Radiological prognosticators of hepatocellular carcinoma treated by hepatectomy
2012-07-10
Hong Kong, China
Radiological prognosticators of hepatocellular carcinoma treated by hepatectomy
Kevin KW Chu, See Ching Chan, Sheung Tat Fan, Kenneth SH Chok, Tan To Cheung, William W Sharr, Albert CY Chan and Chung Mau Lo
Hong Kong, China
BACKGROUND:Hepatectomy is the main curative treatment for hepatocellular carcinoma (HCC), but postoperative longterm survival is poor. Preoperative radiological features of HCC displayed by computed tomography or magnetic resonance imaging could serve as additional prognostic factors. This study aimed to identify preoperative radiological features of HCC that may be of prognostic significance in hepatectomy.
METHODS:Ninety-two patients who underwent hepatectomy for HCC were included in this study. Preoperative radiological features including tumor number, size, location (peripheral, middle, central), portal vein invasion, hepatic vein invasion, and presence of pseudo-capsule were analyzed in relation to survival.
RESULTS:With a median follow-up period of 41.7 months, the 1-, 3- and 5-year overall survival rates were 85%, 65% and 58%, respectively. Univariate analysis showed that portal vein invasion and absence of pseudo-capsule were significant prognostic factors for overall survival, while all the examined radiological features were prognostic factors for disease-free survival. Multivariate analysis for overall survival found no significant factor. On multivariate analysis for disease-free survival, patients who had tumors with portal vein invasion had poorer survival with a hazard ratio of 2.26 (95% CI, 1.05-4.91;P=0.038) and patients with single nodular HCC or pseudo-capsulated HCC had better survival with a hazard ratio of 0.50 (95% CI, 0.27-0.94;P=0.032) and 0.38 (95% CI, 0.14-0.99;P=0.048), respectively.
CONCLUSIONS:Demonstrable pseudo-capsule of HCC and solitary HCC on imaging and absence of portal vein invasionare features associated with better disease-free survival after hepatectomy. These features may guide treatment planning for HCC.
(Hepatobiliary Pancreat Dis Int 2012;11:612-617)
hepatocellular carcinoma;hepatectomy; diagnostic imaging; prognosis; survival analysis
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most common cause of cancer-related deaths worldwide.[1]It has a higher mortality rate among cancers. Hepatectomy, liver transplantation, and local ablative therapies[2]are the treatment modalities which may provide a chance of cure for HCC. The common mode of treatment failure is tumor recurrence.[3,4]Tumor size and tumor number are associated with disease recurrence after hepatectomy[3,5]or liver transplantation.[6]Venous invasion is another consistently reported risk factor for recurrence after hepatectomy, as intrahepatic metastasis via the portal venous system is an important mechanism of cancer spread.[7,8]These factors have been included in the more recent HCC staging systems.[9,10]However, the prognostic value of the presence of pseudo-capsule and the location of HCC has not been fully examined. They could be important in the treatment planning for the disease. We studied the preoperative radiological features of HCC displayed by computed tomography or magnetic resonance imaging with the aim of identifying features which may be of prognostic significance in hepatectomy.
Methods
The study covered patients who underwent resection ofHCC at our hospital from January 2005 to December 2008. Patient data including preoperative imaging details were collected prospectively and stored in a single database. Preoperative imaging details were reviewed blindly, and the data on tumor number and size, portal vein invasion, hepatic vein invasion and presence of pseudo-capsule were analyzed retrospectively. The radiological features are illustrated in Fig. 1. The lesions were categorized into three types: peripheral lesions that did not involve the middle one-third of the liver; middle lesions that did not involve the inner one-third of the liver; and central lesions that involved the inner onethird of the liver. Pseudo-capsules were hypoattenuating or hypointense rings on unenhanced computed tomography scans or magnetic resonance imaging scans and became hyperattenuating or hyperintense in the portal-dominant phase.[11]
Values were expressed as median with range. Nonparametric tests were used for analysis. The primary end-point was overall survival and the second endpoint was disease-free survival. Survival curves were computed with the Kaplan-Meier method and compared between groups by the log-rank test. Cox proportional hazard models were performed to define factors that determined the overall and disease-free survival rates. All statistical analyses were made using statistical software (SPSS 16.0 for Windows, SPSS Inc., Chicago, IL., USA). APvalue <0.05 was considered to be statistically significant.
