Changes of serum alpha-fetoprotein and alpha-fetoprotein-L3 after hepatectomy for hepatocellular carcinoma: prognostic significance
2012-07-10
Shanghai, China
Changes of serum alpha-fetoprotein and alpha-fetoprotein-L3 after hepatectomy for hepatocellular carcinoma: prognostic significance
Xiao-Feng Zhang, Zheng-Feng Yin, Kui Wang, Zong-Qin Zhang, Hai-Hua Qian and Le-Hua Shi
Shanghai, China
BACKGROUND:Alpha-fetoprotein (AFP) is the most established tumor marker of hepatocellular carcinoma (HCC), but one of its limitations is non-specificity. Many studies have demonstrated that alpha-fetoprotein-L3 (AFP-L3) is more specific than AFP in the early diagnosis and prognosis of HCC. However, there is a lack of knowledge about the post-hepatectomy profiles of serum AFP and AFP-L3 values in HCC patients. To identify the profiles after surgical resection of HCC, we analyzed the correlation between the profiles and postoperative HCC recurrence or survival, and evaluated their utility in predicting postoperative therapeutic efficacy and prognosis.
METHODS:From August 2003 to December 2004, 318 patients with positive serum AFP who had received surgical resections were enrolled in this study. Preoperative and postoperative serum AFP and AFP-L3 levels were measured simultaneously and regularly, and their postoperative profiles during a longterm follow-up were recorded and summarized.
RESULTS:A high ratio of AFP-L3 to total AFP was shown to correlate with pathologic features of aggressiveness. The overall 1-, 3-, and 5-year recurrence rates of the whole series were 28%, 57%, and 84%, and the overall survival rates were 86%, 61%, and 33%, respectively. The changes of serum AFP and AFP-L3 after hepatectomy for HCC were classified into 3 groups (group A: AFP-L3 undetectable; group B: AFP-L3 <10%; and group C: AFP-L3 >10%). Patients with positive postoperative AFP-L3had significantly earlier recurrence than those with negative results. The overall survival was significantly shorter in the positive groups than in the groups negative for postoperative AFP-L3.
CONCLUSION:Post-hepatectomy changes in serum AFP and AFP-L3 levels occurred in three distinct patterns, which were closely correlated with HCC recurrence and patient survival with different prognostic values.
(Hepatobiliary Pancreat Dis Int 2012;11:618-623)
hepatocellular carcinoma;hepatectomy; alpha-fetoprotein; alpha-fetoprotein-L3
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cancer and the third most common cause of cancer-related deaths.[1]Its incidence is the highest in South Africa and East Asia, but it is also gradually rising in the United States, Europe and other regions.[2]Surgical resection and liver transplantation remain the mainstay of treatment. However, postoperative residual cancer cells are likely to cause recurrence.[3,4]
Biomarkers have the potential to predict recurrence, metastasis and prognosis. Alpha-fetoprotein (AFP) is a well-known HCC biomarker.[5,6]However, one of the limitations of AFP is its non-specificity. On the one hand, AFP is elevated in some benign liver diseases such as chronic hepatitis and cirrhosis, and on the other hand, post-therapy changes in AFP do not exactly reflect the response of HCC to various treatments in all cases.[7-10]For example, in cases where postoperative serum AFPwas positive, no intrahepatic metastasis, cancer embolus or residual tumor was found, nor was cancer recurrence detected over time.[11-14]
AFP is a glycoprotein, and each molecule contains only one N-glycan, the structure of which is heterogenic. Lens culinaris agglutinin affinity electrophoresis divides AFP into three bands, namely AFP-L1, AFP-L2 and AFP-L3. Many studies have demonstrated that AFP-L3 is more specific than AFP, not only in the differential diagnosis of benign and malignant liver diseases and the early diagnosis of HCC,[15-19]but also in predicting the prognosis of HCC patients who receive surgical resection.[20-25]However, there is a lack of knowledge about early changes in the patterns and characteristics of serum AFP and AFP-L3 in HCC patients after hepatectomy. These changes may directly reflect the survival and conversion of tumor cellsin vivoafter surgical treatment. In this study, we assessed serum AFP and AFP-L3 simultaneously and regularly before and after hepatectomy in 318 HCC patients to evaluate their characteristic changes and responses to surgery. We proposed that analyses of these patterns and their correlations with HCC recurrence time and patient survival as well as the study of their biological significance may help assess therapeutic effect and track the pathologic condition.
