Inhibiting the expression of hepatocyte nuclear factor 4 alpha attenuates lipopolysaccharide/ D-galactosamine-induced fulminant hepatic failure in mice
2012-07-10
Chengdu, China
Inhibiting the expression of hepatocyte nuclear factor 4 alpha attenuates lipopolysaccharide/ D-galactosamine-induced fulminant hepatic failure in mice
En-Qiang Chen, Dao-Yin Gong, Xiao-Hua Leng, Lang Bai, Cong Liu, Li-Chun Wang and Hong Tang
Chengdu, China
BACKGROUND:Hepatocyte nuclear factor 4 alpha (HNF4α) plays an important role in regulating cytokine-induced inflammatory responses. This study aimed to investigate the role of HNF4αin the development of fulminant hepatic failure (FHF) induced by lipopolysaccharide/D-galactosamine (LPS/D-GalN).
METHODS:The FHF model was induced by simultaneous intraperitoneal injection of LPS/D-GalN in mice. Three days prior to LPS/D-GalN administration, HNF4αshort-hairpin interfering RNA expression plasmid or physiological saline was injected via the tail vein with a hydrodynamics-based procedure. The degree of hepatic damage and cumulative survival rate were subsequently assessed.
RESULTS:The expression of HNF4αwas increased in the early stage after LPS/D-GalN administration. Inhibiting the expression of HNF4αreduced serum levels of alanine aminotransferase and aspartate aminotransferase, alleviated histological injury, and improved the survival of mice with FHF. In addition, both serum and hepatic tumor necrosis factor alpha expression were suppressed when HNF4αexpression was inhibited in mice with FHF.
CONCLUSION:Inhibiting HNF4αexpression protects mice from FHF induced by LPS/D-GalN, but the exact mechanism behind this needs further investigation.
(Hepatobiliary Pancreat Dis Int 2012;11:624-629)
hepatocyte nuclear factor 4α; short-hairpin RNA; fulminant hepatic failure; lipopolysaccharide; D-galactosamine
Introduction
Fulminant hepatic failure (FHF) is a life-threatening clinical syndrome characterized by jaundice, severe coagulopathy, massive hepatic necrosis and high mortality, for which there is still no available effective therapy except for liver transplantation.[1]At present, the pathogenesis of FHF is not clearly clarified, and it may be affected by both host and non-host factors.[2]Hepatocyte nuclear factor 4 alpha (HNF4α) is an important host transcription factor, which is mainly expressed in the liver. Evidence shows that many hepatocyte genes are regulated by HNF4α.[3,4]During embryogenesis, HNF4α is highly conserved and essential for the differentiation and development of the liver. And in adults, HNF4α is also the key regulator of carbohydrate, lipid, cholesterol, amino-acid and bile acid homeostasis and is essential for formation of the epithelial phenotype of hepatocytes and hepatocyte differentiation.[4-9]Recently, evidence also showed that HNF4α plays an important role in regulating the cytokine-induced inflammatory response in hepatocytes.[10-12]Besides, HNF4α also participates in the gene transcription of some inflammatory cytokines, which include c-Jun NH 2-terminal kinase, mitogenactivated protein kinase, sterol 12-hydroxylase, and interleukin-1.[13]Interestingly, the gene promoter of fibrinogen-like protein 2/fibroleukin, an important pathogenic factor in FHF,[14,15]is bound and efficiently activated by HNF4α.[16]Thus, we hypothesized thatHNF4α participates in the progress of liver injury. However, there are limited reports on the effects and mechanisms of HNF4α in the progress of FHF. In the present study, an FHF mouse model was established using lipopolysaccharide/D-galactosamine (LPS/D-GaLN) and the role of HNF4α in the development of FHF was investigated.
Methods
Animals and reagents
Male Balb/c mice (6-8 weeks old, weighing 18-20 g) were purchased from the Huaxi Laboratory Animal Center of Sichuan University (Chengdu, China). The mice were maintained under controlled conditions (24 ℃, 55% humidity and 12-hour day/night rhythm) with free access to food and water. All mice received humane care under the Institutional Review Board in accordance with the Animal Protection Art of Sichuan University.