Results
Ninety-two patients were the subjects of the study. We had their full set of relevant data including radiological details. Table 1 lists the patient demographics and radiological and histopathological features of the HCCs. Radiological imaging showed that 53 patients had amedian largest tumor size of >5 cm, 15 between 3 and 5 cm, and 24 with <3 cm. The majority of patients (75/92, 81.5%) had single nodular HCC. Forty-three (46.7%) had gross hepatic vein invasion and 40 (43.5%) of these had gross portal vein invasion. The feature of pseudocapsule was present in 18 (19.6%) patients.
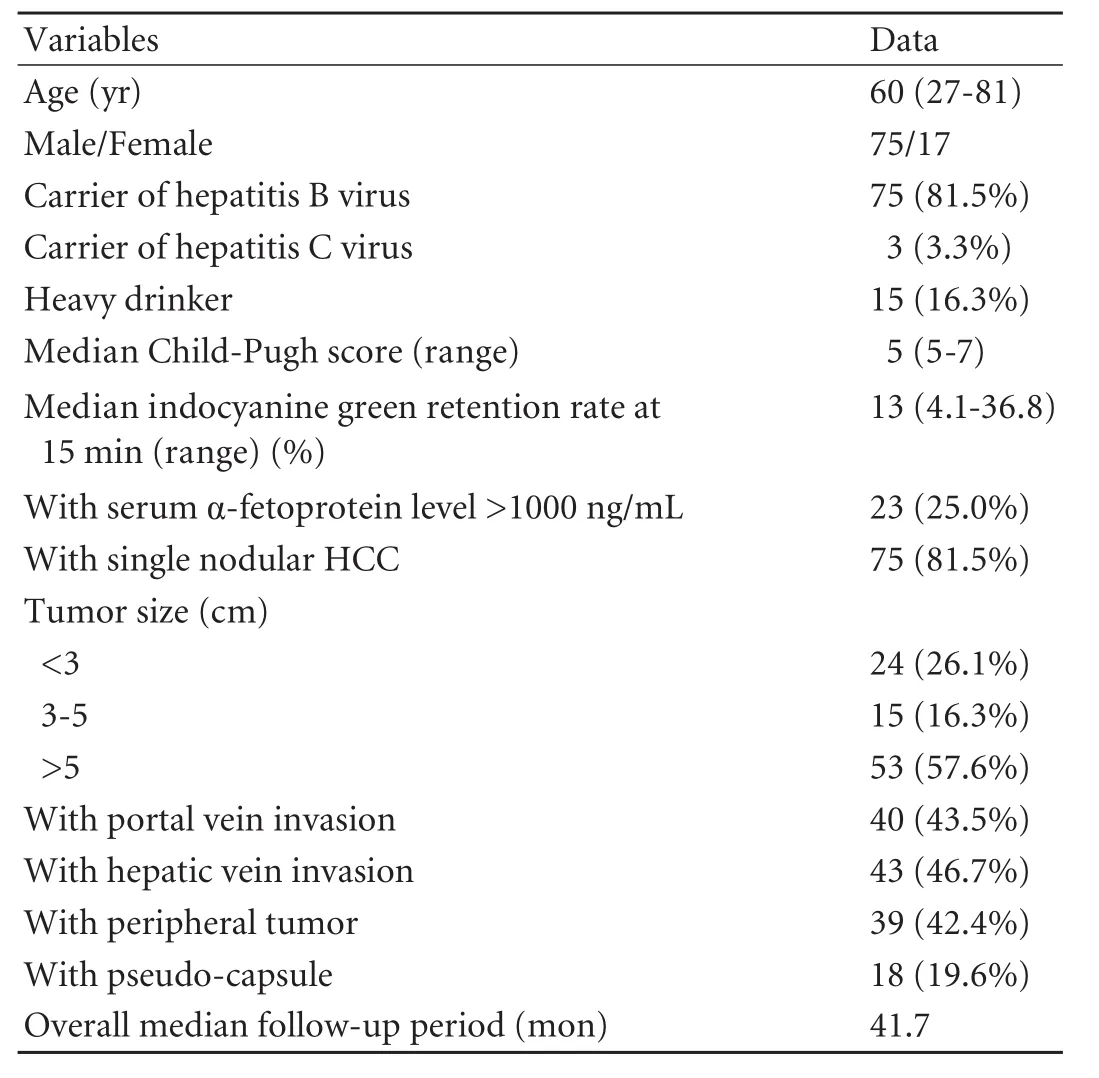
Table 1.Patient demographics and clinicopathological and radiological features of HCCs
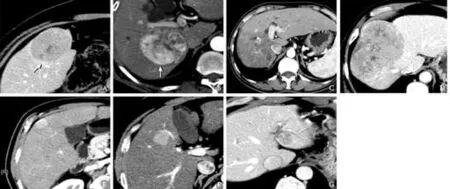
