on the fluxes of CO2absorbed are between 1.2 and 2.3, depending on the [APmim][BF4] concentration. Han et al. [36] evaluated firstly the basicity of IL choline chloride/urea and 1-aminoethyl-3-methyl imidazolium tetrafluoroborate ([AEMIM][BF4]), and 1,1,3,3-tetramethylguanidinium perchlorate ([TMG][ClO4]). Their results suggested that adding CO2of ambient pressure to IL could reduce their basicity significantly, and the basicity of ILs was readily recovered after removing CO2by bubbling N2through the solutions, which can help to understand the reaction mechanism between IL and CO2.
Zhang et al. [37] functionalized both the anion and cation with alkaline group such as NH2. The (3-aminopropyl)tributylphosphonium amino acid ([aP4443][AA]), a dual amino-functional IL, were obtained by neutralizing (3-aminopropyl) tributylphosphonium hydroxide ([aP4443]-[OH]) with 20 natural amino acids. [aP4443][OH] was prepared from (3-aminopropyl)tributyl phosphonium bromide ([aP4443]Br) using an anion-exchange resin. In order to improve the absorption rate, ([aP4443][AA] was then supported on the porous SiO2and their CO2absorption was investigated. The results showed that the ratio of chemical absorption of CO2by [aP4443[Gly], [aP4443][leu], [aP4443][Ala], [aP4443][Val] was close to 1 mol CO2per mol IL within 80 min, which is consistent with the absorption mechanism proposed in [33, 34]. Although the CO2absorption capacity and the rate of this IL decreased steadily, the recovered IL can be repeatedly recycled with out detectable loss for five cycles.
Mu et al. [38] synthesized a new dual amino IL [aemmim][Tau] with amino-functionalized imidazolium cation and taurine anion. The CO2absorption capacity of this IL reaches 0.9 mol CO2per mol IL at 303.15 K and 0.1 MPa. The dissolved CO2can be easily desorbed at higher temperature or under vacuum, so [aemmim][Tau] can be reused and no significant loss of capability was observed after six recycles.
2.2.2Attach the anion of IL with functional group
Gurkan et al. [39] found a case of intra molecular proton transfer by using one larger phosphonium cations with steric hindrance to block the intermolecular proton transfer process and synthesized [P66614][Pro] and [P66614][Met]. The proposed reaction mechanism between these ILs and CO2is different (Scheme 2). The CO2capacity in these ILs approached the theoretical maximum (1.0 mol CO2per mol IL) (Fig. 4). Then they synthesized six amine-functionalized anion-tethered ILs, trihexyl(tetradecyl)phosphonium glycinate ([P66614] [Gly]), alanate ([P66614][Ala]), sarcosinate ([P66614][Sar]), valinate ([P66614][Val]), leucinate ([P66614][Leu]), and isoleucinate ([P66614][Ile]), and showed the capacity of these four ILs was above 0.5 mol CO2per mol IL at CO2pressures of less than 0.1 MPa. This indicated that the predominance of the 1∶1 mechanism, where the CO2reacts with one IL to form a carbamic acid, over further reaction with another IL to make a carbamate (the 1∶2 mechanism). The chemically absorbed CO2increased dramatically the viscosity of the IL, but this can be mitigated to some extent by decreasing the number of hydrogens on the anion available for hydrogen bonding [40]. Furthermore, from temperature dependent isotherms, they estimated the heat of absorption is−63 kJ·mol−1CO2for the 1∶1 reaction of CO2with [P66614][Pro], whose viscosity was not increased, likely due to its ring structure limiting hydrogen atoms to form a hydrogen bonding network.

Scheme 2 Reaction mechanism of CO2with [P66614][Met] (top) and [P66614][Pro] (bottom) [39]

Figure 4 CO2absorption by [P66614][Pro] and [P66614)][Met] at 22 °C [39]○ [P66614][Met];[P66614][Met] React-IR;[P66614][Met] calc.▲ [P66614][Pro]; ▽ [P66614][Pro] React-IR; [P66614][Pro] calc.
In short, the FIL can enhance the CO2absorption capacity with ascending pressure, while the aqueous amine solutions reached their maximum capacity at about 0.2 MPa [41, 42]. But the viscosity was increased sharply after CO2absorption by these ILs [43, 44], which seriously limited their application on industrial scale. Yu and Zhang [45] studied the structure and inter-ionic interaction of the IL by molecular orbital simulation and showed the intermolecular NH2associated hydrogen bonds can substantially increase the cation-anion interaction which increased the viscosity of the IL, but the in-deep reaction mechanism is still worthwhile to study.
2.3 CO2capture with supported ionic liquids membranes (SILM)
In order to get better absorption efficiency, some studies explored the prospects of supported ionic liquid on membranes. The supported ionic liquids membranes (SILM) are made by using IL as liquid phase of membrane. The properties of IL such as viscosity and nonvolatility can stop the membrane solvent flowing out from porous membrane, which prolongs the life of the SILM greatly without diminishing the ability and selectivity of separation. The high-thermal stability and non-flammation of IL is suitable for capturing CO2from the flue-gases at high temperature, and SILM can enhance the contact area between gas and IL [46, 47].
2.3.1Supported ionic liquids membranes based with conventional IL
Scovazzo et al. [48] supported ILs on the porous hydrophilic polyethersulfone (PES) and evaluate the penetrability and selectivity of this CIL-membranes towards CO2, N2and CH4. They also studied the influence of the CIL-membranes having the following four water stable anions: bis(trifluoromethanesulfonyl) amide [Tf2N]−, trifluoromethanesulfone [CF3SO3]−, chloride [Cl]−, and dicyanamide [Dca]−. The experimental results showed CO2permeabilities of 2.6×10−8cm3·cm·cm−2·s−1·kPa−1(for [Cl]−) to 7.5×10−8cm3·cm·cm−2·s−1·kPa−1(for [Tf2N]−) together with ideal CO2/N2selectivities of 15 (for [Cl]−) to 61 (for [DCA]−), and the CO2/C2H4ideal selectivities ranged from 4 (for [Cl]−) to 20 (for [DCA]−). Note that these permeability/selectivities of above CIL-membranes were relatively good and even better in comparison to polymer membranes. Baltus et al. [49] achieved good CO2absorption capacity by fixing [C4min][Tf2N] to porous Al2O3. Moriya et al. [50] supported IL like [C4mim][PF6] to a porous (ceramic or zeolite) material for CO2separation by putting pressurized gas on one side and collecting the CO2-depleted gas stream downstream of the porous medium. Then Park et al. [51] supported [C4min][BF4] on the PVDF (poly-vinylidene fluorolide) as polymer materials for membrane to absorb and separate the mixture gases of CO2and CH4. To investigate the permeation properties, the SILMs were tested with CO2, H2S and CH4at various operating conditions. Since CO2and H2S have higher affinity toward CIL than CH4, the permeability coefficients of these two acidic gases were found to be considerably high at (0.225-1.35)×10−8cm3·cm·cm−2·s−1·kPa−1and (0.12-0.825)×10−7cm3·cm·cm−2·s−1·kPa−1, respectively. Moreover, the selectivity of CO2/CH4and H2S/CH4were found to be 25-45 and 130-260, respectively.