LPS (Escherichia coli, 0111:B4) and D-GalN were from Sigma (St. Louis, MO), and dissolved in pyrogenfree physiological saline (NS) for use. HNF4α shorthairpin RNA expression plasmid (pHNF4α shRNA) has been constructed in our laboratory,[17]and was used to inhibit HNF4α expression in the liver in this study. The primary antibody to HNF4α (catalog: sc-8987) was from Santa Cruz Biotechnology, and the secondary antibody biotinylated goat anti-rabbit IgG was from Sigma. The reverse transcriptase kit was from Invitrogen Corp. The primers for tumor necrosis factor-alpha (TNF-α) and GAPDH were synthesized by Invitrogen (Shanghai, China).
Experimental design
The FHF model was induced by simultaneous intraperitoneal injection of 20 µg/kg LPS plus 800 mg/kg D-GalN, both in NS. The present study comprised three separate experiments.
To investigate the dynamic change of HNF4α expression in mice with FHF, the mice were sacrificed by dislocation of cervical vertebra and liver tissues were harvested at different time points (0, 2, 4 and 6 hours) after LPS/D-GalN injection. The HNF4α expression in hepatocytes was assessed by immunohistochemistry.
To investigate the results after inhibiting HNF4α expressionin vivo, NS (Group A) and pHNF4α shRNA (Group B) were given by the tail-vein 3 days before LPS/ D-GalN administration. Eight hours after LPS/D-GalN administration, the mice were sacrificed and blood and liver samples were harvested and stored for analysis.
To calculate the survival rate, another 16 mice were randomly grouped and given NS or pHNF4α shRNA 3 days before LPS/D-GalN administration, and then the 12-hour cumulative survival rate was calculated.
Hepatic damage assessment
In order to assess hepatocyte damage, serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using an automatic biochemical analyzer. For pathological analysis, liver tissue was fixed in 10% neutral-buffered formalin and then embedded in paraffin. Tissue sections were stained with hematoxylin-eosin using a standard protocol and analyzed by a light microscope under 400× magnification. The morphological criteria of vacuolization, swollen cytoplasm with disrupted cells and organelle membranes, and lytic nuclear changes served to determine necrosis.[18]
HNF4α detection by immunohistochemistry
For immunohistochemical analysis, paraffin sections were deparaffinized, rehydrated and incubated with 30 mL/L H2O2at 37 ℃ for 15 minutes. Antigen retrieval was achieved using supersonic wave repair for 1 minute. After blocking with 3% bovine serum albumin, the sections were incubated with primary antibody (anti-HNF4, 1:200) overnight at 4 ℃. The sections were then incubated with biotinylated secondary antibody for 30 minutes. After washing, the sections were stained with diaminobenzideine (DAB) and counterstained with hematoxylin. The sum of the scores for proportion of positive cells and staining intensity was the final score for HNF4α expression using the Axiotis score standard by examination of 5 randomlychosen fields at 400× magnification.[19]The scoring criteria for the proportion of positive cells were: 0, 0%-10% positive cells; 1, 11%-25% positive cells; 2, 26%-50% positive cells; 3, 51%-75% positive cells; and 4, 76%-100% positive cells. And the scoring criteria for staining intensity in positive cells were: 0, no color; 1, yellow; 2, brown; and 3, tan. To describe the results more directly and easily, staining results were also divided into four grades: negative (-) for scores 0-0.5, weakly positive (+) for scores 1-2.5, positive (++) for scores 3-4.5, and strongly positive (+++) for scores 5-7.
TNF-α detection
The serum levels of TNF-α were measured by immunoradioassay, and were completed by Kingmed Diagnostics (Chengdu, China). The mRNA level of TNF-α in liver tissue was analyzed by reverse transcriptionpolymerase chain reaction. Total RNA was isolated from liver tissue using Trizol reagent (Invitrogen) according to the manufacturer's protocol. A two-microgram sample of total RNA was used for synthesis of first-strand cDNA. The primers for TNF-α were 5'-GGC AGG TCT ACTTTG GAG TCA TTG C-3' (sense) and 5'-ACA TTC GAG GCT CCA GTG AAT TCG G-3' (antisense). The primers for GAPDH were 5'-ACT TGA AGG GTG GAG CCA AA-3' (sense) and 5'-CCA GGA AAT GAG CTT GAC A-3' (antisense).