Fig. 1.HCCs on contrast CT scans.A, B:Two tumors with pseudo-capsule (arrows);C:A tumor invading the right portal vein;D:A tumor invading the right hepatic vein;E-G:Tumors at the peripheral (E), middle (F), and central (G) one-third of the liver.
With a median follow-up period of 41.7 months, the 1-, 3-, and 5-year overall survival rates were 85%, 65%, and 58%, respectively (Fig. 2A). The median diseasefree survival rate was 22.1 months (Fig. 3A). Patients who had HCC with pseudo-capsule had better overall survival (P=0.038) and disease-free survival (P=0.01) (Fig. 2E, 3E). Patients who had HCC with portal vein invasion had poorer overall survival (P=0.004) and disease-free survival (<0.001). Patients who had central lesions tended to have poorer overall survival (P=0.057). Patients who had solitary, small, or peripheral tumors had better disease-free survival (P<0.05) but not overall survival. Patients who had hepatic vein invasion had poorer disease-free survival (P=0.016) (Fig. 3G) but not overall survival (Fig. 2G).
Multivariate analysis found that no factor was significantly associated with overall survival, and that patients who had tumors with portal vein invasion had poorer disease-free survival with a hazard ratio of 2.26 (95% CI, 1.05-4.91;P=0.038) and patients who had single nodular HCC or pseudo-capsulated HCC had better disease-free survival with hazard ratios of 0.50 (95% CI, 0.27-0.94;P=0.032) and 0.38 (95% CI, 0.14-0.99;P=0.048), respectively (Table 2).
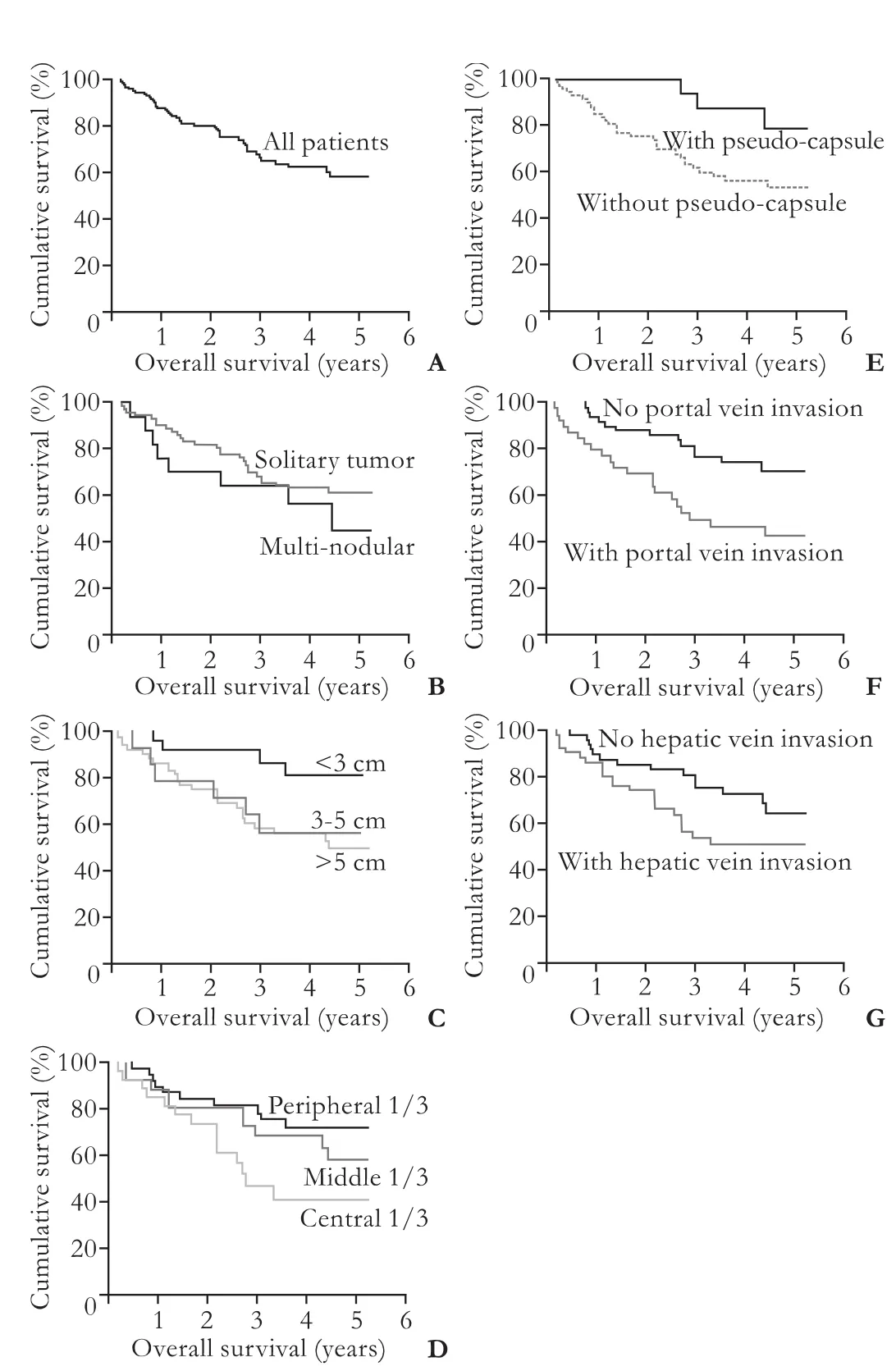
Fig. 2.Overall survival curves of all patients (A) and patient groups with different radiological HCC features: number (B,P=0.301), size (C,P=0.077), location (D,P=0.057), pseudocapsulated (E,P=0.038), portal vein invasion (F,P=0.004), hepatic vein invasion (G,P=0.076).