Neves et al. [52] studied the potential of using supported IL membranes (SILM) for CO2/N2and CO2/CH4gas separations. Their results showed that the SILMs prepared with the most hydrophobic support are more stable than those based on the hydrophilic support, and had a high affinity for CO2over other gases. It also indicated that the presence of water vapour in gas stream increases the SILMs gas permeability but decreased their CO2/N2and CO2/CH4selectivity significantly. This decrease in selectivity was due to the formation of water clusters inside the membrane, because this effect is more significant for the less hydrophobic CIL.
The permeability coefficient of CO2and CH4increased dramatically with the CIL content in the membrane. It also increased with the increase in operating temperature which enlarged the free volumes inside the polymer membranes. But the selectivity of CO2/CH4decreased with the increasing temperature because the permeability coefficient of CH4was more likely to depend on the membrane structure rather than interaction between CH4and membrane.
Furthermore, the new SILMs demonstrated the selectivity of CO2/CH4increased with increasing pressure and maintained mechanical stability at the same time. Kim et al. [53] supported [C2mim][Tf2N] on the polymeric hollow fiber, and found the structure of the support has a large impact on membrane stability, and the sponge-like structure has higher stability than a finger-like one. Compared to a cylindrical void structure, the support with a structure consisting of dense polymer chains, i.e., a torturous structure, showed stronger resistance to pressure.
Gonzalez-Miquel et al. [54] found the [SCN]−based ILs have generally low gas solubilities but it is possible to increase the CO2solubility by enhancing the van der Waals intermolecular interactions of CO2with the ILs. While exhibiting no affinity for N2.The reason was the CO2/N2selectivity also depends on the counter anion structure, which makes it possible to select the cation family and its alkyl substitute to tune CO2/N2selectivity and other properties of interest of the ILs, such as density or viscosity, to optimize the CO2/N2separation in SILMs.
However, the CIL-membranes were found to be unsuitable for gas separation at high pressures because of decreased gas solubility. The properties of CIL membranes were found to be enhanced significantly by the addition of an organic salt with an amine group functionality [55].
2.3.2Supported ionic liquids membranes based with functionalized IL
Haniola et al. [56] and Shishatskiy et al. [57] supported 1-butyl-3-methylimidazoliumbis (trifluoromethylsulfonyl)-imide ([C4mim][Tf2N]), N-aminopropyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([C3NH2mim][Tf2N]) and N-aminopropyl-3-methylimidazolium trifluoromethanesulfone ([C3NH2mim] [CF3SO3]) on the porous hydrophilic polytetrafluoroethylene (PTFE) membrane to separate the mixture gas of CO2and CH4. The experimental results suggested the permeability coefficients of CO2in [C3NH2mim][Tf2N] and [C3NH2mim][CF3SO3] are higher than that in [C4mim][Tf2N]. The reason was that CO2permeated across the SILM by the simple solution-diffusion mechanism in [C4mim][Tf2N] while CO2permeated by chemical reaction mechanism in the case of [C3NH2mim][Tf2N] and [C3NH2mim][CF3SO3], which worked as transport-carrier in the CO2permeation. These SILMs had high stability, without detectable loss, after performance of 260 days. The high temperature prevented the reaction between CO2and amine moiety, so the permeability coefficient of CO2decreased with the increasing temperature [58]. The combination of SILMs with FIL may be a better choice for capture at elevated temperatures and pressures [59]. The CH4permeation was based on the simple solutiondiffusion mechanism, the permeability coefficient of CH4in [C3NH2mim][Tf2N] and [C3NH2mim][CF3SO3] was lower than that in [C4mim][Tf2N]. The reason was that the viscosities of [C3NH2mim][Tf2N] and [C3NH2mim][CF3SO3] were too high (2180 mPa·s and 3760 mPa·s respectively). The CO2/CH4selectivity was quite high even at low CO2partial pressure. At 2.5 kPa of CO2, the SLMs based on [C3NH2mim][Tf2N] and [C3NH2mim][CF3SO3] achieved the selectivity of approximately 100 and 120, respectively.
Meanwhile, the supported IL membranes were made by placing cross-linked nylon supports in a container and depositing [H2NC3H6mim][Tf2N] on top of the membrane with a pipette [62]. The separation of CO2from H2in this amino-functional IL ([C6mim][Tf2N] as reference) was studied. The results suggested when the temperature rises above 85 °C, this IL based facilitated transport membrane will have lower permeability of CO2, which was similar to the Hanioka’s results [56]. The reason was that the high temperature impacts the stability of carbonate, which made the diffusion phenomenon start to dominate. Considering the selectivity and permeability together, the properties of [C6mim][Tf2N] was relatively good but lower than [H2NC3H6mim][Tf2N]. In case of hydrophilic composite membranes, the presence of moisture in flue gas affected the CO2separation performance. Moist feed seemed to increase permeability up to 35-fold without any detectable loss in CO2/H2or CO2/N2selectivity as compared to dry feed [57].
From the above results, we find that though the supported IL membrane has several drawbacks such as the thick membrane (150 μm or even thicker [48, 61, 62]), unapplicability in separating CO2from the flue-gases at high pressures, it is regarded as a potential technology for CO2capture. To develop more efficient and cost-effective SILMs requires in-depth study into the role of anion/cation in optimization of molar volume of constituent ILs and the structure of the support materials, so as to produce more stable, more permeable but thinner membranes [63].