Results
Expression of HNF4α increased in the early stage of FHF in mice
Expression of HNF4α was nucleus-positive, and the expression level of HNF4α protein was time-related in the liver after mice were given LPS/D-GalN (Figs. 1 and 2). At hour 2, both the percentage of positive cells and positive staining intensity were significantly increased, and the score for HNF4α was significantly higher than that at any other time-point. However, as time went by, the HNF4α scores showed a downward trend from hour 2 (Fig. 2). These results suggested that HNF4α expression was increased in the early stage of LPS/D-GalN-induced FHF.
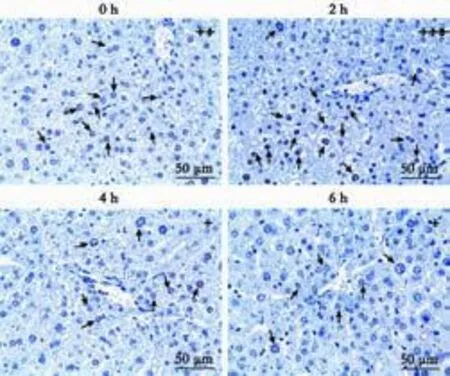
Fig. 1.Dynamic change of HNF4α expression in mice after LPS/ D-GalN administration. The percentage of positive cells and staining intensity at hour 2 were significantly higher than those at any other time-point.
Inhibiting HNF4α expression attenuated hepatic damage of mice with FHF
The serum levels of ALT and AST at 8 hours after LPS/D-GalN administration are presented in Fig. 3. In groups A and B, ALT levels were 2697±701 and 184± 95 IU/L, and AST levels 1670±329 and 185±51 IU/L, respectively. These results showed that hepatic injury was markedly reduced when HNF4α expression was inhibited (P<0.05).
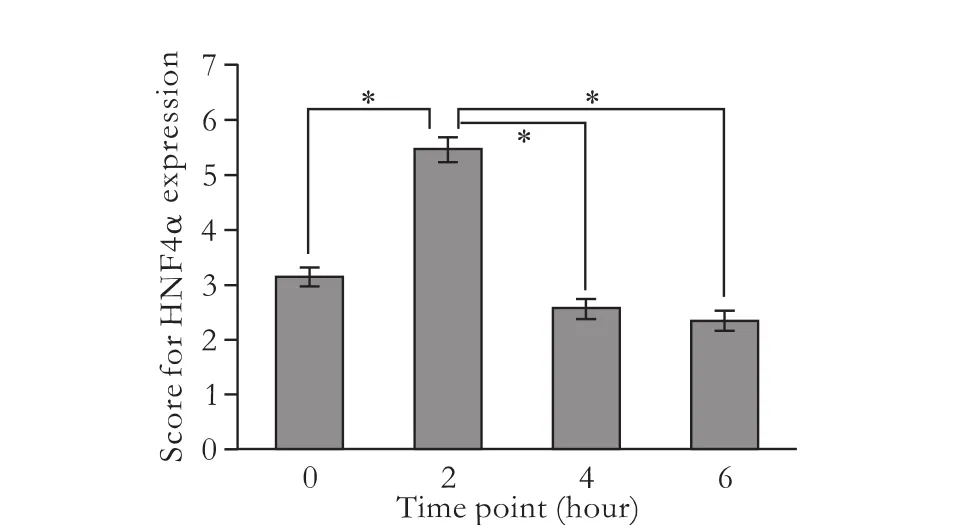
Fig. 2.Scores for HNF4α expression in mice at different time points after LPS/D-GalN administration. The mean scores for hour 0, 2, 4, and 6 were 3.11±0.60, 5.44±0.73, 2.56±0.53 and 2.33±0.50, respectively. *:P<0.05, compared between the two groups.