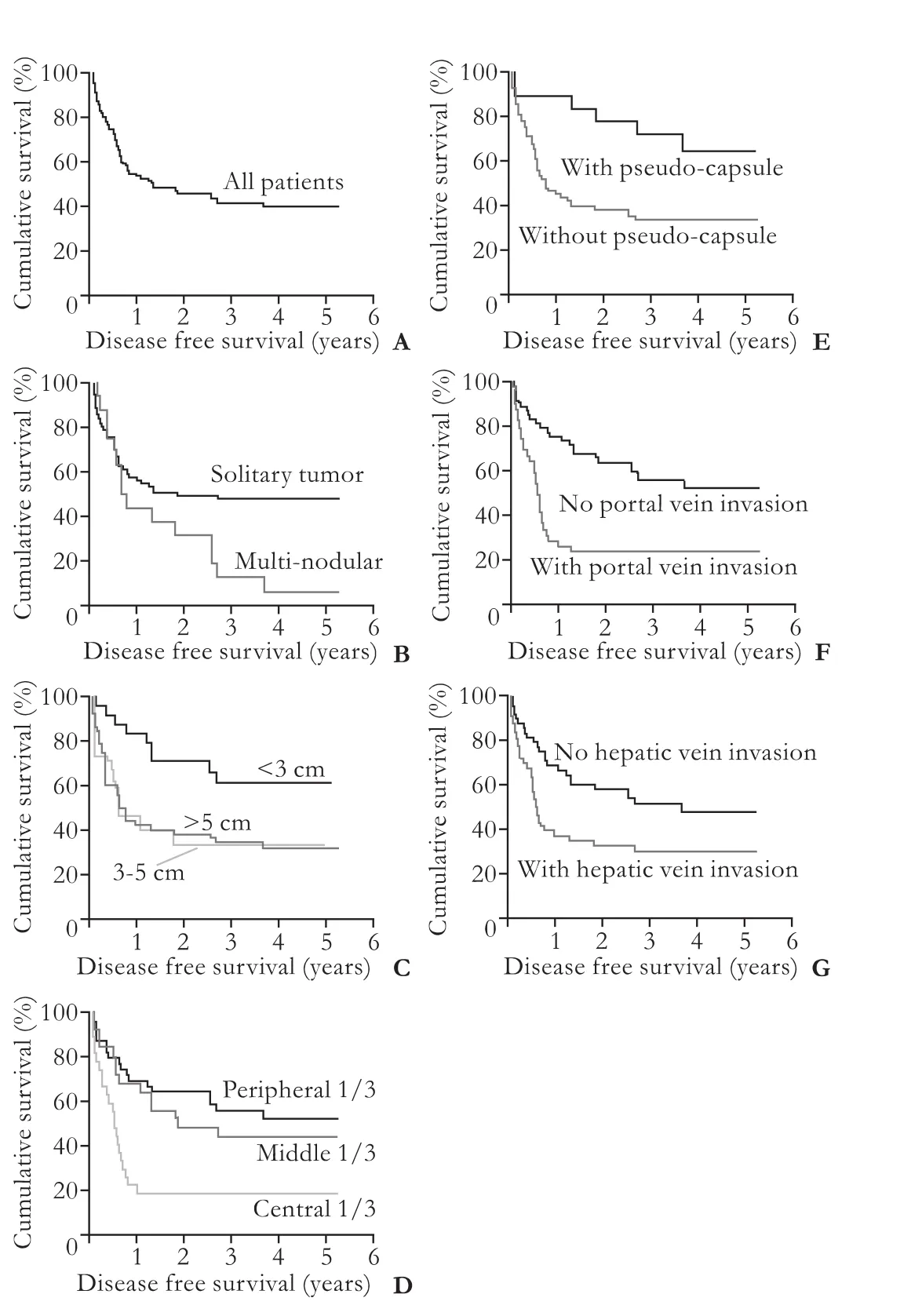
Fig. 3.Disease-free survival curves of all patients (A) and patient groups with different radiological HCC features: number (B,P=0.01), size (C,P=0.027), location (D,P=0.001), pseudocapsulated (E,P=0.01), portal vein invasion (F,P=0.0002), hepatic vein invasion (G,P=0.016).