2.4 CO2capture with polymerized ionic liquids (PIL)
One of the disadvantages of SILM is to leach the liquid through membrane pores when the pressure drop surpasses the liquid stabilizing forces within the matrix. In order to reduce the time of absorption/ desorption and overcome this disadvantage, Tang et al. [64] firstly made IL into polymeric form which significantly increased the CO2sorption capacity compared with conventional IL. The experiment showed that the polymers of tetraalkylammonium-based IL (e.g. poly[p-vinylbenzyltrimethylammonium tetrafluoroborate] (P[VBTMA][BF4]) has CO2sorption capacity 7.6 timesthan those conventional IL e.g. [C4mim][BF4]. The CO2absorption and desorption by these polymers are much faster than in IL. The polymers can also absorb selectively CO2in N2/CO2mixed gas and did not absorb N2or O2at 78.97 kPa and 22 °C [64, 65]. They also found when polyethylene glycol (PEG) was grafted onto ionic polymers, such as P[VBTMA][BF4], it can produce thermally, chemically and mechanically stable CO2-selective membranes [66]. Bara et al. [67] found the solubility, permeability and diffusion of CO2CH4and N2in both types of poly-CIL increased dramatically, and the sorption/desorption was completely reversible, which made these poly-ILs very promising as sorbent and membrane materials for CO2separation.
Tang et al. [68-72] studied the CO2solubility in an ammonium-type ionic and other type polymers, and probed the structure effects on the CO2sorption. They showed the CO2sorption capacities of the PILs with different cations decreased in the order: ammonium>pyridinium>phosphnium>imidazolium, and those of the PILs with different anions were in the sequence of4BF−>PF6≫Tf2N. P[VBTMA][BF4] with a polystyrene backbone had a higher CO2absorption capacity than P[MATMA][BF4] with a polymethylmethacrylate backbone. Long alkyl substituents on the cation and cross-linking decreased the CO2sorption capacity. The results also illustrated that the CO2sorption process consists of dissolution in the polymer matrix and Langmuir sorption inside the microvoid.
Bara et al. [67, 73] tethered oligo(ethylene glycol) or nitrile-terminated alkyl substituents to CIL monomers and these polymer membranes were found to exhibit 50% greater CO2/N2and CO2/CH4separation factors than those with comparable length n-alkyl substituents, but with similar CO2permeability. The OEG-functionalized poly-CIL exhibited several times higher permeability than those with CnCN functional groups. They mixed CIL and poly-CIL as a composite gas separation membrane to absorb CO2and showed that incorporation of an appropriate amount of CIL and introducing free ion pairs into the polymer membrane, the CO2permeability and CO2/N2selectivity increased up to 300%-600% and with 25%-33%, respectively, relative to the analogous poly-CIL membrane lacking any free ion pairs [74, 75].
The polymerized-ILs are very viscous at room temperature and easy to be applied as membrane materials, so if the techniques of combing the polymerized IL and supported IL membranes were used, it will produce higher CO2absorption capacity than the small molecular ILs. Bara et al. [76] summarized the using of polymerized-ILs as membrane materials in the CO2capture and they agreed that this kind of membranes is a relatively good option for separating and absorbing CO2.
2.5 CO2capture with mixture solvent of ionic liquids and molecular solvents
The viscosity of the conventional IL at 33 °C is forty time higher than that of aqueous alkanolamine solutions such as MEA (30%), which brought great influence on the CO2absorption capacity and rate of the absorption of IL [77]. Some scientists synthesized the absorbent by mixing IL with organic solvents or water.
2.5.1IL mixing with water or methanol
Zhang et al. [34] reported that in the presence of water (1%), the IL with one amino group could absorb equimolar amounts of CO2and formed the product of carbonate, which is different from the product of carbamate without water (Scheme 3).
Ventura et al. [78] studied systematically the impact of water content on the viscosity of IL and the CO2absorption capacity. The results suggested the addition of water can reduce the viscosity but decrease the CO2absorption capacity. Li et al. [29] compared [Choline][Pro] with or without the poly-ethyleneglycol at temperature between 35-80 °C at normal pressure. Their conclusion, similar to Ventura et al. [78]: the addition of poly ethyleneglycol can reduce the viscosity of IL and improve the CO2absorption or desorption rate, but the solubility of CO2decreased with added poly ethyleneglycol.
Han et al. [79] studied the phase behavior of CO2-methanol-[C4mim][PF6] system under different conditions by a static method. It showed the three-phase region exists only in the temperature range from 35 to 44.5 °C at 7.6 MPa. Increase of pressure and decrease of temperature were favorable to enlarging the distribution coefficients of IL and methanol. They also studied the phase behavior of CO2-water-[C4mim][BF4] system at different conditions [80], and the results suggestedthe concentrations of CO2in the IL-rich phase and water-rich phase increase as the pressure rises and decreases with increasing temperature. The phase behavior of CO2-acetone-[C4mim][PF6] systems at 313.15 K and at pressures up to 15 MPa was also studied, which illustrated CO2distribution coefficient decreased with increasing pressure while acetone distribution coefficient increased with pressure [81].

Scheme 3 Proposed mechanism for the CO2capture by IL: (a) , (b) without water and (c) with water[34]
2.5.2IL mixing with alkanolamines
In recent years, some researchers proposed a more practical absorbent of mixing IL with aqueous alkanolamine to absorb CO2, and the experimental results showed the non-volatility of IL can restrain the volatility of the aqueous alkanolamine solvent to some degree. Meanwhile, the compound absorbent can provide more physical properties than single absorbent. Camper et al. [82] mixed MEA and [C6mim][Tf2N] to investigate the CO2solubility. They found that the CO2solubility in this compound absorbent approached the maximum 0.5 mol CO2per mol amine and it also can reduce the energy of desorption effectively. This compound absorbent really shed light to a new way to the CO2capture.