Methods
Patients
Assessment of serum AFP and AFP-L3
The same blood sample was used for measurements of AFP and AFP-L3. Serum AFP was measured by enzyme-linked immunosorbent assay, and an AFP level>20 ng/mL was considered positive. Serum AFP-L3 was assessed by lectin-affinity electrophoresis coupled with antibody-affinity blotting, and the result was expressed as the percentage of AFP-L3 in total AFP. An AFP-L3 value >10% was considered positive.[17]Measurements were performed within one week prior to surgery and every 1-3 weeks after surgery four consecutive times, lasting a total of >9 weeks.
Follow-up and confirmation of recurrence
The patients were subjected to a long-term postoperative follow-up, during which clinical data were collected and summarized by the same team of surgeons. This study was censored in January 2010. The median follow-up was 44.0 months (range 1.0-76.0). Apart from tumor markers, the follow-up included abdominal ultrasonography and liver function tests every 1-2 weeks in the first two months, every 1-2 months within 6 months and every 4-6 months for the rest of the period. When tumor recurrences or metastases were suspected, the patients were further examined by computed tomography scan and/or magnetic resonance imaging. Recurrence time referred to the interval from the first surgical resection to the time of documented recurrence. The time of documented recurrence or metastasis was the end point of follow-up for tumor-free survival, and patient death was the end point of overall survival.
Statistical analysis
Patient demographic data, pathological features, postoperative course, and disease progression were collected. End-point measurements were recurrence rate and overall survival. Continuous data were expressed as mean±SD or median (range). Categorical variables were compared by the Chi-square test or Fisher's exact test, and continuous variables were compared by Student'sttest. The survival and recurrence of different groups were calculated by the Kaplan-Meier method, and differences in survival and recurrence rates between the groups were analyzed by the log-rank test. All statistics were analyzed with SPSS 15.0 for Windows (SPSS Inc., Chicago, IL., USA). AP<0.05 was considered statistically significant.
Results
Basic characteristics of the patients
The basic characteristics of the 318 patients are shown in Table 1. Before treatment, serum AFP-L3 ranged from 0.6% to 82.7% with a median of 26.3%, and 274 patientswere positive (86.2%), with 44 negative for AFP-L3 (13.8%). AFP became negative in 219 of the 318 patients within eight weeks after hepatectomy, and remained positive in the remaining 99. Meanwhile, AFP-L3 was not detected or decreased markedly (>5%) in 249 patients, remained unchanged in 56 and increased markedly (>5%) in 13.
Different patterns of serum AFP and AFP-L3 changes after hepatectomy
The influences of patterns of serum AFP and AFP-L3 changes after hepatectomy on the prognosisof patients were the focal concern of the present study. The postoperative AFP and AFP-L3 changes were categorized into three types based on the last followup measurements: in group A the AFP-L3 disappeared, group B had AFP-L3 <10%, and group C had AFP-L3>10% (Fig. 1). The clinical characteristics of group A were compared with those of groups B and C (Table 2), showing that a high AFP-L3 ratio was closely associated with the elevated total AFP and pathologic features of aggressiveness.

Table 1.Clinical characteristics of the 318 patients
Characteristics of the 219 patients with a type 1 pattern (group A): in all patients in this group, AFP-L3 disappeared when AFP became negative. In this group, postoperative serum AFP decreased quickly and became negative in about 8 weeks. However, the percentage ofAFP-L3 did not decrease gradually as was the case with AFP, but remained at a relatively stable level before AFP conversion.
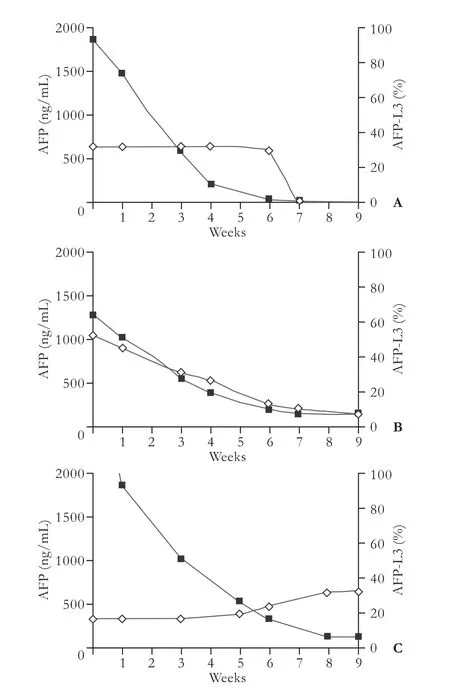
Fig. 1.Typical curves of serum AFP and AFP-L3 after hepatectomy for HCC. ■: AFP; ◊: AFP-L3.A:AFP-L3 disappeared with AFP conversion after hepatectomy.B:AFP decreased markedly but remained unconverted after hepatectomy, while AFP-L3 converted from positive to negative.C:AFP decreased markedly but remained unconverted after hepatectomy, while AFP-L3 remained positive with a rising trend.