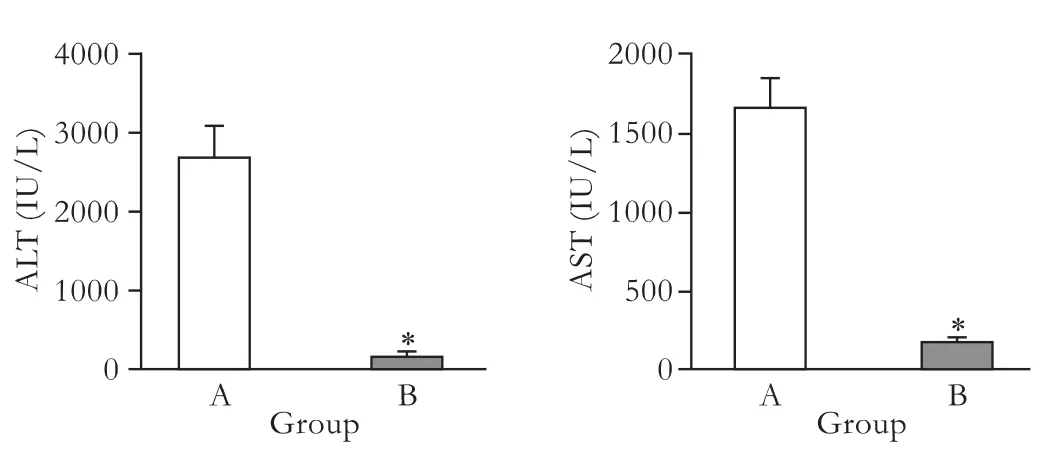
Fig. 3.Serum levels of ALT and AST were determined in the NS-injected (group A) and pHNF4α shRNA-injected (group B). *:P<0.05, compared with the NS-injected group.
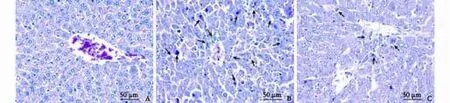
Fig. 4.Histology of liver sections stained with HE.A:Normal hepatic lobules in normal mouse;B:Severe hemorrhage, massive liver necrosis and inflammation around the central veins in the NS-injected mice (group A);C:Minimal hepatic necrosis and inflammation in the pHNF4α shRNA-injected mice (group B).
The liver sections of normal mice showed an integrated structure of hepatic lobules and no denatured or necrotic hepatocytes (Fig. 4A). Injection of LPS/ D-GalN induced markedly hepatic injury accompanied by hemorrhage, massive necrosis and inflammatory infiltration in the NS-injected mice (Fig. 4B), but did not in the pHNF4α shRNA-injected mice (Fig. 4C). This finding further suggested that inhibiting HNF4α expression attenuated the hepatic damage in mice with FHF induced by LPS/D-GalN.
Inhibiting HNF4α expression decreased mortality of mice with FHF
To evaluate whether inhibiting HNF4α expression affected the outcome in mice with FHF induced by LPS/D-GalN, the survival rates were compared (Fig. 5). Kaplan-Meier survival curves showed that LPS/D-GalN administration caused 75% (6/8) of dead mice within 12 hours; however, inhibiting HNF4α expression improved the survival to 75% (6/8) in group B at hour 12.
Inhibition of HNF4α expression reduced TNF-α expression in mice with FHF

Fig. 5.Survival rate within 12 hours after LPS/D-GalN administration in the two groups. Log-rank test (GraphPad Prism 5.0) showed that the difference in survival curves was statistically significant (P=0.032).
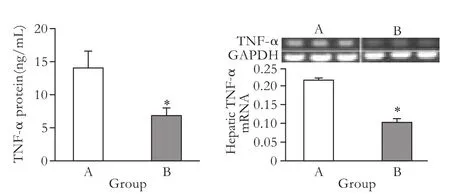
Fig. 6.Serum TNF-α protein and tissue TNF-α mRNA in the NS-injected (group A) and pHNF4α shRNA-injected (group B). *:P<0.05, compared with the NS-injected group.