Zhang et al. [83] synthesized four ILs: tetramethylammonium glycinate ([Gly]), tetraethylammonium glycinate ([N2222][Gly]), tetramethylammonium lysinate ([N1111][Lys]) and tetraethylammonium lysinate ([N2222][Lys]) and mixed with water or N-methyldiethanolamine (MDEA) aqueous solutions to form new solvents for the uptake of CO2. The results indicated that IL enhanced greatly the absorption and raised the absorption rate of CO2in aqueous MDEA solutions. The CO2absorption capacity in the pure [N2222][Gly] is very small and the absorption rate is slow which cannot reach equilibrium in 100 min, but the CO2absorption capacity and rate are improved after the addition of MDEA.
Zhang et al. [84] studied three compound absorbents such as amines-IL-H2O, IL-H2O, and amines-H2O at temperature ranging from 303.15 to343.15 K with different IL mass fractions. The results indicated the absorbent of amines-IL-H2O shows the best performance on CO2capture. Zhao et al. [85] investigated the solubilities of CO, H2, N2, O2and CO2in IL ([MDEA][Cl]) at different pressures from 1.22 to 8.62 MPa and temperature from 313.15 to 333.15 K, showing that the solubility of CO2was much higher than the other four gases. And 1 g solvent of MCHP (MDEA-[MDEA][Cl]-H2O-PZ) could absorb approximately 0.1584 g of CO2[86], while 0.15 g of CO2for 1 g solvent of MCHP (MDEA-[MDEA][PF6]-H2O-PZ) at 303.15 K and 1.50 MPa [87]. Their research on mechanism of CO2capture in [MDEA][Cl] and [MDEA][PF6] indicated that both of them could capture CO2by physisorption and chemisorption.
Aroua et al. [88] found that the CO2loading in IL-MDEA (MEDA 4.0 mol·L−1) mixtures increased with increasing CO2partial pressure and decreased with increasing temperature and the CO2loading decreases significantly as the IL concentration increases, but this reduction in solutions contained [C4mim][BF4] was less than other types of imidazolium-based ILs.
2.5.3IL mixing with super-base or other organic solvents
Wang et al. [89, 90] combined bicyclic amidine (DBU) with hydroxyl modified IL as a superbase to trap the proton produced during sorption process of CO2, which proved to be an effective way in achieving an equimolar of sorption (Fig. 5). Superbase-derived protic ILs were further synthesized with the equilibrium sorption molar ratio approached 1∶1. The sorption mechanism between CO2and anions with proton trapped by the superbase to form cations is shown in Scheme 4.
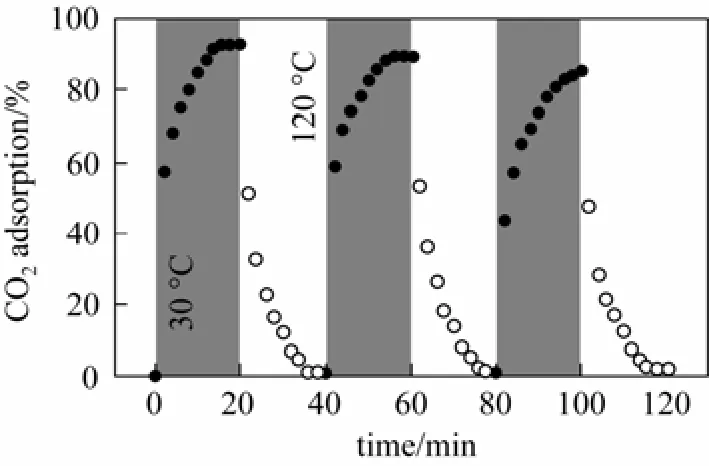
Figure 5 Three consecutive cycles of CO2absorption (grey, 30 °C) and release (white, 120 °C) by the [Im21OH][Tf2N]-DBU system (100% CO2uptake denotes 1.0 mol CO2per mol IL [89]

Scheme 4 The sorption mechanism of CO2absorption by the anions of superbase-derived protic ILs [90]
Zhang et al. [91] mixed IL 1-butyl-3-methylimidazolium chloride ([C4mim][Cl]) with chitin or chitosan as a mixed solvent to capture CO2. Chitin has two hydroxyl groups in the cellulose-like polymeric structure, whereas chitosan has an additional amine group. For the chitosan/IL mixture, the measured absorption capacity exceeds the theoretical capacity due to the physical absorption of CO2in these liquids. Physical absorption is also observed for the chitin/IL mixture. Although this approach can not provide as high absorption capacities as pure FIL, it really has the advantage of bringing a fully recyclable, less corrosive and non-volatile CO2absorbent.
In summary, the viscosity of this kind of solvent is lower than that of pure IL and easier to regeneratethan the traditional alkanolamines. So it maybe another potential option to CO2capture in the future.
3 CONCLUSIONS AND OUTLOOK
The unique properties of IL such as non-volatility, high thermal stability, strong solubility capacity, and tunability of structure and property made it be potential candidate materials for CO2capture. This paper summarized the current research progress on the ionic liquids and IL-based materials which are used in CO2capture. It showed that functionalized IL, supported IL membranes, polymerized IL and the mixtures of IL with molecular solvents have good prospects for CO2capture. Some companies in the world have intended to use the IL-based materials for industrial-scale separating CO2from natural gas or flue gas CO2[92, 93]. The future research should be focused on the following issues.
(1) Understanding the mechanism of the CO2capture with IL combing molecular simulation and experimental characteristics, and revealing the relationships between structures of IL and the performance of CO2capture;
(2) Developing the new IL with low cost, low viscosity and high absorption capacity with high selectivity, which will reduce the absorption rate and decrease the energy demand for desorption. The CO2desorption process is also crucial with respect to the energy consumption required by industries;
(3) Studying the transport phenomena which will be very important for the suitable design and operation of industrial unit, especially at low pressure and high temperature (like flue gas from coal-fired power plants) used for CO2capture with ionic liquids.
Though there are lots of problems in using ILs to capture CO2, we believe all the problems will be resolved one by one with in depth investigation and then it is possible to use ILs to capture CO2on large scales in the future.
REFERENCES
1 CO2Emissions From Fuel Combustion Highlights, International Energy Agency, Paris (2011).
2 Hasib-ur-Rahman, M., Siaj, M., Larachi, F., “Ionic liquids for CO2capture—Development and progress”, Chem. Eng. Process.,49(4), 313-322 (2010).
3 Oyenekan, B.A., Rochelle, G.T., “Energy performance of stripper configurations for CO2capture by aqueous amines”, Ind. Eng. Chem. Res.,45(8), 2457-2466 (2006).