Characteristics of the 55 patients with a type 2 pattern (group B): Postoperative AFP decreased markedly (<10%) while AFP-L3 converted from positivity to negativity or remained negative. In this group, AFP decreased markedly after surgery but remained unconverted at week 8 and remained stable below 400 ng/mL, while AFP-L3 converted from positive to negative or remained negative after surgery.
Characteristics of the 44 patients with a type 3 pattern (group C): AFP decreased markedly but remained unconverted after surgery, and AFP-L3 remained positive (>10%) or elevated. Serum AFP decreased quickly within 4 weeks after operation in most patients, but remained unconverted even after 8 weeks. Despite the large reduction in postoperative serum AFP, changes in serum AFP-L3 were insignificant or even elevated.Correlations between patterns of serum AFP-L3 changes and total recurrence
Recurrences were found in 266 patients during a postoperative follow-up. The median recurrence interval was 29.0±2.8 months and the overall 1-, 3-, and 5-yearrecurrence rates were 28%, 57%, and 84%, respectively (Table 2). Patients with positive postoperative AFP-L3 had a significantly higher recurrence rate and a shorter interval of recurrence than those with negative results (Fig. 2).
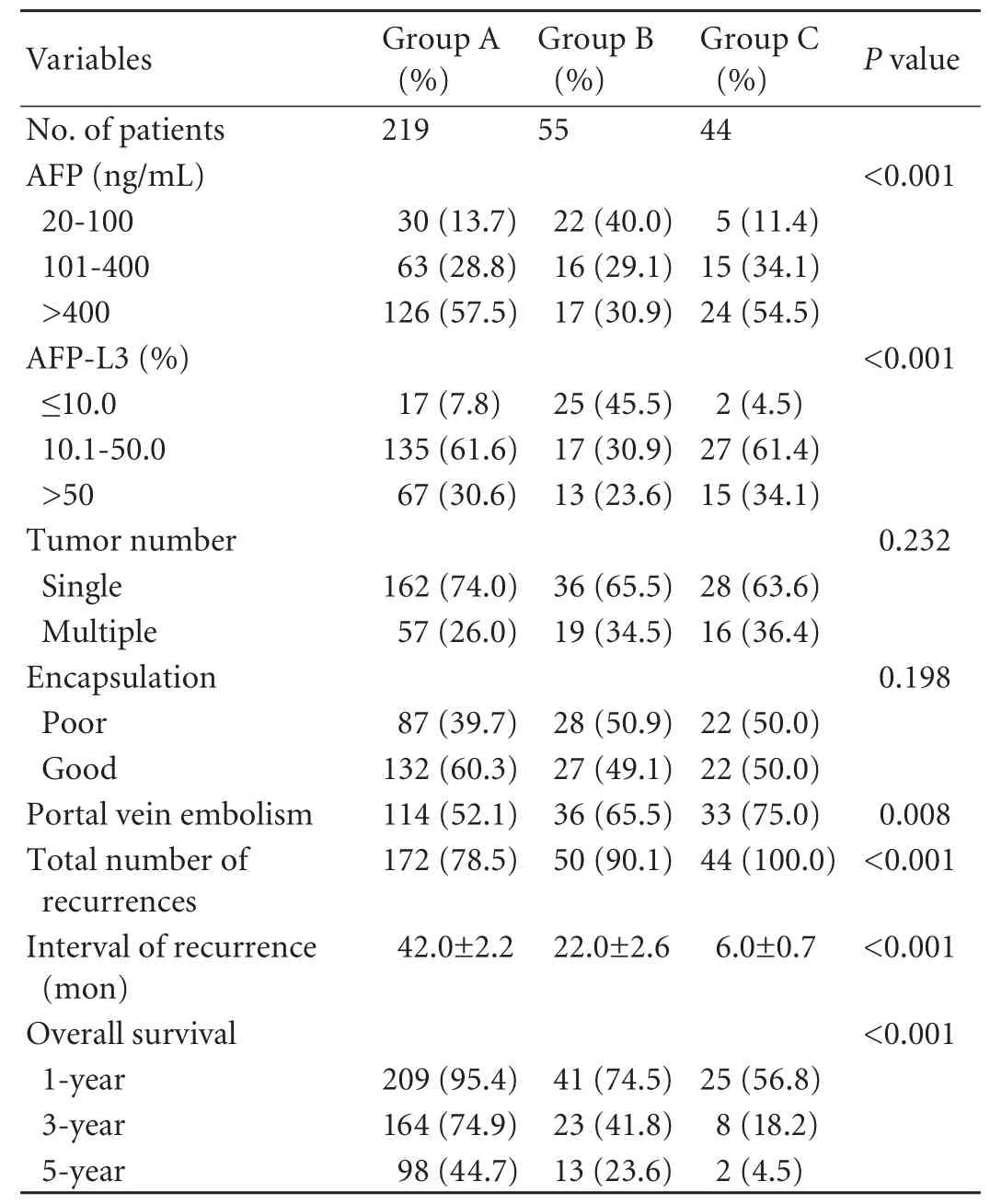
Table 2.Clinical characteristics and outcomes of the three groups
Correlations between patterns of serum AFP-L3 changes and total survival
For all 318 patients, the median survival was 49.0±2.7 months and the overall 1-, 3-, and 5-year survival rates were 86%, 61%, and 33%, respectively (Table 2). The overall survival was significantly longer in the negative postoperative AFP-L3 group compared with the other two groups (Fig. 2). There was an association between the overall survival and the AFP-L3 percentage levels after treatment.