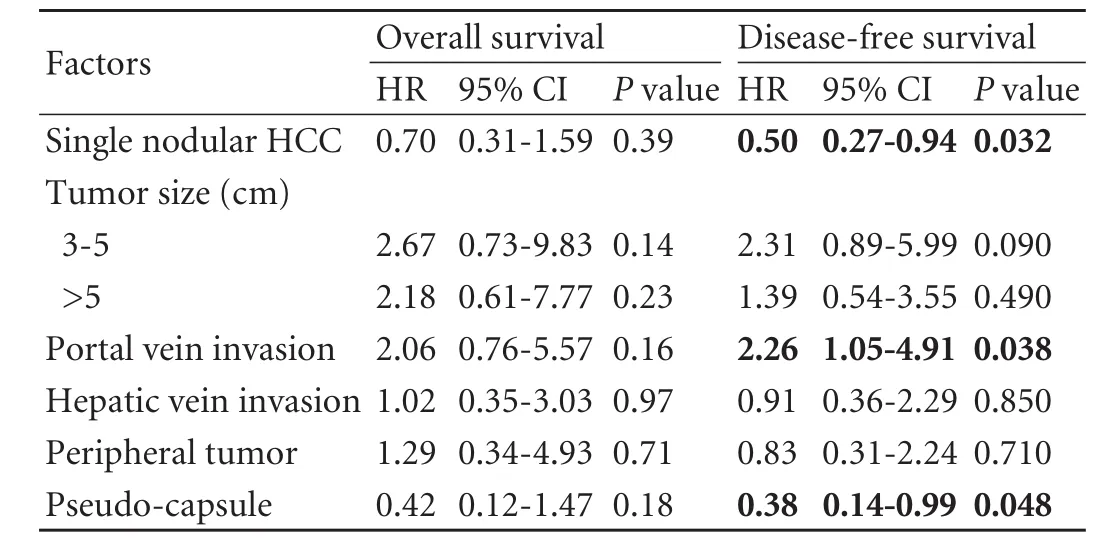
Table 2.Multivariate analysis of factors affecting overall and disease-free survival
Discussion
The presence of pseudo-capsule was found to be a favorable prognostic factor in the study. Nevertheless, this feature is not included in existing HCC staging systems. In fact, more aggressive treatment can be considered for patients who have HCC with pseudocapsule, particularly potential candidates for primary or salvage liver transplantation, despite a large HCC. In the study, pseudo-capsules were present in 19.6% of patients with HCCs between 1.8 and 9.8 cm. It has been reported that solitary large HCCs (>5 cm in diameter) growing expansively within an intact capsule or pseudocapsule have a clinical outcome similar to that of small HCCs and significantly better than that of nodular HCCs,[12]and that histologically confirmed encapsulated HCCs entail a better long-term outcome.[13,14]Pseudocapsulated HCC with a peripheral enhancing rim can be detected by computed tomography or magnetic resonance imaging.[15-17]Histopathologically, the pseudo-capsule is composed of an inner layer rich in collagen and an outer layer containing various amounts of the portal vein, bile duct, compressed artery and collagen fibers.[18,19]Encapsulation is associated with markedly lower tumor invasiveness represented by a lower incidence of venous permeation, liver invasion, and tumor microsatellite formation as well as a higher incidence of negative surgical margins.[20]
HCC is characterized by its propensity for vascular invasion. Portal venous invasion is widely accepted as an important mechanism of intrahepatic recurrence.[21]T3b (major vascular invasion of portal or hepatic veins) in the 7th AJCC staging system has been shown to have poorer prognosis than T3a (multiple tumors, any >5 cm).[22]Microvascular invasion is also regarded as a histological prognostic factor, and irregular circumferential peritumoral enhancement on magnetic resonance imaging has been reported to be a surrogate marker.[23]It was not surprising to find a poorer outcome in HCC patients with portal venous invasion in our study. HCC with pseudo-capsule shown in radiological imaging entailed a better outcome after hepatectomy. The findings are consistent with a previous pathological finding that encapsulated tumors show a lower incidence of direct liver invasion, tumor microsatellite formation, and venous permeation compared with nonencapsulated ones.[16]
In the present study, disease recurrence increased with portal vein invasion and decreased with HCC which was solitary or with pseudo-capsule. In principle, anatomical resection should eradicate the spread of HCC via the portal vascular territory of the supplied segments. However, such a surgical plan may not be executed precisely in all cases in practice. On the other hand, HCC which is solitary or with pseudo-capsule is a feature of expanding instead of permeating growth of the tumor, albeit malignant. Achieving adequate and clear margins will be easier if reference is made to preoperative imaging or intraoperative ultrasonography. In the present study, patients with HCCs which were central in location had poorer disease-free survival (P=0.001) and tended to have poorer overall survival (P=0.057). The central location of the lesions increased the chance of involvement of more proximal branches of the portal vein and thus intrahepatic spread of the tumor.
The present study only included patients with full documentation of preoperative imaging in the hospital network system, so these were not consecutive cases, which could be a potential source of bias. Nonetheless, all the images were from one single institute using one single protocol, which lowered the chance of error caused by variation in image quality.