4 Fisher, K.S., Rochelle, G.T., Ziaii, S., Schubert, C., “Advanced amine solvent formulations and process integration for near-term CO2capture success”, Trimeric Corporation, Pennsylvania (2007).
5 Dawodu, O.F., Meisen, A., “Degradation alkanolamine blends by carbon dioxide”, Can J. Chem. Eng.,74(6), 960-966 (1996).
6 Ahn, S., Song, H.J., Park, J.W., Lee, J.H., Lee, I.Y., Jang, K.R.,“Characterization of metal corrosion by aqueous amino acid salts for the capture of CO2”, Kor. J. Chem. Eng.,27(5), 1576-1580 (2010).
7 Karadas, F., Atilhan, M., Aparicio, S., “Review on the use of ionic liquids (ILs) as alternative fluids for CO2capture and natural gas sweetening”, Energy Fuels,24(11), 5817-5828 (2010).
8 Rogers, R.D., “Materials science—Reflections on ionic liquids”, Nature,447(7147), 917-918 (2007).
9 Blanchard, L.A., Hancu, D., Beckman, E.J., Brennecke, J.F., “Green processing using ionic liquids and CO2”, Nature,399(6371), 28-29 (1999).
10 Blanchard, L.A., Gu, Z.Y., Brennecke, J.F., “High-pressure phase behavior of ionic liquid/CO2systems”, J. Phys. Chem. B,105(12), 2437-2444 (2010).
11 Baltus, R.E., Culbertson, B.H., Dai, S., Luo, H.M., DePaoli, D.W.,“Low-pressure solubility of carbon dioxide in room-temperature ionic liquids measured with a quartz crystal microbalance”, J. Phys. Chem. B,108(2), 721-727 (2004).
12 Zhang, S.J., Yuan, X.L., Chen, Y.H., Zhang, X.P., “Solubilities of CO2in 1-btyl-3-methylimidazolium hexafluorophosphate and 1,1,3,3-tetramethylguanidium lactate at elevated pressures”, J. Chem. Eng. Data,50(5), 1582-1585 (2005).
13 Yuan, X.L., Zhang, S.J., Chen, Y.H., Lu, X.M., Dai, W.B., Mori, R.,“Solubilities of gases in 1,1,3,3-tetramethylguanidium lactate at elevated pressures”, J. Chem. Eng. Data,51(2), 645-647 (2006).
14 Anderson, J.L., Dixon, J.K., Brennecke, J.F., “Solubility of CO2, CH4, C2H6, C2H4, O2and N2in hexyl-3-methylpyridinium bis(trifluoromethylsulfonyl)imide comparison to other ionic liquids”, Acc. Chem. Res.,40(11), 1208-1216 (2007).
15 Muldoon, M.J., Aki, S.N.V.K., Anderson, J.L., Dixon, J.K., Brennecke, J.F., “Improving carbon dioxide solubility in ionic liquids”, J. Phys. Chem. B,111(30), 9001-9009 (2007).
16 Cadena, C., Anthony, J.L., Shah, J.K., Morrow, T.I., Brennecke, J.F., Maginn, E.J., “Why is CO2so soluble in imidazolium-based ionic liquids”, J. Am. Chem. Soc.,126(16), 5300-5308 (2004).
17 Aki, S., Mellein, B.R., Saurer, E.M., Brennecke, J.F., “High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids”, J. Phys. Chem. B,108(52), 20355-20365 (2004).
18 Schilderman, A.M., Raeissi, S., Peters, C.J., “Solubility of carbon dioxide in the ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide”, Fluid Phase Equilib.,260(1), 19-22 (2007).
19 Zhang, X.C., Liu, Z.P., Wang, W.C., “Screening of ionic liquids to capture CO2by COSMO-RS and experiments”, AIChE J.,54(10), 2717-2728 (2008).
20 Jalili, A.H., Mehdizadeh, A., Shokouhi, M., Sakhaeinia, H., Taghikhani, V., “Solubility of CO2in 1-(2-hydroxyethyl)-3-methylimidazolium ionic liquids with different anions”, J. Chem. Thermodyn.,42(6), 787-791 (2010).
21 Anthony, J.L., Maginn, E.J., Brennecke, J.F., “Solubilities and thermodynamic properties of gases in the ionic liquid 1-n-butyl-3-methylimidazolium hexafluorophosphate”, J. Phys. Chem. B,106(29), 7315-7320 (2002).
22 Brennecke, J.F., Maginn, E.J, “Purification of gas with ionic liquids compounds”, US Pat., 6579343 B2 (2003).
23 Yu, T., Weiss, R.G., Yamada, T., George, M., “Reversible room-temperature ionic liquids”, WO Pat., 094846 A1 (2008).
24 Shiflett, M.B., Niehaus, A.M.S., Yokozeki, A., “Separation of N2O and CO2using room-temperature ionic liquid [bmim][BF4]”, J. Phys. Chem. B,115(13), 3478-3487 (2011).
25 Reddy, R.G., Zhang, Z.J., Arenas, M.F., Blake, D.M., “Thermal stability and corrosivity evaluations of ionic liquids as thermal energy storage media”, High Temp. Mater. Process.,22(2), 87-94 (2003).
26 Perissi, I., Bardi, U., Caporali, S., Lavacchi, A., “High temperature corrosion properties of ionic liquids”, Corrosion Sci.,48(9), 2349-2362 (2006).
27 Veawab, A., Tontiwachwuthikul, P., Chakma, A., “Investigation of low-toxic organic corrosion inhibitors for CO2separation process using aqueous MEA solvent”, Ind. Eng. Chem. Res.,40(22), 4771-4777 (2001).
28 Soosaiprakasam, I.R., Veawab, A., “Corrosion and polarization behavior of carbon steel in MEA-based CO2capture process”, Int. J. Greenhouse Gas Contr.,2(4), 553-562 (2008).
29 Li, X.Y., Hou, M.Q., Zhang, Z.F., Han, B.X., Yang, G.Y., Wang, X.L., Zou, L.Z., “Absorption of CO2by ionic liquid/polyethylene glycol mixture and the thermodynamic parameters”, Green Chem.,10(8), 879-884 (2008).