Discussion
Different patterns of changes in post-hepatectomy serum AFP and AFP-L3
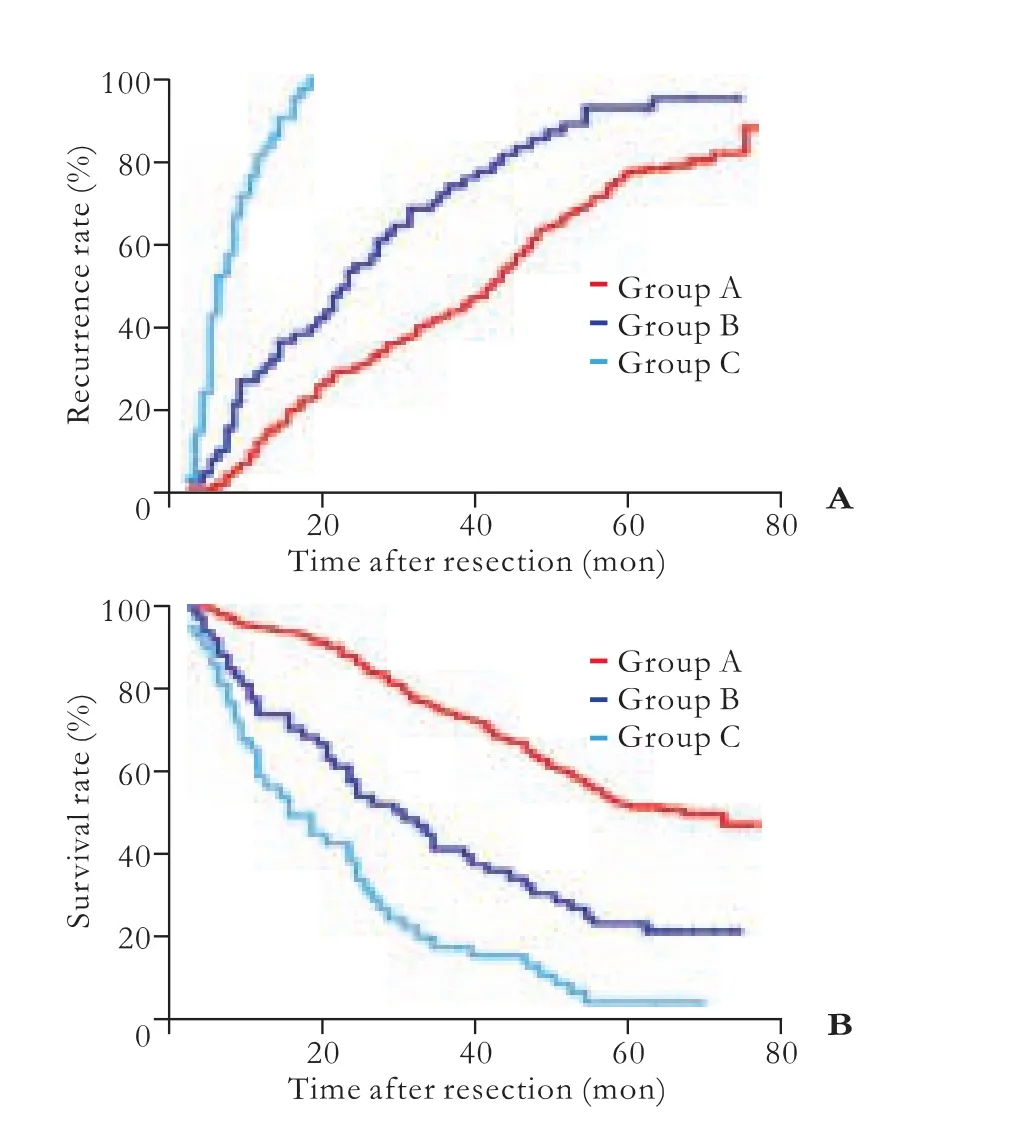
Fig. 2.The Kaplan-Meier method for analyses of recurrence (A) and overall survival (B) of the three groups (P<0.001).
Enzymatic metabolic disturbances in HCC cells cause increased AFP fucosylation. Core fucose is formed by transporting fucose to N-acetylglucosamine on the most medial side of the sugar chain under the action of α-1, 6-fucosyltransferase.[26-29]The glycan structures of AFP produced by HCC cells are clearly different from thoseproduced in benign liver diseases, and therefore are used as an HCC biomarker that is far more specific than AFP quantification.[15-25]AFP glycosylation occurs within cells and the product is then released to the blood. Because the peripheral blood is not a suitable environment for glycosyl hydrolase activity, no significant change in AFP glycosylation is detected.
In addition, since AFP-L3 was expressed as a percentage, it was not affected by AFP concentration and half-life. This is the reason why changes in AFP-L3 were different from changes in AFP after HCC resection. The characteristics of the AFP-L3 changes more accurately reflected the presence or absence of HCC, and therefore postoperative detection of AFP-L3 has more prognostic significance than preoperative detection.
Correlations of patterns of post-hepatectomy serum AFP and AFP-L3 changes with therapeutic efficacy and prognosis