The presence of pseudo-capsule and portal vein invasion were associated with overall survival (P<0.05, univariate analysis) but they were not independent predictive factors (P>0.05, multivariate analysis). Actually, there was a negative correlation between portal vein invasion and the presence of pseudo-capsule; nearly all tumors with portal vein invasion (38 of 40 patients, 95%) were not encapsulated.
Prognostication is of value for patients to know their chance of cure. If disease-free survival is much worse than overall survival, a radical change of treatment modality should be considered. A staging system should also direct treatment strategy. Primary liver transplantation, which incorporates total native liver hepatectomy, is the most radical oncological resection of HCC and gives patients the lowest chance of local recurrence. Also a radical secondary prevention measure,it eliminates the development of new HCC.[24]
In current patient selection systems for liver transplantation, for example the Milan,[6]UCSF[25]and Hangzhou criteria,[26]tumor morphology is not included but only size and number. The presence of multiple tumors (n>3) is more likely to entail post-transplant recurrence and is related to poorer patient survival after transplantation.[27]However, it has been shown that the correlation between imaging findings and explant histology in tumor size and number is not accurate enough, especially for small lesions.[28]We have found in this study that demonstrable pseudo-capsule of HCC and solitary HCC on imaging and absence of portal vein invasion are features implying better disease-free survival after hepatectomy. Therefore, the inclusion of tumor morphology may improve the prognostic power of these selection systems.
Contributors:CKKW, CKSH, CTT, SWW and CACY collected data. CKKW analyzed the data and drafted the manuscript. CSC and FST made critical revisions and approved the manuscript. LCM approved the manuscript. CSC is the guarantor.
Funding:None.
Ethical approval:Not needed
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-2917.
2 Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST, et al. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg 2004;239:441-449.
3 Hwang S, Moon DB, Lee SG. Liver transplantation and conventional surgery for advanced hepatocellular carcinoma. Transpl Int 2010;23:723-727.
4 Poon RT, Fan ST. Hepatectomy for hepatocellular carcinoma: patient selection and postoperative outcome. Liver Transpl 2004;10:S39-45.
5 Shimada K, Sakamoto Y, Esaki M, Kosuge T, Morizane C, Ikeda M, et al. Analysis of prognostic factors affecting survival after initial recurrence and treatment efficacy for recurrence in patients undergoing potentially curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol 2007;14:2337-2347.
6 Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-699.
7 Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 2000;89:500-507.
8 Taketomi A, Toshima T, Kitagawa D, Motomura T, Takeishi K, Mano Y, et al. Predictors of extrahepatic recurrence after curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol 2010;17:2740-2746.
9 Cho YB, Lee KU, Lee HW, Cho EH, Yang SH, Cho JY, et al. Outcomes of hepatic resection for a single large hepatocellular carcinoma. World J Surg 2007;31:795-801.
10 Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol 2002;20:1527-1536.
11 Itai Y, Ohtomo K, Kokubo T, Yamauchi T, Minami M, Yashiro N, et al. CT of hepatic masses: significance of prolonged and delayed enhancement. AJR Am J Roentgenol 1986;146:729-733.
12 Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ, Wu F. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg 2009;249:118-123.
13 Ng IO, Lai EC, Ng MM, Fan ST. Tumor encapsulation in hepatocellular carcinoma. A pathologic study of 189 cases. Cancer 1992;70:45-49.
14 Okuda K, Musha H, Nakajima Y, Kubo Y, Shimokawa Y, Nagasaki Y, et al. Clinicopathologic features of encapsulated hepatocellular carcinoma: a study of 26 cases. Cancer 1977; 40:1240-1245.
15 Ebara M, Ohto M, Watanabe Y, Kimura K, Saisho H, Tsuchiya Y, et al. Diagnosis of small hepatocellular carcinoma: correlation of MR imaging and tumor histologic studies. Radiology 1986;159:371-377.