It has been shown that TNF-α is an important cytokine responsible for hepatocyte apoptosis and neutrophil transmigration in the LPS/D-GalN-induced FHF model.[20]In the present study, the serum levels of TNF-α protein in group A was 14.08±5.18 and in group B 6.83±2.22 ng/mL (Fig. 6A), and the levels of hepatic TNF-α mRNA in the two groups were 0.22±0.01 and 0.11±0.02, respectively (Fig. 6B). There were significant differences in serum TNF-α protein and tissue TNF-α mRNA between the two groups. This finding indicated that the attenuation of hepatic damage by HNF4α inhibition may be correlated with decreased TNF-α expression.
Discussion
HNF4α has been reported to play important roles in regulating cytokine-induced inflammatory responses in hepatocytes, and relatively high levels of HNF4α expression also occur in the liver tissue of patients with fulminant hepatitis B (unpublished data). In the mouse FHF model induced by hepatitis virus type 3, HNF4α is also necessary for virus-induced fibrinogenlike protein 2 gene transcription, which plays a pivotal role in the pathogenesis of FHF.[16]However, the data concerning HNF4α in FHF development is still limited. In order to investigate the pathogenic role of HNF4α in the development of FHF, an LPS/D-GalN-induced FHF mouse model, which closely resembles human fulminant hepatitis in both morphological and functional features, was established.[20]We found that inhibiting HNF4α expression significantly attenuated the hepatic damage in these mice.
Recently, studies reported that HNF4α is an important component of the inflammatory response in the liver.[12,16,21]However, the data on HNF4α expression under the condition of acute inflammatory stimulation are limited. In the present study, we first observed the dynamic expression of HNF4α in the liver tissue of mice after LPS/D-GalN administration, and found that the HNF4α expression level increased in the early phase of FHF development. Though other studies reported that HNF4α expression is reduced in seriously damaged hepatic cells, this finding did not contradict our results. In those studies, HNF4α was assessed in the end-stage of FHF, and in this period, hepatocyte necrosis is very serious, and can easily lead to protein structural changes and degradation when the nuclear membrane is damaged. So it is not surprising that reduced HNF4α in FHF was observed. In fact, reduced HNF4α was also found in the present study. At hour 6 after LPS/D-GalN administration, the intensity of the signal in liver tissue was significantly reduced compared with that in normal mice.
To further investigate the possible role of HNF4α in the development of FHFin vivo, endogenous HNF4αin hepatocytes was inhibited by RNA interference. Our results showed that inhibiting HNF4α expression alleviated hepatic damage and significantly increased the survival rate of mice with FHF induced by LPS/D-GalN. The findings of the present study form a basis for future functional study of HNF4α in liver inflammation and necrosis, and may also provide a new therapeutic target for FHF.
Evidence suggests that the generation of large numbers of inflammatory cytokines plays important roles in extensive apoptosis and necrosis,[22-25]which result in the development of hepatic damage. For example, in the hepatic damage of FHF induced by LPS/GalN, the effect of iNOS activation has been investigated.[25]Interestingly, another study reported that HNF4α acts as an activator of hepatocyte iNOS expression at the levels of protein, mRNA, and promoter activation, and in the absence of HNF4α, this enhancement is ablated.[12]These studies indicated that there may be a correlation between HNF4α and inflammatory cytokine expression.
To further investigate the mechanism of HNF4α in the development of FHF, we studied cytokines associated with apoptosis/necrosis in the peripheral blood and liver tissue of FHF mice, and after HNF4α expression was inhibited in these mice, we found both the serum TNF-α protein and liver tissue TNF-α mRNA levels decreased. In fact, many other studies also reported that TNF-α plays important roles in the acute inflammation caused by LPS/D-GalN administration,[26-29]so in the present study, the role of HNF4α in the development of FHF may be achieved through regulation of TNF-α expression. However, this is a speculation, and needs further studies for confirmation.