Since postoperative serum AFP-L3 changes more accurately reflect the presence or absence of HCC, different patterns of change may indicate different prognoses. A type 1 pattern denotes that the surgical resection of HCC was complete and radical (Fig. 1A). In patients of this group, the total survival was the highest, and the total recurrence the lowest. The reasons for these differences between AFP and AFP-L3 are that AFP glycosylation does not occur extracellularly and the total amount of AFP in the blood decreases continuously over the half-life. The content of AFP-L3 also decreased continuously, and therefore the percentage of AFP-L3 in total AFP remained at a relatively stable level and was not affected by the total amount of AFP and its half-life.
However, a type 2 pattern indicates that the surgical resection was complete, but active hepatitis or cirrhosis existed in the residual liver (group B, Fig. 1B). It is known that the glycan structure of AFP produced by cirrhosis is different from that produced in HCC. It is, therefore, easy to understand why AFP did not convert while the percentage of AFP-L3 was significantly reduced in patients with active cirrhosis after the HCC was resected. In these patients, the short-term prognosis was relatively better than in group C patients.
A type 3 pattern indicates either that surgical resections are incomplete or there are small satellite nodules and extrahepatic metastases (group C, Fig. 1C). As a result, the overall survival was low and the overall recurrence was high in patients of this group. This type of pattern is due to the disappearance of the main tumor cells that secrete AFP and the persistence of the same clonal residual HCC cells, which continue to secrete AFP similar to that secreted by the main tumors. Although the absolute values of AFP were decreased, the percentage of AFP-L3 in total AFP remained unchanged. Clearly, the prognosis of this group of patients was poor, and the survival and recurrence were significantly different from the other patterns.