16 Imaeda T, Kanematsu M, Mochizuki R, Goto H, Saji S, Shimokawa K. Extracapsular invasion of small hepatocellular carcinoma: MR and CT findings. J Comput Assist Tomogr 1994;18:755-760.
17 Nino-Murcia M, Olcott EW, Jeffrey RB Jr, Lamm RL, Beaulieu CF, Jain KA. Focal liver lesions: pattern-based classification scheme for enhancement at arterial phase CT. Radiology 2000;215:746-751.
18 Kadoya M, Matsui O, Takashima T, Nonomura A. Hepatocellular carcinoma: correlation of MR imaging and histopathologic findings. Radiology 1992;183:819-825.
19 Ueda K, Matsui O, Kawamori Y, Nakanuma Y, Kadoya M, Yoshikawa J, et al. Hypervascular hepatocellular carcinoma: evaluation of hemodynamics with dynamic CT during hepatic arteriography. Radiology 1998;206:161-166.
20 Ng IO, Lai EC, Fan ST, Ng MM, So MK. Prognostic significance of pathologic features of hepatocellular carcinoma. A multivariate analysis of 278 patients. Cancer 1995;76:2443-2448.
21 Arii S, Tanaka J, Yamazoe Y, Minematsu S, Morino T, Fujita K, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992;69:913-919.
22 Chun YH, Kim SU, Park JY, Kim do Y, Han KH, Chon CY, et al. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. Eur J Cancer 2011;47:2568-2575.
23 Kim H, Park MS, Choi JY, Park YN, Kim MJ, Kim KS, et al. Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol 2009;19:1744-1751.
24 Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K,Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200-207.
25 Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-1403.
26 Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-1732
27 Chan KM, Chou HS, Wu TJ, Lee CF, Yu MC, Lee WC. Characterization of hepatocellular carcinoma recurrence after liver transplantation: perioperative prognostic factors, patterns, and outcome. Asian J Surg 2011;34:128-134.
28 Bhattacharjya S, Bhattacharjya T, Quaglia A, Dhillon AP, Burroughs AK, Patch DW, et al. Liver transplantation in cirrhotic patients with small hepatocellular carcinoma: an analysis of pre-operative imaging, explant histology and prognostic histologic indicators. Dig Surg 2004;21:152-160.
Accepted after revision June 1, 2012
News
Joint Liver Cancer Center established by Cleveland Clinic and the First Affiliated Hospital, Zhejiang University in Hangzhou, China

A joint liver cancer center has been launched by signing a cooperative agreement between Cleveland Clinic at Cleveland, Ohio, USA and the First Affiliated Hospital, Zhejiang University on November 16, 2012 at Hangzhou, China. In recent years, a series of cooperative projects have been carried out between medical centers of other countries and the First Affiliated Hospital. This endeavor facilitates international cooperation between the First Affiliated Hospital and other oversea centers in faculty training and basic research in liver cancer.
January 27, 2012
10.1016/S1499-3872(12)60233-1)

Author Affiliations: Department of Surgery (Chu KKW, Chan SC, Fan ST, Chok KSH, Cheung TT, Sharr WW, Chan ACY and Lo CM), and State Key Laboratory for Liver Research (Chan SC, Fan ST and Lo CM), The University of Hong Kong, 102 Pokfulam Road, Hong Kong, China
See Ching Chan, Professor, Department of Surgery, The University of Hong Kong, 102 Pokfulam Road, Hong Kong, China (Tel: 852-22553025; Fax: 852-28165284; Email: seechingchan@ gmail.com)
© 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
doi: 10.1016/S1499-3872(12)60232-X
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer
- Risk factors and incidence of acute pyogenic cholangitis
- Endoscopic sphincterotomy associated cholangitis in patients receiving proximal biliary self-expanding metal stents
- Biliary drainage for obstructive jaundice caused by unresectable hepatocellular carcinoma: the endoscopic versus percutaneous approach
- Gallstone-related complications after Roux-en-Y gastric bypass: a prospective study
- Inhibiting the expression of hepatocyte nuclear factor 4 alpha attenuates lipopolysaccharide/ D-galactosamine-induced fulminant hepatic failure in mice