30 Sanchez, L.M.G., Meindersma, G.W., de Haan, A.B., “Solvent properties of functionalized ionic liquids for CO2absorption”, Chem. Eng. Res. Des.,85(A1), 31-39 (2007).
31 Carvalho, P.J., Alvarez, V.H., Marrucho, I.M., Aznar, M., Coutinho,J.A.P., “High pressure phase behavior of carbon dioxide in 1-butyl-3-methylimidazoliumbis (trifluoromethylsulfonyl)imide and 1-butyl-3-methylimidazolium dicyanamide ionic liquids”, J. Supercrit. Fluids,50(2), 105-111 (2009).
32 Carvalho, P.J., Alvarez, V.H., Schroeder, B., Gil, A.M., Marrucho, I.M., Aznar, M., Santos, L.M.N.B.F., Coutinho, J.A.P., “Specific solvation interactions of CO2on acetate and trifluoroacetate imidazolium based ionic liquids at high pressures”, J. Phys. Chem. B,113(19), 6803-6812 (2009).
33 Bates, E.D., Mayton, R.D., Ntai, I., Davis, J.H., “CO2capture by a task-specific ionic liquid”, J. Am. Chem. Soc.,124(6), 926-927 (2002).
34 Zhang, J.M., Zhang, S.J., Dong, K., Zhang, Y.Q., Shen, Y.Q., Lv, X.M., “Supported absorption of CO2by tetrabutylphosphonium amino acid ionic liquids”, Chem. Eur. J.,12(15), 4021-4026 (2006).
35 Sanchez, L.M.G., Meindersma, G.W., de Haan, A.B., “Kinetics of absorption of CO2in amino-functionalized ionic liquids”, Chem. Eng. J.,166(3), 1104-1115 (2011).
36 Li, W.J., Zhang, Z.F., Han, B.X., Hu, S.Q., Song, J.L., Xie, Y., Zhou, X.S., “Switching the basicity of ionic liquids by CO2”, Green Chem.,10(11), 1142-1145 (2008).
37 Zhang, Y.Q., Zhang, S.J., Lu, X.M., Zhou, Q., Fan, W., Zhang, X.P.,“Dual amino-functionalised phosphonium ionic liquids for CO2capture”, Chem. Eur. J.,15(12), 3003-3011 (2009).
38 Xue, Z.M., Zhang, Z.F., Han, J., Chen, Y., Mu, T.C., “Carbon dioxide capture by a dual amino ionic liquid with amino-functionalized imidazolium cation and taurine anion”, Int. J. Greenhouse Gas Contr.,5(4), 628-633 (2011).
39 Gurkan, B.E., de la Fuente, J.C., Mindrup, E.M., Ficke, L.E., Goodrich, B.F., Price, E.A., Schneider, W.F., Brennecke, J.F., “Equimolar CO2absorption by anion-functionalized ionic liquids”, J. Am. Chem. Soc.,132(7), 2116-2117 (2010).
40 Goodrich, B.F., de la Fuente, J.C., Gurkan, B.E., Zadigian, D., Price, E.A., Huang, Y., Brennecke, J.F., “Experimental measurements of amine-functionalized anion-tethered ionic liquids with carbon dioxide”, Ind. Eng. Chem. Res.,50(1), 111-118 (2011).
41 Shen, K.P., Li, M.H., “Solubility of carbon dioxide in aqueous mixtures of monoethanolamine with methyldiethanolamine”, J. Chem. Eng. Data,37(1), 96-100 (1992).
42 Sanchez, L.M.G., Meindersma, G.W., de Haan, A.B., “Solvent properties of functionalized ionic liquids for CO2absorption”, Chem. Eng. Res. Des.,85(A1), 31-39 (2007).
43 Soutullo, M.D., Odom, C.I., Wicker, B.F., Henderson, C.N., Stenson, A.C., Davis, J.H., “Reversible CO2capture by unexpected plastic-, resin-, and gel-like ionic soft materials discovered during the combi-click generation of a TSIL library”, Chem. Mater.,19(15), 3581-3583 (2007).
44 Gutowski, K.E., Maginn, E.J., “Amine-functionalized task-specific ionic liquids: A mechanistic explanation for the dramatic increase in viscosity upon complexation with CO2from molecular simulation”, J. Am. Chem. Soc.,130(44), 14690-14704 (2008).
45 Yu, G.R., Zhang, S.J., “Insight into the cation-anion interaction in 1,1,3,3-tetramethylguanidinium lactate ionic liquid”, Fluid Phase Equilib.,255(1), 86-92 (2007).
46 Luis, P., Neves, L.A., Afonso, C.A.M., Coelhoso, I.M., Crespo, J., Garea, A., Irabien, A., “Facilitated transport of CO2and SO2through supported ionic liquid membranes (SILMs)”, Desalination,245(1-3), 485-493 (2009).
47 Cserjesi, P., Nemestothy, N., Belafi-Bako, K., “Gas separation properties of supported liquid membranes prepared with unconventional ionic liquids”, J. Membr. Sci.,349(1-2), 6-11 (2010).
48 Scovazzo, P., Kieft, J., Finan, D.A., Koval, C., DuBois, D., Noble, R., “Gas separations using non-hexafluorophosphate [PF6]−anion supported ionic liquid membranes”, J. Membr. Sci.,238(1-2), 57-63 (2004).
49 Baltus, R.E., Counce, R.M., Culbertson, B.H., Luo, H.M., DePaoli, D.W., Dai, S., Duckworth, D.C., “Examination of the potential of ionic liquids for gas separations”, Sep. Sci. Technol.,40(1-3), 525-541 (2005).
50 Moriya, Y., Sasaki,T., Yanase, T., “Gas collection method and apparatus therefor”, US Pat., 0084344 A1 (2007).
51 Park, Y.I., Kim, B.S., Byun, Y.H., Lee, S.H., Lee, E.W., Lee, J.M.,“Preparation of supported ionic liquid membranes (SILMs) for the removal of acidic gases from crude natural gas”, Desalination,236(1-3), 342-348 (2009).
52 Neves, L.A., Crespo, J.G., Coelhoso, I.M., “Gas permeation studies in supported ionic liquid membranes”, J. Membr. Sci.,357(1-2), 160-170 (2010).