In conclusion, failure of AFP conversion after hepatectomy does not necessarily mean that the resection is incomplete. On the other hand, failure of AFP-L3 conversion or further elevation is a warning signal and significantly correlated with the interval of tumor recurrence and patient survival. Based on the results of the present study, we suggest that AFP-L3 should be assessed together with AFP about 6 weeks after hepatectomy for HCC, and an assessment based on the different patterns of post-hepatectomy serum AFP and AFP-L3 changes should be made to truly understand the pathologic condition and give effective information for follow-up and individualized therapy.
Contributors:YZF and SLH proposed the study. ZXF and QHH performed research and wrote the first draft. WK and ZZQ collected and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. SLH is the guarantor.
Funding:The study was supported by grants from the National High Technology Research and Development Program of China (2007AA02Z461) and the China National Key Projects for Infectious Disease (2008ZX10002-021).
Ethical approval:This study was approved by the ethics committee of the hospital (2003-005).
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001;94:153-156.
2 Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology 2008;48:137-145.
3 Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007;141:330-339.
4 El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008;134:1752-1763.
5 Liaw YF, Tai DI, Chu CM, Lin DY, Sheen IS, Chen TJ, et al. Early detection of hepatocellular carcinoma in patients with chronic type B hepatitis. A prospective study. Gastroenterology 1986;90:263-267.
6 Debruyne EN, Delanghe JR. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: new aspects and applications. Clin Chim Acta 2008;395:19-26.
7 Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha-fetoprotein in cirrhotic patientsmonitored for development of hepatocellular carcinoma. Hepatology 1994;19:61-66.
8 Chu CW, Hwang SJ, Luo JC, Lai CR, Tsay SH, Li CP, et al. Clinical, virologic, and pathologic significance of elevated serum alpha-fetoprotein levels in patients with chronic hepatitis C. J Clin Gastroenterol 2001;32:240-244.
9 Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol 2005;43:434-441.
10 Paul SB, Gulati MS, Sreenivas V, Madan K, Gupta AK, Mukhopadhyay S, et al. Evaluating patients with cirrhosis for hepatocellular carcinoma: value of clinical symptomatology, imaging and alpha-fetoprotein. Oncology 2007;72:117-123.
11 Sterling RK, Jeffers L, Gordon F, Sherman M, Venook AP, Reddy KR, et al. Clinical utility of AFP-L3% measurement in North American patients with HCV-related cirrhosis. Am J Gastroenterol 2007;102:2196-2205.
12 Huo TI, Huang YH, Lui WY, Wu JC, Lee PC, Chang FY, et al. Selective prognostic impact of serum alpha-fetoprotein level in patients with hepatocellular carcinoma: analysis of 543 patients in a single center. Oncol Rep 2004;11:543-550.
13 Kemmer N, Neff G, Kaiser T, Zacharias V, Thomas M, Tevar A, et al. An analysis of the UNOS liver transplant registry: high serum alpha-fetoprotein does not justify an increase in MELD points for suspected hepatocellular carcinoma. Liver Transpl 2006;12:1519-1522.
14 Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, et al. Diagnostic and prognostic role of alphafetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006;101:524-532.
15 Taketa K, Endo Y, Sekiya C, Tanikawa K, Koji T, Taga H, et al. A collaborative study for the evaluation of lectin-reactive alpha-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res 1993;53:5419-5423.
16 Cheng HT, Chang YH, Chen YY, Lee TH, Tai DI, Lin DY. AFP-L3 in chronic liver diseases with persistent elevation of alpha-fetoprotein. J Chin Med Assoc 2007;70:310-317.
17 Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009;137:110-118.
18 Sato Y, Nakata K, Kato Y, Shima M, Ishii N, Koji T, et al. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med 1993;328:1802-1806.
19 Shiraki K, Takase K, Tameda Y, Hamada M, Kosaka Y, Nakano T. A clinical study of lectin-reactive alpha-fetoprotein as an early indicator of hepatocellular carcinoma in the follow-up of cirrhotic patients. Hepatology 1995;22:802-807.
20 Yamashita F, Tanaka M, Satomura S, Tanikawa K. Prognostic significance of Lens culinaris agglutinin A-reactive alpha-fetoprotein in small hepatocellular carcinomas. Gastroenterology 1996;111:996-1001.