In conclusion, inhibition of HNF4α expression attenuates the liver damage in mice with FHF induced by LPS/D-GalN, and the exact mechanism needs to be further investigated.
Contributors:CEQ and TH proposed the study. CEQ, LXH and WLC performed research and wrote the first draft. GDY, BL and LC collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. TH is the guarantor.
Funding:This study was supported by grants from the 973 Program of China (2007CB512902), the National Natural Science Foundation of China (30972622), and the National Science and Technology Major Project of China (2008ZX10002-006).
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev Gastroenterol Hepatol 2009;6:542-553.
2 Lee WM, Squires RH Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology 2008;47:1401-1415.
3 Costa RH, Kalinichenko VV, Holterman AX, Wang X. Transcription factors in liver development, differentiation, and regeneration. Hepatology 2003;38:1331-1347.
4 Mogilenko DA, Dizhe EB, Shavva VS, Lapikov IA, Orlov SV, Perevozchikov AP. Role of the nuclear receptors HNF4 alpha, PPAR alpha, and LXRs in the TNF alpha-mediated inhibition of human apolipoprotein A-I gene expression in HepG2 cells. Biochemistry 2009;48:11950-11960.
5 Clotman F, Lannoy VJ, Reber M, Cereghini S, Cassiman D, Jacquemin P, et al. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development 2002;129:1819-1828.
6 Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol 2001;21:1393-1403.
7 Inoue Y, Yu AM, Inoue J, Gonzalez FJ. Hepatocyte nuclear factor 4alpha is a central regulator of bile acid conjugation. J Biol Chem 2004;279:2480-2489.
8 Nagaki M, Moriwaki H. Transcription factor HNF and hepatocyte differentiation. Hepatol Res 2008;38:961-969.
9 Ganjam GK, Dimova EY, Unterman TG, Kietzmann T. FoxO1 and HNF-4 are involved in regulation of hepatic glucokinase gene expression by resveratrol. J Biol Chem 2009;284:30783-30797.
10 Wiwi CA, Waxman DJ. Role of hepatocyte nuclear factors in growth hormone-regulated, sexually dimorphic expression of liver cytochromes P450. Growth Factors 2004;22:79-88.
11 Li X, Salisbury-Rowswell J, Murdock AD, Forse RA, Burke PA. Hepatocyte nuclear factor 4 response to injury involves a rapid decrease in DNA binding and transactivation via a JAK2 signal transduction pathway. Biochem J 2002;368:203-211.
12 Guo H, Wei J, Inoue Y, Gonzalez FJ, Kuo PC. Serine/ threonine phosphorylation regulates HNF-4alpha-dependent redox-mediated iNOS expression in hepatocytes. Am J Physiol Cell Physiol 2003;284:C1090-1099.
13 Jahan A, Chiang JY. Cytokine regulation of human sterol 12alpha-hydroxylase (CYP8B1) gene. Am J Physiol Gastrointest Liver Physiol 2005;288:G685-695.
14 Chan CW, Kay LS, Khadaroo RG, Chan MW, Lakatoo S, Young KJ, et al. Soluble fibrinogen-like protein 2/fibroleukin exhibits immunosuppressive properties: suppressing T cell proliferation and inhibiting maturation of bone marrowderived dendritic cells. J Immunol 2003;170:4036-4044.
15 Zhu F, Ning Q, Chen Y, Tao XX, Yan WM, Xi D, et al. Hfgl2/fibroleukin expression in liver and peripheral blood mononuclear cells (PBMC) and its correlation with disease severity. Zhonghua Gan Zang Bing Za Zhi 2004;12:385-388.
16 Ning Q, Lakatoo S, Liu M, Yang W, Wang Z, Phillips MJ, et al. Induction of prothrombinase fgl2 by the nucleocapsid protein of virulent mouse hepatitis virus is dependent on host hepatic nuclear factor-4 alpha. J Biol Chem 2003;278:15541-15549.
17 Song XQ, Chen EQ, Wang YB, Zhou TY, Liu L, Liu C, et al. Construction of a plasmid vector for liver-specific inhibition of hepatocyte nuclear factor 4 alpha expression. Plasmid 2012;67:60-66.