53 Kim, D.H., Baek, I.H., Hong, S.U., Lee, H.K., “Study on immobilized liquid membrane using ionic liquid and PVDF hollow fiber as a support for CO2/N2separation”, J. Membr. Sci.,372(1-2), 346-354 (2011).
54 Gonzalez-Miquel, M., Palomar, J., Omar, S., Rodriguez, F., “CO2/N2selectivity prediction in supported ionic liquid membranes (SILMs) by COSMO-RS”, Ind. Eng. Chem. Res.,50(9), 5739-5748 (2011).
55 Iarikov, D.D., Hacarlioglu, P., Oyama, S.T., “Supported room temperature ionic liquid membranes for CO2/CH4separation”, Chem. Eng. J.,166(1), 401-406 (2011).
56 Hanioka, S., Maruyama, T., Sotani, T., Teramoto, M., Matsuyama, H., Nakashima, K., Hanaki, M., Kubota, F., Goto, M., “CO2separation facilitated by task-specific ionic liquids using a supported liquid membrane”, J. Membr. Sci.,314(1-2), 1-4 (2008).
57 Shishatskiy, S., Pauls, J.R., Nunes, S.P., Peinemann, K.V., “Quaternary ammonium membrane materials for CO2separation”, J. Membr. Sci.,359(1-2), 44-53 (2010).
58 Jindaratsamee, P., Shimoyama, Y., Morizaki, H., Ito, A., “Effects of temperature and anion species on CO2permeability and CO2/N2separation coefficient through ionic liquid membranes”, J. Chem. Thermodyn.,43(3), 311-314 (2011).
59 Scovazzo, P., Havard, D., McShea, M., Mixon, S., Morgan, D.,“Long-term, continuous mixed-gas dry fed CO2/CH4and CO2/N2separation performance and selectivities for room temperature ionic liquid membranes”, J. Membr. Sci.,327(1-2), 41-48 (2009).
60 Myers, C., Pennline, H., Luebke, D., Ilconich, J., Dixon, J.K., Maginn, E.J., Brennecke, J.F., “High temperature separation of carbon dioxide/hydrogen mixtures using facilitated supported ionic liquid membranes”, J. Membr. Sci.,322(1), 28-31 (2008).
61 Bara, J.E., Gabriel, C.J., Carlisle, T.K., Camper, D.E., Finotello, A., Gin, D.L., Noble, R.D., “Gas separations in fluoroalkyl-functionalized room-temperature ionic liquids using supported liquid membranes”, Chem. Eng. J.,147(1), 43-50 (2009).
62 Ilconich, J., Myers, C., Pennline, H., Luebke, D., “Experimental investigation of the permeability and selectivity of supported ionic liquid membranes for CO2/He separation at temperatures up to 125 °C”, J. Membr. Sci.,298(1-2), 41-47 (2007).
63 Scovazzo, P., “Determination of the upper limits, benchmarks, and critical properties for gas separations using stabilized room temperature ionic liquid membranes (SILMs) for the purpose of guiding future research”, J. Membr. Sci.,343(1-2), 199-211 (2009).
64 Tang, J.B., Tang, H.D., Sun, W.L., Plancher, H., Radosz, M., Shen, Y.Q., “Poly(ionic liquid)s: A new material with enhanced and fast CO2absorption”, Chem. Commun.,26, 3325-3327 (2005).
65 Tang, J.B., Sun, W.L., Tang, H.D., Radosz, M., Shen, Y.Q., “Enhanced CO2absorption of poly(ionic)s”, Macromolecules,38(6), 2037-2039 (2005).
66 Hu, X.D., Tang, J.B., Blasig, A., Shen, Y.Q., Radosz, M., “CO2permeability, diffusivity and solubility in polyethylene glycol-grafted polyionic membranes and their CO2selectivity relative to methane and nitrogen”, J. Membr. Sci.,281(1-2), 130-138 (2006).
67 Bara, J.E., Lessmann, S., Gabriel, C.J., Hatakeyama, E.S., Noble, R.D., Gin, D.L., “Synthesis and performance of polymerizable room-temperature ionic liquids as gas separation membranes”, Ind. Eng. Chem. Res.,46(16), 5397-5404 (2007).
68 Blasig, A., Tang, J.B., Hu, X.D., Tan, S.P., Shen, Y.Q., Radosz, M.,“Carbon dioxide solubility in polymerized ionic liquids containing ammonium and imidazolium cations from magnetic suspension balance P[VBTMA][BF4] and P[VBMI][BF4]”, Ind. Eng. Chem. Res.,46(17), 5524-5547 (2007).
69 Blasig, A., Tang, J.B., Hu, X.D., Shen, Y.Q., Radosz, M., “Magnetic suspension balance study of carbon dioxide solubility in ammonium-based polymerized ionic liquids: Poly(p-vinylbenzyltrimethyl ammonium tetrafluoroborate) and poly ([2-(methacryloyloxy)ethyl] trimethyl ammonium tetrafluoroborate) ”, Fluid Phase Equilib.,256(1-2) , 75-80 (2007).
70 Tang, J.B., Tang, H.D., Sun, W.L., Radosz, M., Shen, Y.Q.,“Low-pressure CO2sorption in ammonium-based poly(ionic liquid)s”, Polymer,46(26) , 12460-12467 (2005).
71 Tang, J.B., Tang, H.D., Sun, W.L., Radosz, M., Shen, Y.Q.,“Poly(ionic liquid)s as new materials for CO2absorption”, J. Polym. Sci. Pol. Chem.,43(22), 5477-5489 (2005).
72 Tang, J.B., Shen, Y.Q., Radosz, M., Sun, W.L., “Isothermal carbon dioxide sorption in poly(ionic liquid)s”, Ind. Eng. Chem. Res.,48(20), 9113-9118 (2009).
73 Bara, J.E., Gabriel, C.J., Hatakeyama, E.S., Carlisle, T.K., Lessmann, S., Noble, R.D., Gin, D.L., “Improving CO2selectivity in polymerized room-temperature ionic liquid gas separation membranes through incorporation of polar substituents”, J. Membr. Sci.,321(1), 3-7 (2008).
74 Bara, J.E., Hatakeyama, E.S., Gin, D.L., Noble, R.D., “Improving CO2permeability in polymerized room-temperature ionic liquid gas separation membranes through the formation of a solid composite with a room-temperature ionic liquid”, Polym. Adv. Technol.,19(10), 1415-1420 (2008).