21 Hayashi K, Kumada T, Nakano S, Takeda I, Sugiyama K, Kiriyama S, et al. Usefulness of measurement of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein as a marker of prognosis and recurrence of small hepatocellular carcinoma. Am J Gastroenterol 1999;94:3028-3033.
22 Igarashi H, Aoyagi Y, Suda T, Mita Y, Kawai K. Studies on the correlation among the fucosylation index, concentration of alpha-fetoprotein and des-gamma-carboxy prothrombin as prognostic indicators in hepatocellular carcinoma. Hepatol Res 2003;27:280-288.
23 Tada T, Kumada T, Toyoda H, Kiriyama S, Sone Y, Tanikawa M, et al. Relationship between Lens culinaris agglutininreactive alpha-fetoprotein and pathologic features of hepatocellular carcinoma. Liver Int 2005;25:848-853.
24 Tateishi R, Shiina S, Yoshida H, Teratani T, Obi S, Yamashiki N, et al. Prediction of recurrence of hepatocellular carcinoma after curative ablation using three tumor markers. Hepatology 2006;44:1518-1527.
25 Zhang XF, Lai EC, Kang XY, Qian HH, Zhou YM, Shi LH, et al. Lens culinaris agglutinin-reactive fraction of alphafetoprotein as a marker of prognosis and a monitor of recurrence of hepatocellular carcinoma after curative liver resection. Ann Surg Oncol 2011;18:2218-2223.
26 Nakagawa T, Miyoshi E, Yakushijin T, Hiramatsu N, Igura T, Hayashi N, et al. Glycomic analysis of alpha-fetoprotein L3 in hepatoma cell lines and hepatocellular carcinoma patients. J Proteome Res 2008;7:2222-2233.
27 Noda K, Miyoshi E, Uozumi N, Yanagidani S, Ikeda Y, Gao C, et al. Gene expression of alpha1-6 fucosyltransferase in human hepatoma tissues: a possible implication for increased fucosylation of alpha-fetoprotein. Hepatology 1998;28:944-952.
28 Mita Y, Aoyagi Y, Suda T, Asakura H. Plasma fucosyltransferase activity in patients with hepatocellular carcinoma, with special reference to correlation with fucosylated species of alphafetoprotein. J Hepatol 2000;32:946-954.
29 Noda K, Miyoshi E, Kitada T, Nakahara S, Gao CX, Honke K, et al. The enzymatic basis for the conversion of nonfucosylated to fucosylated alpha-fetoprotein by acyclic retinoid treatment in human hepatoma cells: activation of alpha1-6 fucosyltransferase. Tumour Biol 2002;23:202-211.
Received November 23, 2011
Accepted after revision March 7, 2012
nsecutive patients who had
hepatectomy for HCC in our hospital between August 2003 and December 2004, 318 were included in this study. The excluded were 26 patients who died from other diseases and 15 patients who failed to return for follow-up examination and interview. All the 318 patients met the following criteria: 1) HCC was the primary preoperative diagnosis without extrahepatic metastasis; 2) preoperative serum AFP was positive (>20 ng/mL); 3) there was no evidence of extrahepatic causes contributing to AFP elevation; 4) there was no preoperative history of chemotherapy, radiotherapy or other treatments; and 5) hepatectomy was the first surgical treatment. The diagnoses of HCC were confirmed by both surgery and pathology in all patients. This study protocol was approved by the Biomedical Ethics Committee of our hospital.
Author Affiliations: The 4th Department of Hepatic Surgery (Zhang XF, Wang K, Zhang ZQ and Shi LH) and Molecular Oncology Laboratory (Yin ZF and Qian HH), Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China
Le-Hua Shi, MD, the 4th Department of Hepatic Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China (Tel: 86-21-81875241; Fax: 86-21-65562400; Email: slhehbh@126.com)
© 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(12)60234-3
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer
- Risk factors and incidence of acute pyogenic cholangitis
- Endoscopic sphincterotomy associated cholangitis in patients receiving proximal biliary self-expanding metal stents
- Biliary drainage for obstructive jaundice caused by unresectable hepatocellular carcinoma: the endoscopic versus percutaneous approach
- Gallstone-related complications after Roux-en-Y gastric bypass: a prospective study
- Inhibiting the expression of hepatocyte nuclear factor 4 alpha attenuates lipopolysaccharide/ D-galactosamine-induced fulminant hepatic failure in mice