18 Kerr JF, Gobé GC, Winterford CM, Harmon BV. Anatomical methods in cell death. Methods Cell Biol 1995;46:1-27.
19 Long Y, Chen E, Liu C, Huang F, Zhou T, He F, et al. The correlation of hepatocyte nuclear factor 4 alpha and 3 beta with hepatitis B virus replication in the liver of chronic hepatitis B patients. J Viral Hepat 2009;16:537-546.
20 Silverstein R. D-galactosamine lethality model: scope and limitations. J Endotoxin Res 2004;10:147-162.
21 Wang Z, Bishop EP, Burke PA. Expression profile analysis of the inflammatory response regulated by hepatocyte nuclear factor 4α. BMC Genomics 2011;12:128.
22 Leung TM, Tipoe GL, Liong EC, Lau TY, Fung ML, Nanji AA. Endothelial nitric oxide synthase is a critical factor in experimental liver fibrosis. Int J Exp Pathol 2008;89:241-250.
23 Tipoe GL, Leung TM, Liong E, So H, Leung KM, Lau TY, et al. Inhibitors of inducible nitric oxide (NO) synthase are more effective than an NO donor in reducing carbontetrachloride induced acute liver injury. Histol Histopathol 2006;21:1157-1165.
24 Pan Q, Liu Y, Zheng J, Lu X, Wu S, Zhu P, et al. Protective effect of chloral hydrate against lipopolysaccharide/ D-galactosamine-induced acute lethal liver injury and zymosan-induced peritonitis in mice. Int Immunopharmacol 2010 May 25.
25 Liu LM, Zhang JX, Wang XP, Guo HX, Deng H, Luo J. Pim-3 protects against hepatic failure in D-galactosamine (D-GalN)-sensitized rats. Eur J Clin Invest 2010;40:127-138.
26 Gong X, Luo FL, Zhang L, Li HZ, Wu MJ, Li XH, et al. Tetrandrine attenuates lipopolysaccharide-induced fulminant hepatic failure in D-galactosamine-sensitized mice. Int Immunopharmacol 2010;10:357-363.
27 Song HL, Lv S, Liu P. The roles of tumor necrosis factor-alpha in colon tight junction protein expression and intestinal mucosa structure in a mouse model of acute liver failure. BMC Gastroenterol 2009;9:70.
28 Olleros ML, Vesin D, Fotio AL, Santiago-Raber ML, Tauzin S, Szymkowski DE, et al. Soluble TNF, but not membrane TNF, is critical in LPS-induced hepatitis. J Hepatol 2010;53:1059-1068.
29 Holmgren C, Esplin MS, Hamblin S, Molenda M, Simonsen S, Silver R. Evaluation of the use of anti-TNF-alpha in an LPS-induced murine model. J Reprod Immunol 2008;78:134-139.
October 10, 2011
Accepted after revision March 16, 2012
Author Affiliations: Center for Infectious Diseases, West China Hospital and State Key Laboratory of Biotherapy (Chen EQ, Leng XH, Bai L, Liu C, Wang LC and Tang H); Department of Forensic Pathology, College of Basic Medicine and Forensic Medicine (Gong DY), Sichuan University, Chengdu 610041, China
Hong Tang, MD, Center for Infectious Diseases, West China Hospital, Sichuan University, Chengdu 610041, China (Tel: 86-28-85422650; Fax: 86-28-85422650; Email: htang6198@hotmail.com)
© 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(12)60235-5
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer
- Risk factors and incidence of acute pyogenic cholangitis
- Endoscopic sphincterotomy associated cholangitis in patients receiving proximal biliary self-expanding metal stents
- Biliary drainage for obstructive jaundice caused by unresectable hepatocellular carcinoma: the endoscopic versus percutaneous approach
- Gallstone-related complications after Roux-en-Y gastric bypass: a prospective study
- Changes of serum alpha-fetoprotein and alpha-fetoprotein-L3 after hepatectomy for hepatocellular carcinoma: prognostic significance