75 Bara, J.E., Gin, D.L., Noble, R.D., “Effect of anion on gas separation performance of polymer-room-temperature ionic liquid composite membranes”, Ind. Eng. Chem. Res.,47(24), 9919-9924 (2008).
76 Bara, J.E., Camper, D.E., Gin, D.L., Noble, R.D., “Room-temperature ionic liquids and composite”, Acc. Chem. Res.,43(1), 152-159 (2010).
77 Wolsky, A.M., Daniels, E.J., Jody, B.J., “CO2capture from the flue gas of conventional fossil-fuel-fired power plants”, EnViron. Prog.,13(3), 214-219 (1994).
78 Ventura, S.P.M., Pauly, J., Daridon, J.L., Lopes da Silva, J.A., Marrucho, I.M., Dias, A.M.A., Coutinho, J.A.P., “High pressure solubility data of carbon dioxide in (tri-iso-butyl(methyl)phosphonium tosylate + water) systems”, J. Chem. Thermodyn.,40(8), 1187-1192 (2008).
79 Zhang, Z.F., Wu, W., Liu, Z.M., Han, B.X., Gao, H.X., Jiang, T., “A study of tri-phasic behavior of ionic liquid-methanol-CO2systems”, Phys. Chem. Chem. Phys.,6(9) 2352-2357 (2004).
80 Zhang, Z.F., Wu, W.Z., Gao, H.X., Han, B.X., Wang, B., Huang, Y.,“Tri-phase behavior of ionic liquid-water-CO2system at elevated pressures”, Phys. Chem. Chem. Phys.,6(21), 5051-5055 (2004).
81 Zhang, Z.F., Wu, W.Z., Wang, B., Chen, J.W., Shen, D., Han, B.X.,“High-pressure phase behavior of CO2/acetone/ionic liquid system”, J. Supercrit. Fluids,40(1), 1-6 (2007).
82 Camper, D., Bara, J.E., Gin, D.L., Noble, R.D., “Room-temperature ionic liquid amine solutions tunable solvents for efficient and reversible capture of CO2”, Ind. Eng. Chem. Res.,47(21), 8496-8498 (2008).
83 Zhang, F., Fang, C.G., Wu, Y.T., Wang, Y.T., Li, A.M., Zhang, Z.B.,“Absorption of CO(2)in the aqueous solutions of functionalized ionic liquids and MDEA”, Chem. Eng. J.,160(2), 691-697 (2010).
84 Zhao, Y.S., Zhang, X.P., Zeng, S.J., Zhou, Q., Dong, H.F., Tian, X., Zhang, S.J., “Density, viscosity, and performances of carbon dioxide capture in 16 absorbents of amine + ionic liquid + H2O, ionic liquid + H2O, and amine + H2O systems”, J. Chem. Eng. Data,55(9), 3513-3519 (2010).
85 Zhao, Y.S., Zhang, X.P., Dong, H.F., Zhen, Y.P., Li, G.H., Zeng, S.J., Zhang, S.J., “Solubilities of gases in novel alcamines ionic liquid 2-[2-hydroxyethyl (methyl) amino] ethanol chloride”, Fluid Phase Equilib.,302(1-2), 60-64 (2011).
86 Zhao, Y.S., Zhang, X.P., Zhen, Y.P., Dong, H.F., Zhao, G.Y., Zeng, S.J., Tian, X., Zhang, S.J., “Novel alcamines ionic liquids based solvents: Preparation, characterization and applications in carbon dioxide capture”, Int. J. Greenhouse Gas Contr.,5(2), 367-373 (2011).
87 Zhang, S.J., Zhang, X.P., Zhao, Y.S., Zhao, G.Y., Yao, X.Q., Yao, H.W., “A novel ionic liquids-based scrubbing process for efficient CO2capture”, Sci. China-Chem.,53(7), 1549-1553 (2010).
88 Ahmady, A., Hashim, M.A., Aroua, M.K., “Absorption of carbon dioxide in the aqueous mixtures of methyldiethanolamine with three types of imidazolium-based ionic liquids”, Fluid Phase Equilib.,309(1), 76-82 (2011).
89 Wang, C.M., Mahurin, S.M., Luo, H.M., Baker, G.A., Li, H.R., Dai, S., “Reversible and robust CO(2)capture by equimolar task-specific ionic liquid-superbase mixtures”, Green Chem.,12(5), 870-874 (2010).
90 Wang, C.M., Luo, H.M., Jiang, D.E., Li, H.R., Dai, S., “Carbon dioxide capture by superbase-derived protic ionic liquids”, Angew. Chem. Int. Edit.,49(34), 5978-5981 (2010).
91 Xie, H.B., Zhang, S.B., Li, S.H., “Chitin and chitosan dissolved in ionic liquids as reversible sorbents of CO2”, Green Chem.,8(7), 630-633 (2006).
92 ION Engineering, “ION Engineering introduces ionic liquid CO2capture technology”, Carbon Capture J., Feb. 24, http://www.carboncapturejournal.com (2009).
93 Bara, J.E., Camper, D.E., Gin, D.L., Noble, R.D., “Ionic liquids and methods for using the same”, US Pat., 0291872 A1 (2009).
2011-11-18, accepted 2011-12-19.
* Supported by the Key Program of National Natural Science Foundation of China (21036007) and the National High Technology Research and Development Program of China (2011AA050606).
** To whom correspondence should be addressed. E-mail: xpzhang@home.ipe.ac.cn
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Festschrift in Honor of the 90thBirthday of Prof. Chen Jiayong
- Ternary System of Fe-based Ionic Liquid, Ethanol and Water for Wet Flue Gas Desulfurization*
- Synthesis of PGMA Microspheres with Amino Groups for High-capacity Adsorption of Cr(VI) by Cerium Initiated Graft Polymerization*
- Solvothermal Synthesis and Optical Performance of One-dimensional Strontium Hydroxyapatite Nanorod*
- Effects of Additives and Coagulant Temperature on Fabrication of High Performance PVDF/Pluronic F127 Blend Hollow Fiber Membranes via Nonsolvent Induced Phase Separation
- Polymer/Ceramic Composite Membranes and Their Application in Pervaporation Process
